Although subcutaneous treatments for multiple sclerosis (MS) have been shown to be effective, adverse reactions and pain may adversely affect treatment satisfaction and adherence. This study presents an adapted and validated Spanish version of the Multiple Sclerosis Treatment Concerns Questionnaire© (MSTCQ), which evaluates satisfaction with the injection device (ID) across 4 domains: injection system (A), side effects (B) (flu-like symptoms, reactions, and satisfaction), experience with treatment (C) and benefits (D).
MethodsTwo study phases: (1) Cultural adaptation process with input from experts (n=6) and patients (n=30). (2) Validation obtained by means of an observational, cross-sectional, multi-centre study evaluating 143 adult MS patients using an ID. Tools employed: MSTCQ©, Patient-Reported Indices for Multiple Sclerosis (PRIMUS©), and Treatment Satisfaction Questionnaire for Medication (TSQM©). Psychometric properties: Feasibility (percentage of valid cases and floor/ceiling effects); Reliability (Cronbach α) and test–retest correlation (n=41, intraclass correlation coefficient, ICC); and construct validity (factor analysis of domains A and B) and convergent validity (Spearman rank-order correlation for MSTCQ© vs TSQM©).
ResultsMean age (SD) was 41.94 (10.47) years, 63% of the group were women, and 88.11% presented relapsing-remitting MS. Mean (SD) EDSS score was 2.68 (1.82) points. MSTCQ© completion was high (0%-2.80% missing data). Internal consistency was high at α=0.89 for the total score (A+B) and α=0.76, 0.89, and 0.92 for domains A, B, and C, respectively. The version demonstrated excellent test–retest reliability for the total (ICC=0.98) and for domains A, B, and C: ICC=0.82, 0.97, and 0.89, respectively. Factor analysis corroborated the internal structure of the original questionnaire. The association between total and domain scores on both the MSTCQ© and the TSQM© was moderately strong (Rho=0.42-0.74) and significant (P<.05 and P<.01).
ConclusionThe Spanish version of MSTCQ© demonstrates appropriate psychometric properties.
A pesar de la efectividad de los tratamientos inyectables para la esclerosis múltiple (EM), las reacciones adversas y el dolor pueden implicar problemas de satisfacción y adherencia. Se presenta la validación de la versión española del Multiple Sclerosis Treatment Concerns Questionnaire (MSTCQ)©, que evalúa la satisfacción con el dispositivo de autoinyección (DA), 4 dimensiones: sistema de inyección (A), efectos secundarios (B) (síntomas pseudogripales, reacciones, satisfacción), experiencia con el tratamiento (C) y beneficios (D).
MétodosDos fases de estudio: 1) Adaptación cultural con expertos (n=6) y pacientes (n=27). 2) Estudio observacional, transversal y multicéntrico de validación. Se evaluaron 143 pacientes adultos con EM que utilizaban el DA Extaviject™30G. Cuestionarios: MSTCQ©; Patient-Reported Indices for Multiple Sclerosis (PRIMUS©), y Treatment Satisfaction Questionnaire for Medication (TSQM©). Propiedades psicométricas: factibilidad (% casos válidos y distribución de puntuaciones); fiabilidad (α-Cronbach) y test-retest (n=41, coeficiente correlación intraclase [CCI]), y validez de constructo (análisis factorial A y B, [AF]) y convergente (Spearman-rho MSTCQ© vs TSQM©).
ResultadosEdad media (DT) 41,94 (10,47) años, 63% mujeres, 88,11% con EM remitente-recurrente, media (DT) EDSS 2,68 (1,82) puntos. Alta cumplimentación del MSTCQ© (perdidos 0-2,80%). Alta consistencia interna: puntuación total (A+B) α=0,89, por dimensiones (A, B y C) α=0,76, 0,89 y 0,92, respectivamente. Excelente concordancia test-retest en las puntuación total (CCI=0,98), por dimensiones (A, B y C) CCI=0,82, 0,97 y 0,89, respectivamente. El AF corroboró la estructura interna del cuestionario original. Correlación moderada (Rho=0,42-0,74) y significativa (p<0,05 y p<0,01) entre las puntuación total y por dimensiones del MSTCQ© y el TSQM©.
ConclusionesSe constatan adecuadas propiedades psicométricas de la versión española del MSTCQ©.
Multiple sclerosis (MS) is a chronic demyelinating and immune-mediated neurological disease affecting the central nervous system (CNS).1,2 Three main clinical subtypes3 have been distinguished: the relapsing-recurring course (RRMS), characterised by acute recurring episodes of neurological symptoms (exacerbations) followed by total or partial remission of symptoms with no progression between episodes; the secondary progressive course (SPMS), a more advanced form of RR in which symptoms worsen progressively but are unrelated to exacerbations; and the primary progressive course (PPMS), which worsens steadily from onset apart from a few temporary stable periods. Clinically isolated syndrome (CIS) refers to the first episode of neurological symptoms caused by inflammation or demyelination within the CNS.4
The main treatment objectives in MS are to control exacerbations and reduce both their frequency and the progression of related disability using disease modifying therapies (DMT). First line treatments include interferon beta or glatiramer acetate; second line treatments are fingolimod or natalizumab.4 IFN beta-1b (IFNβ-1b) (Betaferon®/Extavia®) has been proved an effective and safe means of reducing the number and severity of clinical exacerbations,5–7 and its efficacy has been shown to exceed that of IFNβ-1a.8 It is indicated for patients with CIS, RRMS, and SPMS with exacerbations. Nevertheless, the route and schedule of administration (subcutaneously, every other day) leads to injection site reactions (ISR) and pain,9 as well as flu-like side effects. These consequences may give rise to low patient satisfaction and poor treatment adherence.10,11
In general, a patient who is satisfied with a specific treatment prescribed by his/her doctor will show better adherence.12 Adherence to MS treatment with IFNβ-1b improves with self-injection using fine needles, since these measures have been clinically shown to reduce ISRs.13 This finding led to the development of safer self-injection devices (IDs) that tend to alarm patients less than conventional needles do. One such ID is the ExtaviJect™30G for subcutaneous administration of IFNβ-1b. Its advantages include the small diameter (0.31mm) of the needle and its system for selecting different injection depths within the optimal subcutaneous area (8, 10, or 12mm below the surface of the skin).14
The Multiple Sclerosis Treatment Concerns Questionnaire (MSTCQ©) was designed and validated in English as a means of measuring the satisfaction of MS patients who use ID to administer IFNβ-1a.15 The Cronbach alpha coefficient showed an acceptable level of internal consistency for the original version of this instrument (that is, all items on each dimension measure the same construct); furthermore, the intraclass correlation coefficient (ICC) indicted that scores showed a high degree of temporal stability and acceptable construct validity (scores were significantly correlated with the analogue visual pain scale and with the short form of the McGill Pain Questionnaire).
The MSTCQ© consists of 9 items measuring satisfaction with the ‘injection system’ (dimension A), α=0.70, ICC=0.68; and 11 items measuring the impact of the most common adverse effects (AE) in patients treated with IFNβ-1a (dimension B). Dimension B is composed of 3 subscales: flu-like AEs, α=0.82, ICC=0.86; injection site reactions (ISR), α=0.68, ICC=0.73; and overall satisfaction, α=0.75, ICC=0.77. All answers are given on 5-point Likert scales. The total score on the questionnaire is the sum of dimensions A and B (20 items).
Furthermore, the questionnaire's follow-up version includes an ‘experience with treatment’ section (dimension C). This dimension includes 10 items referring to changes in the patient's experience with the treatment since beginning to use a new ID. An additional item, 11, asks patients for an overall assessment of the experience of changing the device using an 11-point visual analogue scale (VAS) ranging from −5 to 5 points. The last section examines preferences (dimension D); patients are asked to rank 4 out of 5 benefits of the device in order of importance.
This study was designed so that clinical neurologists in Spain would be able to use the MSTCQ© to measure satisfaction in patients with MS who use the ExtaviJect™30G device to administer IFNβ-1b. It entailed providing a cultural adaptation of the questionnaire and validating the version in Spanish. As a secondary objective, we evaluated satisfaction, acceptance, tolerance, adherence to treatment, and health-related quality of life (HRQOL) among patients who use the ExtaviJect™30G device.
Patients and methodsThis study was designed in 2 stages: the first stage involved adapting the MSTCQ© to a Spanish setting15; after that, we evaluated feasibility, reliability, and validity (construct and convergent) for the psychometric properties.
Cultural adaptationBetween March and July 2011, 2 independent expert translators, one from Spain and the other from the UK, provided an English to Spanish translation of the original MSTCQ©15 and a back-translation from Spanish to English. A translation task force consisting of the 2 translators from the first phase and 2 psychologists with ample training in the cultural development of health questionnaires determined the final wording of the Spanish version.
Using an ad hoc form, a panel of expert neurologists and nurses with experience caring for MS patients (n=6), together with adult patients with MS selected by consecutive sampling (n=27) evaluated the pertinence and readability of each item on the Spanish version of the MSTCQ© on a scale of 0 to 4 (from the least to the most favourable rating). Participants in this panel were permitted to leave alternative wordings and comments on the different items using an open field on the form.
Both experts and patients rated the questionnaire well for relevance and readability. Qualitative analysis of the open responses led to the conclusion that items on this new version of the questionnaire were appropriate. The Spanish version of the MSTCQ© was then approved.
Assessment of psychometric propertiesThis observational, transversal, and multi-centre study was approved by the Ethics Committee at Parc de Salut Mar in Barcelona. Between October 2011 and July 2012 (9 months), we gathered data from 143 patients seen in 15 neurology clinics pertaining to public hospitals all over Spain.
Before participating in the study, patients gave their informed consent in writing. To be included, patients had to be aged 18 or older, diagnosed with CIS, RRMS, or SPMS with exacerbations, and show a history of at least 3 months of treatment with IFNβ-1b delivered using the ExtaviJect™30G device.
Patients were assessed during single visits on 2 separate occasions (test and retest). During tests, neurologists recorded sociodemographic and basic clinical data (Table 1), while nursing staff completed the ad hoc Trainer User Trial Questionnaire. Patients filled in the following self-administered questionnaires: MS Treatment Concerns Questionnaire (MSTCQ©),15 Patient-Reported Indices for Multiple Sclerosis instrument (PRIMUS©),16 Treatment Satisfaction Questionnaire for Medication (TSQM©),17 Patient/Trainer User Trial Questionnaire, Patient Injection Site Reaction and Injection Site Pain (ISR&ISP),13 and the Morisky–Green treatment adherence test (M-G). In the retest performed to calculate the stability of scores on the adapted version of the MSTCQ©, 41 patients out of the total sample, representing 6 participating hospitals, repeated the questionnaire at 7±2 days after the initial session. Experts also applied the Clinical Global Impressions Scale for Improvement (CGI-I) to detect any clinically significant changes occurring after the first test.
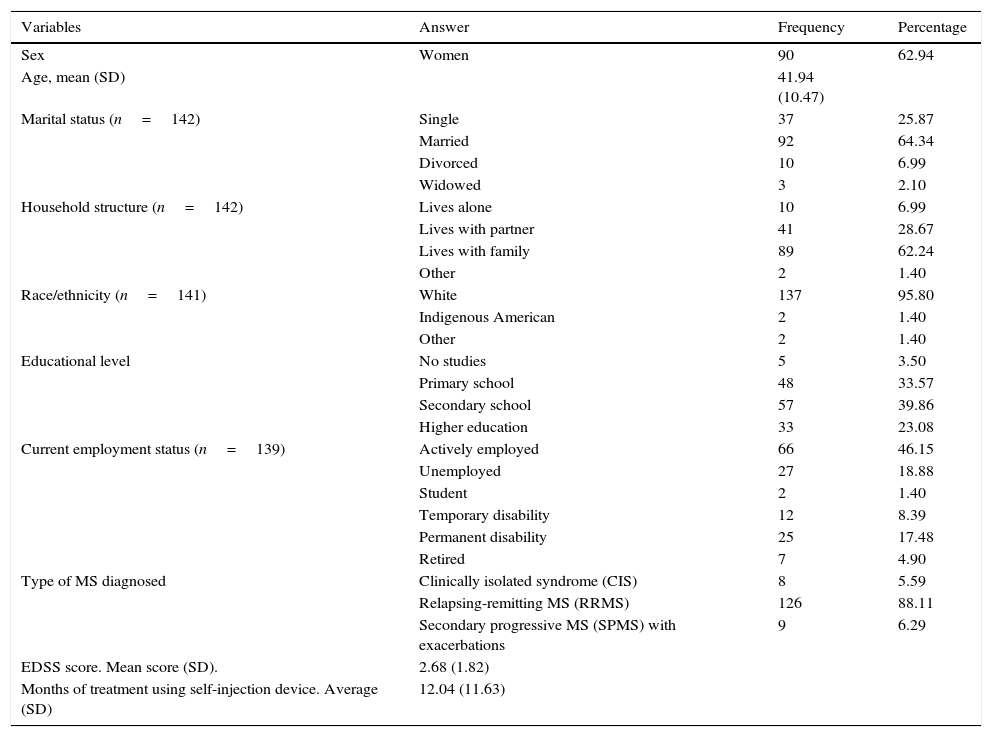
Sociodemographic and clinical variables (n=143).
| Variables | Answer | Frequency | Percentage |
|---|---|---|---|
| Sex | Women | 90 | 62.94 |
| Age, mean (SD) | 41.94 (10.47) | ||
| Marital status (n=142) | Single | 37 | 25.87 |
| Married | 92 | 64.34 | |
| Divorced | 10 | 6.99 | |
| Widowed | 3 | 2.10 | |
| Household structure (n=142) | Lives alone | 10 | 6.99 |
| Lives with partner | 41 | 28.67 | |
| Lives with family | 89 | 62.24 | |
| Other | 2 | 1.40 | |
| Race/ethnicity (n=141) | White | 137 | 95.80 |
| Indigenous American | 2 | 1.40 | |
| Other | 2 | 1.40 | |
| Educational level | No studies | 5 | 3.50 |
| Primary school | 48 | 33.57 | |
| Secondary school | 57 | 39.86 | |
| Higher education | 33 | 23.08 | |
| Current employment status (n=139) | Actively employed | 66 | 46.15 |
| Unemployed | 27 | 18.88 | |
| Student | 2 | 1.40 | |
| Temporary disability | 12 | 8.39 | |
| Permanent disability | 25 | 17.48 | |
| Retired | 7 | 4.90 | |
| Type of MS diagnosed | Clinically isolated syndrome (CIS) | 8 | 5.59 |
| Relapsing-remitting MS (RRMS) | 126 | 88.11 | |
| Secondary progressive MS (SPMS) with exacerbations | 9 | 6.29 | |
| EDSS score. Mean score (SD). | 2.68 (1.82) | ||
| Months of treatment using self-injection device. Average (SD) | 12.04 (11.63) |
SD: standard deviation; MS: multiple sclerosis.
In addition to the 4 dimensions (A, B, C, and D) of the Spanish version of the MSTCQ©, patients filled in the following to respond to all objectives of this study (Table 2):
- •
The PRIMUS questionnaire,16 which measures quality of life and activity in patients with MS. It includes 3 scales providing separate assessments of symptoms (22 yes/no items), activities (15 items answered on a 3-point Likert scale), and quality of life (22 true/false items). The scoring of this questionnaire provides a self-reported assessment of HRQoL focusing on MS.
- •
The Spanish-language version of the TSQM© (v. 1.4)17 is a generic measure of patient satisfaction with prescribed treatments. It consists of 14 items answered on 7- or 5-point Likert scales and which provide independent evaluations of efficacy (3 items), AEs (4 items), convenience of treatment (3 items), and overall satisfaction (3 items). Scores on the TSQM© are used to analyse the convergent validity of the questionnaire being tested.
- •
ISR&ISP13 is an ad hoc instrument with 5 items that describe the state of the site receiving the treatment (erythema, redness, appearance of haematomas, inflammation, and pain); the patient uses this questionnaire to evaluate the tolerability of the ID.
- •
The ad hoc Patient/Trainer User Trial Questionnaires consist of 8 and 9 items, respectively. These questionnaires were administered to evaluate the degree of acceptance of the ID (ease of use and dosing, comfort, etc.) according to both patients and nursing staff.
- •
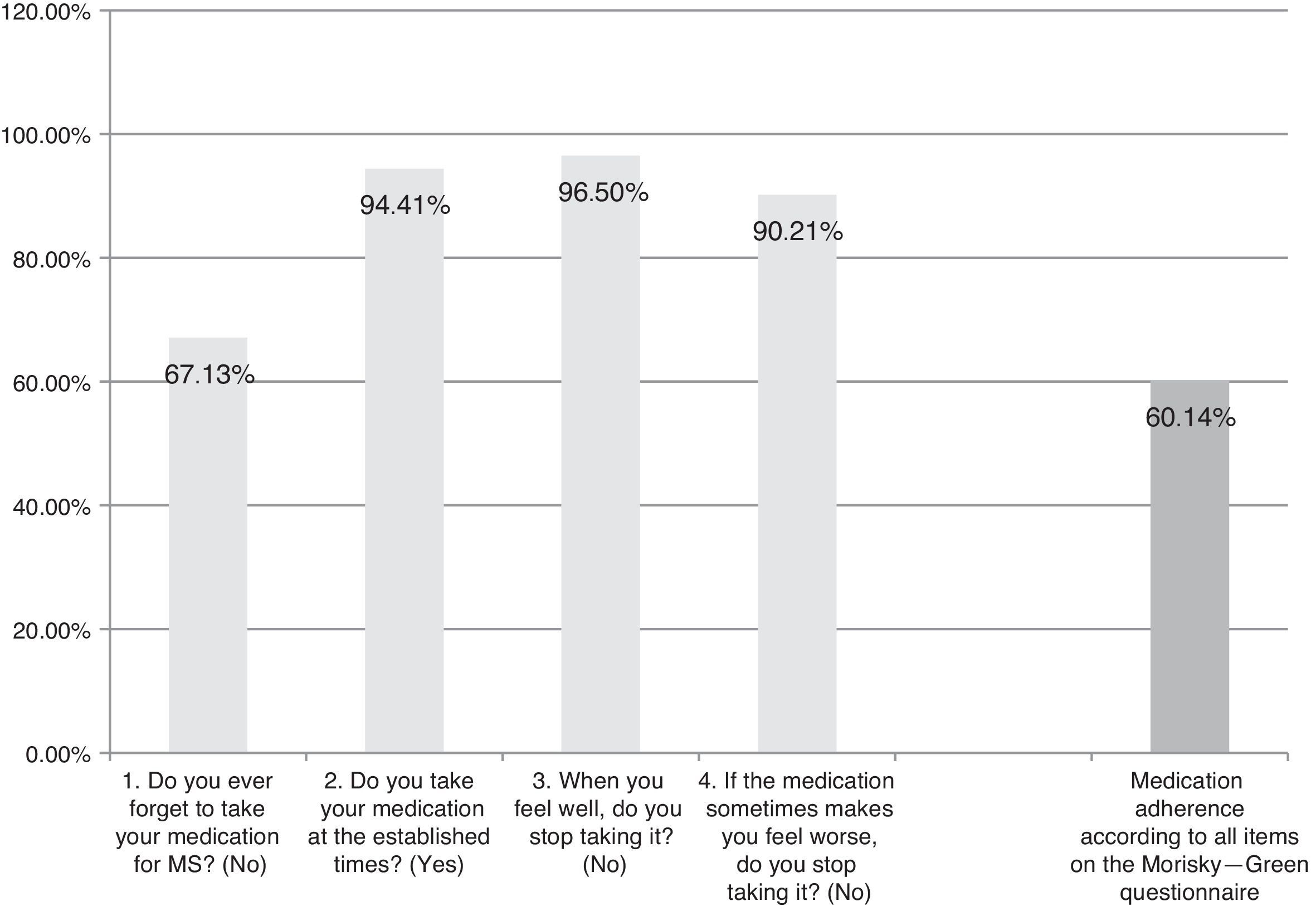
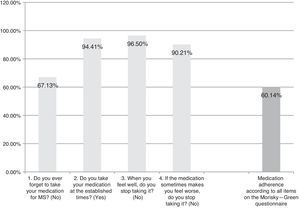
The Spanish-language adaptation of the M-G test, which evaluates patient-reported treatment adherence. Patients are considered compliant if they give optimal answers to the 4 yes/no questions (Fig. 1).
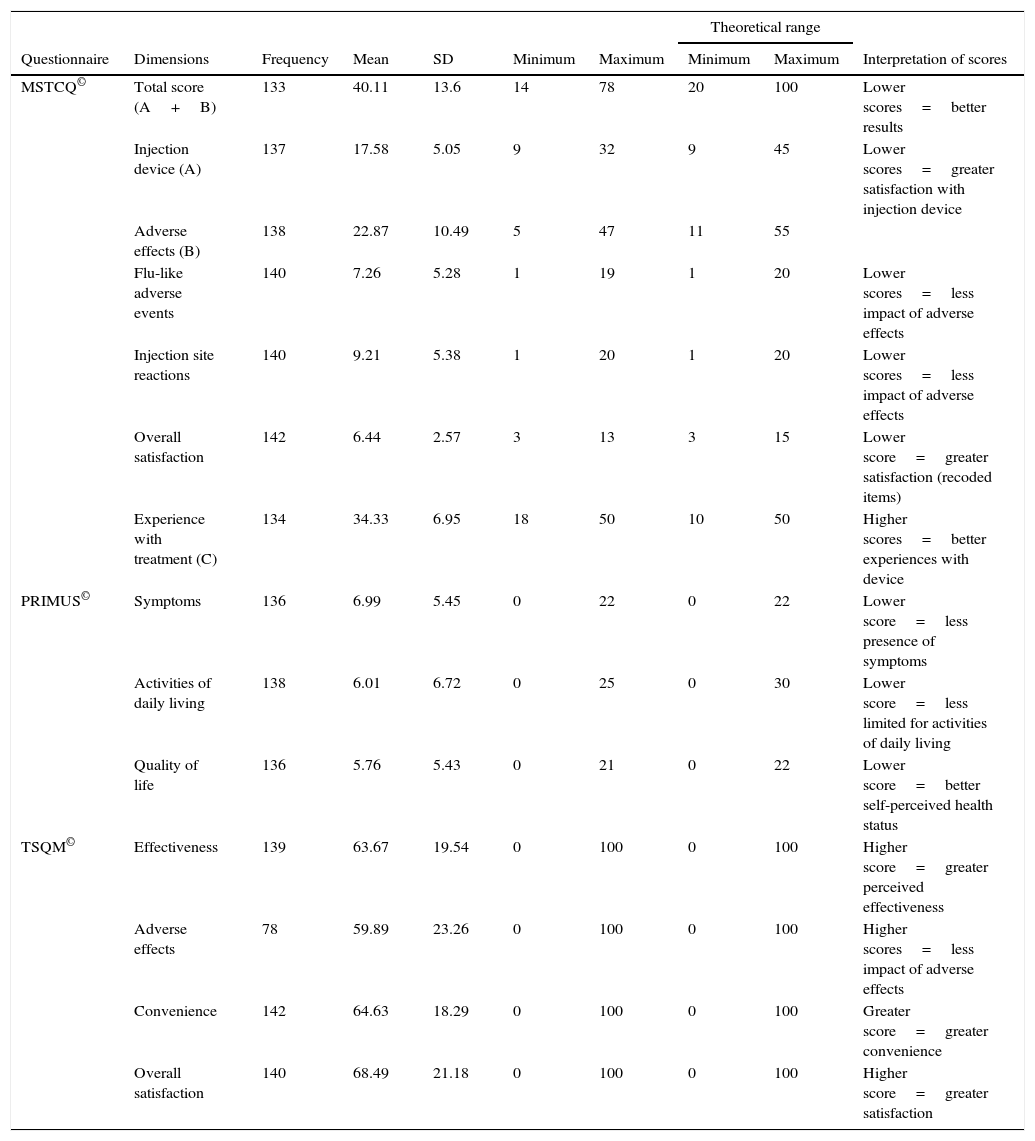
Description of scores and theoretical ranges on the MSTCQ©, PRIMUS©, and TSQM© (n=143).
| Theoretical range | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Questionnaire | Dimensions | Frequency | Mean | SD | Minimum | Maximum | Minimum | Maximum | Interpretation of scores |
| MSTCQ© | Total score (A+B) | 133 | 40.11 | 13.6 | 14 | 78 | 20 | 100 | Lower scores=better results |
| Injection device (A) | 137 | 17.58 | 5.05 | 9 | 32 | 9 | 45 | Lower scores=greater satisfaction with injection device | |
| Adverse effects (B) | 138 | 22.87 | 10.49 | 5 | 47 | 11 | 55 | ||
| Flu-like adverse events | 140 | 7.26 | 5.28 | 1 | 19 | 1 | 20 | Lower scores=less impact of adverse effects | |
| Injection site reactions | 140 | 9.21 | 5.38 | 1 | 20 | 1 | 20 | Lower scores=less impact of adverse effects | |
| Overall satisfaction | 142 | 6.44 | 2.57 | 3 | 13 | 3 | 15 | Lower score=greater satisfaction (recoded items) | |
| Experience with treatment (C) | 134 | 34.33 | 6.95 | 18 | 50 | 10 | 50 | Higher scores=better experiences with device | |
| PRIMUS© | Symptoms | 136 | 6.99 | 5.45 | 0 | 22 | 0 | 22 | Lower score=less presence of symptoms |
| Activities of daily living | 138 | 6.01 | 6.72 | 0 | 25 | 0 | 30 | Lower score=less limited for activities of daily living | |
| Quality of life | 136 | 5.76 | 5.43 | 0 | 21 | 0 | 22 | Lower score=better self-perceived health status | |
| TSQM© | Effectiveness | 139 | 63.67 | 19.54 | 0 | 100 | 0 | 100 | Higher score=greater perceived effectiveness |
| Adverse effects | 78 | 59.89 | 23.26 | 0 | 100 | 0 | 100 | Higher scores=less impact of adverse effects | |
| Convenience | 142 | 64.63 | 18.29 | 0 | 100 | 0 | 100 | Greater score=greater convenience | |
| Overall satisfaction | 140 | 68.49 | 21.18 | 0 | 100 | 0 | 100 | Higher score=greater satisfaction | |
SD: standard deviation.
First of all, SPSS software v. 21 was used to run a descriptive analysis of all sociodemographic and clinical variables (Table 1), and of those scores on questionnaires (Table 2) given as measures of central tendency and dispersion for quantitative variables, with frequency analysis for qualitative variables.
We then proceeded to evaluate the psychometric properties of the MSTCQ© questionnaire. Feasibility was analysed by calculating the percentage of non-response for each item. We verified that scores showed a normal distribution and calculated the ceiling effect (percentage of patients with the highest score) and the floor effect (percentage with the lowest score) for each item. The analyses performed on the original instrument (15) were repeated to determine that the internal consistency and test–retest reliability of the Spanish-language version would equal or exceed those of the original (α=0.70; ICC=0.68). We evaluated reliability using Cronbach α (internal consistency); after using the CGI-I to determine that there had been no clinically significant changes, test–retest reliability was assessed by applying ICC as a parametric test to the answers provided by the 41 patients who completed the retest.
Once sampling adequacy had been established (using KMO and Bartlett's test of sphericity), we used exploratory factor analysis and principal component analysis (PCA) with Varimax rotation to confirm that the original factorial structure was still present in the Spanish-language version, and to confirm test construct validity. Convergent validity was studied using Spearman's rho to examine correlations between MSTCQ© dimension scores and total scores and the dimension and total scores on the TSQM©; we expected the correlations to be significant.
The total number of patients included in the study (n=143) was sufficient to contrast statistics in the analyses we describe (some 5-10 patients per item).18,19
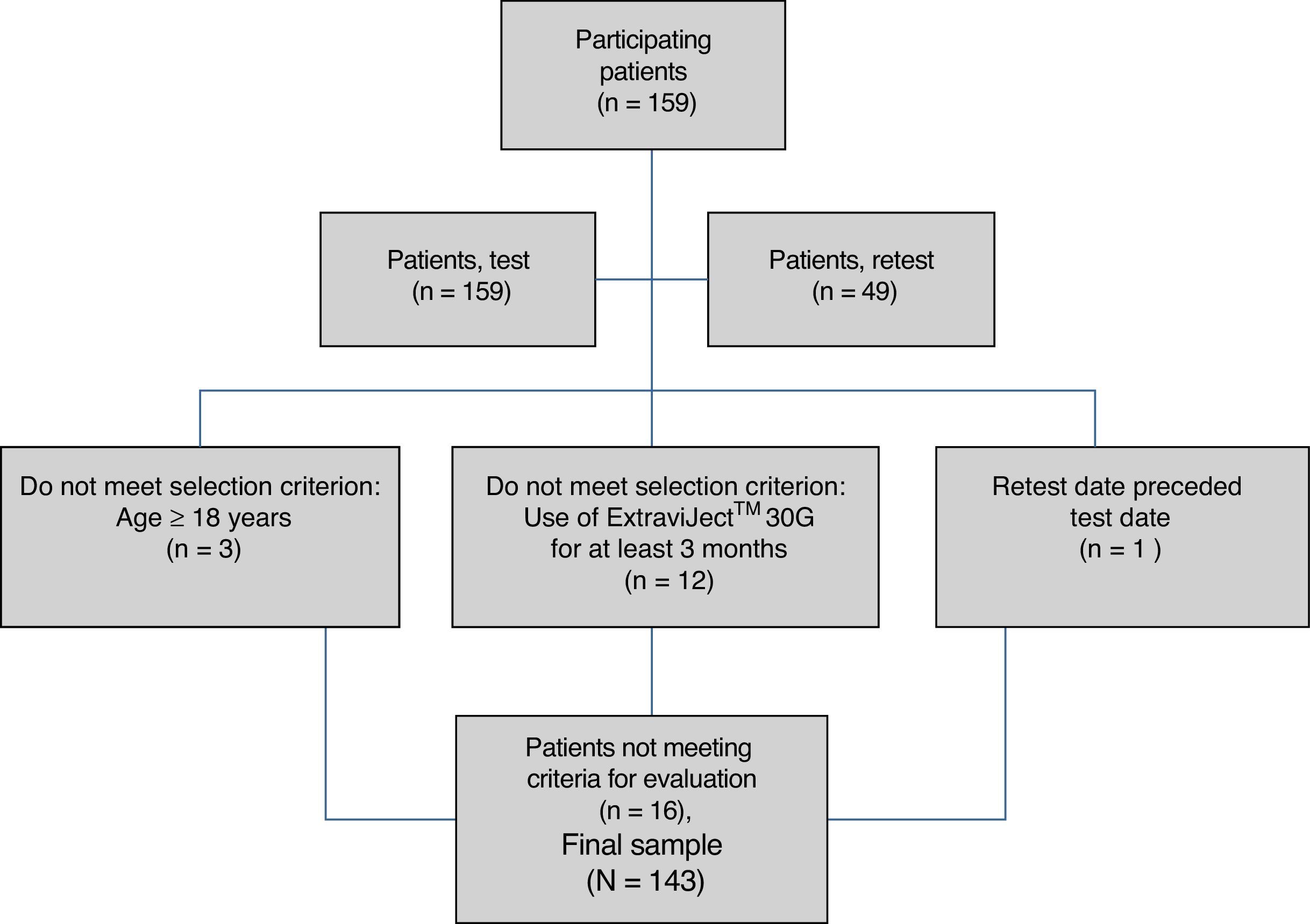
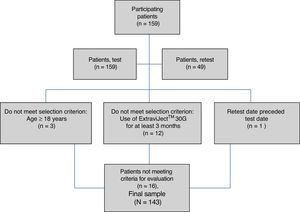
ResultsAlthough we gathered data from a total of 159 patients, 16 had to be excluded since they did not meet selection criteria (Fig. 2). The study sample therefore consisted of 143 patients. Mean patient age (standard deviation, SD) was 41.94 (10.47) years; 62.94% were women, and patients diagnosed with RRMS made up 88.11% of the total. Sociodemographic and clinical variables are described in Table 1. According to the M-G treatment adherence test, 87.50% of the patients with CIS were identified as compliant, vs 58.75% of those with RRMS and 55.55% of those with SPMS. Fig. 1 presents treatment adherence results for the entire sample.
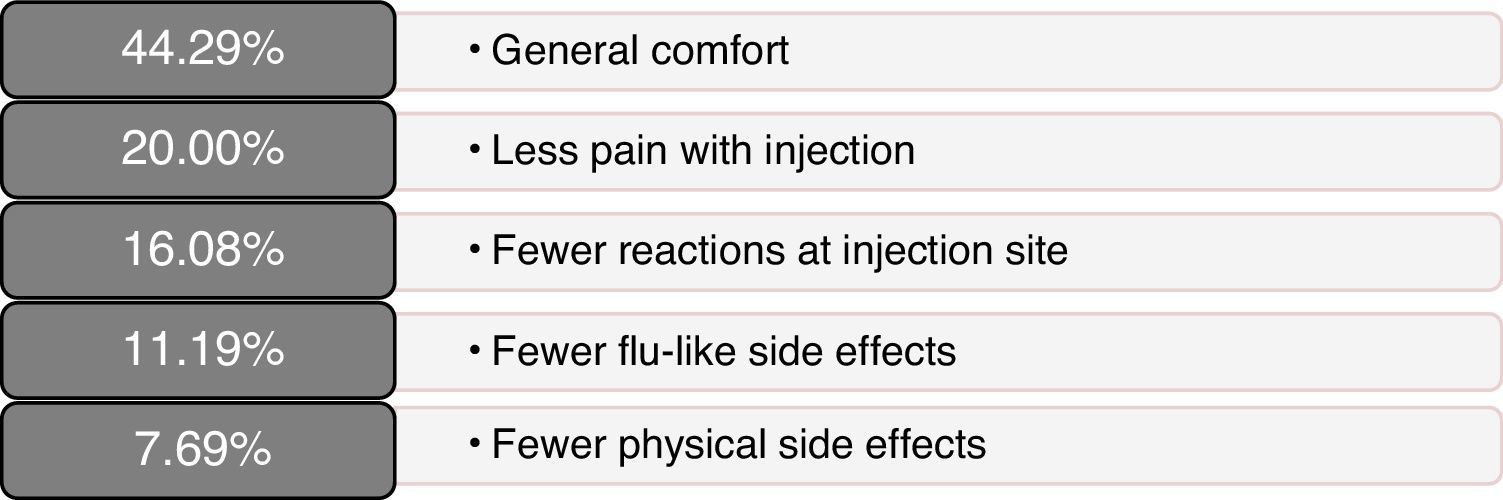
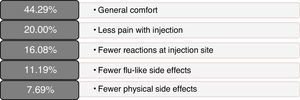
Scores on patients’ self-reported measures of satisfaction and quality of life were favourable; total scores and dimension scores on the MSTCQ©, PRIMUS,© and TSQM© are given in Table 2. Fig. 3 presents the patients’ ranking of the benefits of the ID (dimension D of the MSTCQ©).
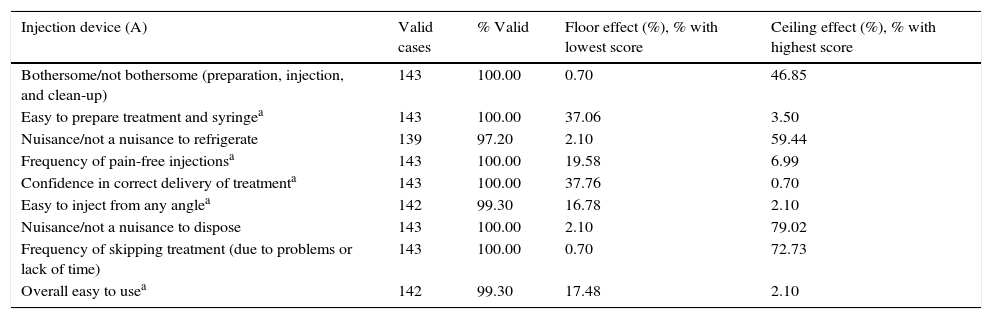
Assessment of the questionnaire's psychometric propertiesThe percentage of completed MSTCQ© forms, an indicator of feasibility, ranged from 97.20% to 100%. Results from the floor and ceiling effect analysis for dimensions A and B (total score) are shown in Table 3 and reflect response variability for this instrument.
Feasibility. Analysis of ceiling and floor effect of the MSTCQ©.
| Injection device (A) | Valid cases | % Valid | Floor effect (%), % with lowest score | Ceiling effect (%), % with highest score |
|---|---|---|---|---|
| Bothersome/not bothersome (preparation, injection, and clean-up) | 143 | 100.00 | 0.70 | 46.85 |
| Easy to prepare treatment and syringea | 143 | 100.00 | 37.06 | 3.50 |
| Nuisance/not a nuisance to refrigerate | 139 | 97.20 | 2.10 | 59.44 |
| Frequency of pain-free injectionsa | 143 | 100.00 | 19.58 | 6.99 |
| Confidence in correct delivery of treatmenta | 143 | 100.00 | 37.76 | 0.70 |
| Easy to inject from any anglea | 142 | 99.30 | 16.78 | 2.10 |
| Nuisance/not a nuisance to dispose | 143 | 100.00 | 2.10 | 79.02 |
| Frequency of skipping treatment (due to problems or lack of time) | 143 | 100.00 | 0.70 | 72.73 |
| Overall easy to usea | 142 | 99.30 | 17.48 | 2.10 |
| Adverse effects (AE) (B) | Valid cases | % Valid | Floor effect (%), better results | Ceiling effect (%), poorer results |
|---|---|---|---|---|
| Flu-like symptoms: how often | 142 | 99.30 | 33.57 | 8.39 |
| Flu-like symptoms: how longb | 140 | 97.90 | 9.09 | 6.99 |
| Flu-like symptoms: bothersomeb | 141 | 98.60 | 5.59 | 1.40 |
| Flu-like symptoms: interference with work, other activitiesb | 141 | 98.60 | 17.48 | 0.70 |
| Injection site reactions: how often | 141 | 98.60 | 22.38 | 13.99 |
| Injection site reactions: how longc | 141 | 98.60 | 6.29 | 40.56 |
| Injection site reactions: bothersome3 | 142 | 99.30 | 9.79 | 4.20 |
| Injection site reactions: interference with work, other activitiesc | 141 | 98.60 | 40.56 | 2.10 |
| Overall satisfaction with current treatmenta | 142 | 99.30 | 24.48 | 2.10 |
| Overall benefits vs bothera | 142 | 99.30 | 39.16 | 1.40 |
| Overall coping with discomfort and side effectsa | 142 | 99.30 | 29.37 | 1.40 |
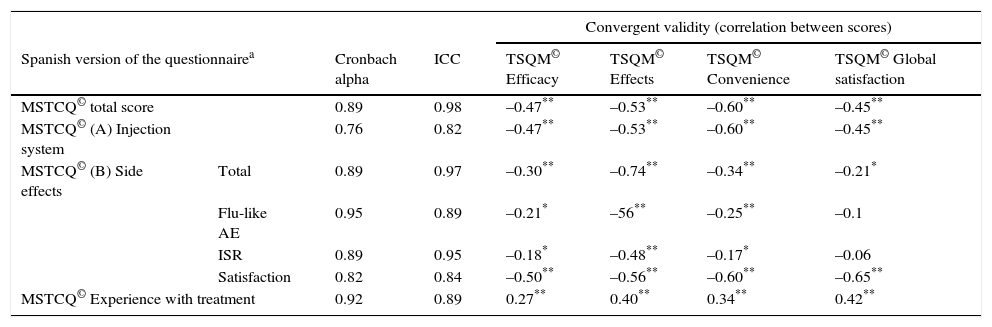
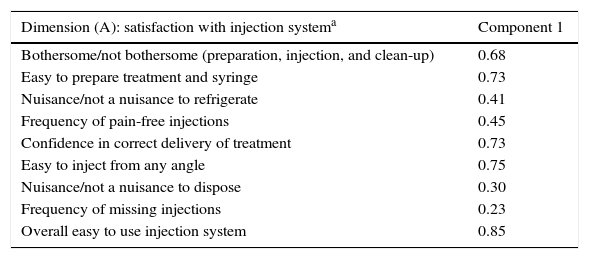
Results from the reliability study for this instrument, referring both to α values and to the ICC for the test–retest study, show a high level of internal consistency for the total score (α=0.89) and for dimension scores (dimension A, α=0.76; B [total], α=0.89; and C, α=0.92). In addition, we observe a high degree of concordance between scores on the test and the re-test of the Spanish version of the MSTCQ© (Table 4). We evaluated the construct validity of the MSTCQ© after determining that the sample was adequate for this analysis (KMO=0.86; Bartlett's sphericity test, P<.05). The construct validity test consisted of a PCA that corroborated the internal structure of the original questionnaire. Table 5 details factorial weights of resulting PCA items. A single factor in dimension A accounted for 36.91% of the explained variance in the scores; in dimension B, 3 factors provided 82.39% of the total explained variance of the scores on the MSTCQ© (factor 1 [33.64%], factor 2 [28.79%], and factor 3 [19.96%]).
Reliability analysis for the Spanish version of the MSTCQ© [Cronbach alpha and ICC] and convergent validity (correlation between scores on Spanish MSTCQ © and scores on TSQM© v. 1.4. (n=143).
| Convergent validity (correlation between scores) | |||||||
|---|---|---|---|---|---|---|---|
| Spanish version of the questionnairea | Cronbach alpha | ICC | TSQM© Efficacy | TSQM© Effects | TSQM© Convenience | TSQM© Global satisfaction | |
| MSTCQ© total score | 0.89 | 0.98 | –0.47** | –0.53** | –0.60** | –0.45** | |
| MSTCQ© (A) Injection system | 0.76 | 0.82 | –0.47** | –0.53** | –0.60** | –0.45** | |
| MSTCQ© (B) Side effects | Total | 0.89 | 0.97 | –0.30** | –0.74** | –0.34** | –0.21* |
| Flu-like AE | 0.95 | 0.89 | –0.21* | –56** | –0.25** | –0.1 | |
| ISR | 0.89 | 0.95 | –0.18* | –0.48** | –0.17* | –0.06 | |
| Satisfaction | 0.82 | 0.84 | –0.50** | –0.56** | –0.60** | –0.65** | |
| MSTCQ© Experience with treatment | 0.92 | 0.89 | 0.27** | 0.40** | 0.34** | 0.42** | |
ICC: intraclass correlation coefficient.
Principal component analysis. MSTCQ© (n=143).
| Dimension (A): satisfaction with injection systema | Component 1 |
|---|---|
| Bothersome/not bothersome (preparation, injection, and clean-up) | 0.68 |
| Easy to prepare treatment and syringe | 0.73 |
| Nuisance/not a nuisance to refrigerate | 0.41 |
| Frequency of pain-free injections | 0.45 |
| Confidence in correct delivery of treatment | 0.73 |
| Easy to inject from any angle | 0.75 |
| Nuisance/not a nuisance to dispose | 0.30 |
| Frequency of missing injections | 0.23 |
| Overall easy to use injection system | 0.85 |
| Component 1 | Component 2 | Component 3 | |
|---|---|---|---|
| Dimension (B): side effectsb | Flu-like symptoms | Injection site reactions | Satisfaction |
| Flu-like symptoms: bothersome | 0.9 | ||
| Flu-like symptoms: how often | 0.89 | ||
| Flu-like symptoms: how long | 0.92 | ||
| Flu-like symptoms: interference with work, other activities | 0.88 | ||
| Injection site reactions: how often | 0.92 | ||
| Injection site reactions: how long | 0.88 | ||
| Injection site reactions: bothersome | 0.87 | ||
| Injection site reactions: interference with work, other activities | 0.68 | ||
| Overall benefits vs bother | 0.87 | ||
| Overall satisfaction | 0.87 | ||
| Overall coping with discomfort and side effects | 0.75 |
The assessment of convergent validity (Table 4) showed statistically significant negative correlations (P<.05 and P<.01, two-tailed) between scores on the MSTCQ© and scores on different dimensions of the TSQM© (except for those pertaining to the subscales for flu-like AEs [P=.253] and ISRs [P=.508] on dimension B of the MSTCQ©, and the global satisfaction scale of the TSQM©).
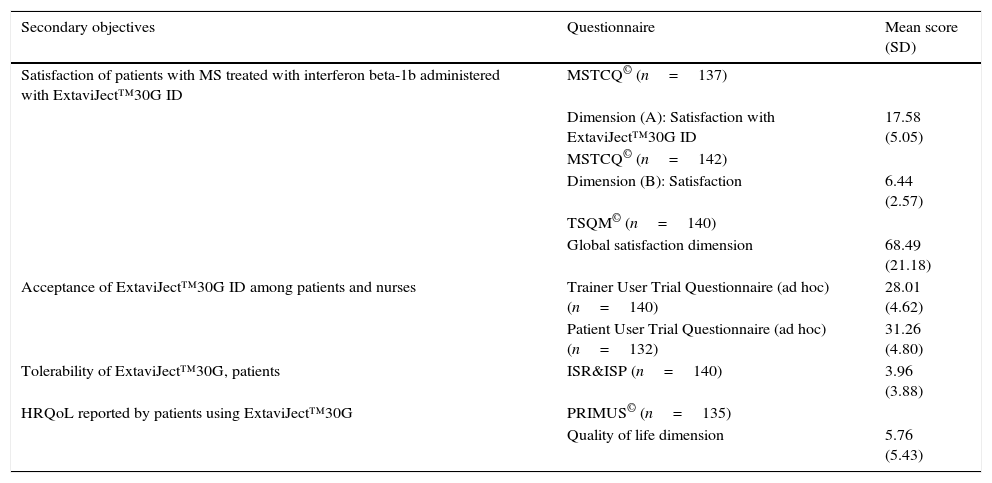
Satisfaction, acceptance, tolerance, treatment adherence, and HRQoL (Table 6)Patient satisfaction with the ExtaviJect™30G is higher than the theoretical mean of the dimensions of the MSTCQ© and TSQM© that have to do with satisfaction. Furthermore, the participants (patients and nursing staff) express a high degree of acceptance of the ID used to administer IFNβ-1b (Patient/Trainer User Trial Questionnaires), and high tolerance for the ISRs linked to the treatment (ISR&ISP scores). Lastly, our participant sample perceived HRQoL as high to very high (PRIMUS quality of life dimension). Results for treatment adherence (M-G test) reveal that a high percentage of patients (60.14%) believe that they comply with treatment according to the 4 items on that test (Table 6).
Secondary objectives: satisfaction, acceptance, tolerance, treatment adherence, and HRQoL with the ExtaviJect™30G ID.
| Secondary objectives | Questionnaire | Mean score (SD) |
|---|---|---|
| Satisfaction of patients with MS treated with interferon beta-1b administered with ExtaviJect™30G ID | MSTCQ© (n=137) | |
| Dimension (A): Satisfaction with ExtaviJect™30G ID | 17.58 (5.05) | |
| MSTCQ© (n=142) | ||
| Dimension (B): Satisfaction | 6.44 (2.57) | |
| TSQM© (n=140) | ||
| Global satisfaction dimension | 68.49 (21.18) | |
| Acceptance of ExtaviJect™30G ID among patients and nurses | Trainer User Trial Questionnaire (ad hoc) (n=140) | 28.01 (4.62) |
| Patient User Trial Questionnaire (ad hoc) (n=132) | 31.26 (4.80) | |
| Tolerability of ExtaviJect™30G, patients | ISR&ISP (n=140) | 3.96 (3.88) |
| HRQoL reported by patients using ExtaviJect™30G | PRIMUS© (n=135) | |
| Quality of life dimension | 5.76 (5.43) |
Results presented here demonstrate that the Spanish-language version of the MSTCQ© is feasible, reliable, and valid for the clinical assessment of patients who administer IFNβ-b1 using ExtaviJect™30G. The instrument evaluated patients’ perception of multiple factors associated with using an ID: ease of use, discomfort, flu-like AEs, ISRs, and overall satisfaction.
The reliability analysis shows that the percentage of lost data for these questionnaires was very low (between 0% and 2.80%). Furthermore, data display a normal distribution and a high level of response variability, meaning that the minimum and maximum values for each score include all possible responses.
The reliability test (Cronbach α) yielded very high scores that resembled those obtained using the TSQM©.17 They were also similar to scores returned by other measures of general satisfaction applied to chronically treated patients, such as the SAT-Q (α=0.80)20 and the SATMED-Q (α=0.87).21 Scores displayed a high level of concordance22 such that results for test–retest reliability were superior to those cited in the validation study of the original version15; they were also similar to those from other instruments used to measure patient satisfaction, such as the SATMED-Q (ICC=0.94).21
The factorial analysis showed that the original factorial structure was preserved by the new version, and the factorial weights in Spain were very similar to those in the original version (Table 5).15 The correlation coefficient showed a statistically significant and moderate to high association between scores on the MSTCQ© and those on the TSQM©. This association was negative since items on each of these instruments are scored in opposite senses (P<.05 and P<.01, two-tailed). We did not find any associations between the ‘flu-like AE’ and ‘ISR’ subscales on the MSTCQ© and the ‘global satisfaction’ scale on the TSQM©. This lack may be explained by the content of these items, which do not link data for overall treatment satisfaction to data on the impact of flu-like AEs or ISRs arising from the same treatment.
MSTCQ© scores for patient satisfaction with the ExtaviJect™30G device, referring to both dimension A (injection system) and dimension B (the global satisfaction subscale), indicate a high level of patient satisfaction with the drug delivery method. This is coherent with results from the generic treatment satisfaction scale TSQM.©17 The ceiling and floor effect analysis of subscale B, a measure of overall satisfaction with low scores indicating greater satisfaction, found a significant floor effect (24.38%–39.16%). This commonly occurs with scales evaluating satisfaction.20,21
Although there was a significant association between flu-like AEs, ISRs, and treatment with Extavia© in patients with RRMS and SPMS (94.40% of the sample), scores on the MSTCQ© indicate that the impact of flu-like AEs was slightly lower than the impact of ISRs. It is true that ISRs are the most frequently cited type of AE,9,23 but these data should be interpreted cautiously; there are no variables able to determine whether or not participants were taking drugs (ibuprofen, low doses of corticosteroids, etc.) to treat flu-like symptoms. Furthermore, the low scores on the ISR&ISP questionnaire indicate a high level of tolerability for the ExtaviJect™30G device. According to that questionnaire, the most frequent ISRs are redness, pain, and itching or burning sensations. Scores on the quality of life scale of the PRIMUS© questionnaire reflect that patients perceive a very high HRQoL, despite the discomfort arising from the reported AEs.
There were favourable results on MSTCQ© items having to do with ease of use of the device, on both dimension A and dimension C, as well as for patient acceptance of ExtaviJect™30G according to the ad hoc patient- or nurse-reported questionnaires on use of the injection device (Patient/Trainer User Trial Questionnaire). The best-rated benefit of the device was the ease of preparing the dose.24
Results for treatment adherence showed room for improvement: 60.14% of the patients demonstrated treatment adherence for all items on the M-G test. The explanation for this percentage of treatment adherence may reside in the time in which patients have already been in treatment for the disease. The literature reports that at longer treatment times, treatment adherence decreases10; since the study design did not call for recording data about the patient's previous treatment duration, we cannot offer any conclusions on this subject. On the other hand, the strongest indicator of poor treatment adherence on the M-G test is the question about forgetting to take the medication (occurring in 67.13% of the sample). This finding is coherent with previous data on patients taking disease-modifying treatment.10,11 Lastly, results from this study support using the MSTCQ© to evaluate satisfaction, flu-like AEs related to treatment, reactions at the injection site, and overall experience in treating patients who use ExtaviJect™30G to administer IFNβ-1b. However, this study was not designed for the purpose of comparing ExtaviJect™30G with any earlier injection systems, and this data is necessary if we are to examine patient preferences. We therefore feel that a retrospective study should be designed as an addition and complement to the current study in order to evaluate sensibility to change in the new Spanish version of the MSTCQ©.
Furthermore, future projects intending to use this version of the MSTCQ© will need larger sample sizes if the statistical comparisons and results they present are to be more representative.
FundingThis study received financial support from Novartis Farmacéutica, S.A.
Conflicts of interestAuthors Elvira Munteis, José Meca-Lallana, Antonio Pato, and Ángel Pérez-Sempere disclose that they have conflicts of interest. All other authors have no conflicts of interest to declare.
Please cite this article as: Muntéis Olivas E, Navarro Mascarell G, Meca Lallana J, Maestre Martínez A, Pérez Sempere Á, Gracia Gil J, et al. Adaptación cultural y validación al español de España del MSTCQ© (Multiple Sclerosis Treatment Concerns Questionnaire). Neurología. 2017;32:29–39.
This study was presented in poster format at the 65th Annual Meeting of the Spanish Society of Neurology (SEN), Barcelona 2013; at the 16th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Dublin 2013; and at the 2014 Annual Meeting of the American Academy of Neurology (AAN).