The last consensus statement of the Spanish Society of Neurology’s Demyelinating Diseases Study Group on the treatment of multiple sclerosis (MS) was issued in 2016. Although many of the positions taken remain valid, there have been significant changes in the management and treatment of MS, both due to the approval of new drugs with different action mechanisms and due to the evolution of previously fixed concepts. This has enabled new approaches to specific situations such as pregnancy and vaccination, and the inclusion of new variables in clinical decision-making, such as the early use of high-efficacy disease-modifying therapies (DMT), consideration of the patient’s perspective, and the use of such novel technologies as remote monitoring.
In the light of these changes, this updated consensus statement, developed according to the Delphi method, seeks to reflect the new paradigm in the management of patients with MS, based on the available scientific evidence and the clinical expertise of the participants.
The most significant recommendations are that immunomodulatory DMT be started in patients with radiologically isolated syndrome with persistent radiological activity, that patient perspectives be considered, and that the term “lines of therapy” no longer be used in the classification of DMTs (> 90% consensus). Following diagnosis of MS, the first DMT should be selected according to the presence/absence of factors of poor prognosis (whether epidemiological, clinical, radiological, or biomarkers) for the occurrence of new relapses or progression of disability; high-efficacy DMTs may be considered from disease onset.
El último documento de consenso del Grupo de Estudio de Enfermedades Desmielinizantes de la Sociedad Española de Neurología sobre el tratamiento de la esclerosis múltiple (EM) data del año 2016. Aunque muchas consideraciones continúan todavía vigentes, desde entonces se han producido significativos cambios en el manejo y tratamiento de esta enfermedad motivados no sólo por la aprobación de nuevos fármacos con diferentes mecanismos de acción, sino también por la evolución de conceptos otrora consolidados. Esto ha permitido abordar situaciones especiales como el embarazo y la vacunación desde otra perspectiva e incluir nuevas variables en la toma de decisiones en práctica clínica, como plantear tratamiento modificador de la enfermedad (TME) de alta eficacia en fases tempranas, considerar la perspectiva del paciente y utilizar nuevas tecnologías como monitorización remota.
Estos cambios han motivado la presente actualización del consenso mediante metodología Delphi, con el objetivo de reflejar el nuevo paradigma de manejo del paciente con EM basándose en la evidencia científica y experiencia clínica de los participantes.
Entre las principales conclusiones destacan como recomendaciones: iniciar TME inmunomodulador en el síndrome radiológico aislado con actividad radiológica persistente, evaluar la perspectiva del paciente y abandonar la terminología “líneas de tratamiento” en la clasificación de los TME (consenso mayor del 90%). Tras el diagnóstico de EM, la elección del primer TME debería considerar la presencia/ausencia de factores de mal pronóstico (epidemiológicos, clínicos, radiológicos y biomarcadores) para la aparición de nuevos brotes o progresión de discapacidad, pudiendo plantear desde el inicio TME de alta eficacia.
Multiple sclerosis (MS) is an inflammatory, demyelinating, neurodegenerative disease of the central nervous system (CNS). The disease typically manifests between 20 and 40 years of age, and is the most frequent non-traumatic cause of disability among young adults, affecting employability and productivity in this population.1 In Spain, MS prevalence has increased in recent years, with a current rate of 80–180 cases/100 000 population.2 Despite its heterogeneous clinical course, 3 phenotypes are recognised: relapsing-remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS).3 Disease progression is mainly characterised according to the presence or absence of clinical or radiological disease activity and progression of disability, which may be dependent or independent of relapses, over a given period of time.
In recent years, consensus guidelines have been developed with a view to improving certain aspects of MS management, both at the regional level in Europe (2018)4 and the Middle East/North Africa (2020)5 and at the national level in some European countries (2021).6 In Spain, the Spanish Society of Neurology’s Demyelinating Diseases Study Group has published 3 consensus statements, in 2010, 2013, and 2017.7–9 The 2017 statement7 mainly focused on the treatment of patients with MS, and did not employ a systematic, structured methodology to formulate the recommendations. Numerous important advances have been made over the last 5 years. Among others, we have seen the approval of new disease-modifying therapies (DMT; ocrelizumab, cladribine, siponimod, ozanimod, ponesimod, diroximel fumarate, ofatumumab; Table S1), updated diagnostic criteria, new evidence on early treatment with high-efficacy DMTs (HE-DMT), increased focus on tools assessing the patient’s perception of the disease patient-reported outcomes (PRO), and efforts to implement in clinical practice the use of remote monitoring tools used in clinical trials.10–12
This consensus statement seeks to provide an updated set of recommendations for the management of MS in Spain, addressing issues related to the diagnosis, treatment, and follow-up of these patients. These recommendations have been reviewed and agreed by the members of the Spanish Society of Neurology’s Demyelinating Diseases Study Group.
MethodsThe Delphi method was followed to establish consensus on the recommendations. The Delphi method is a structured procedure used to establish the opinion of an expert group on complex subjects. It is based on an iterative process, ensures anonymity, and gathers participants’ comments and feedback.
In this project, we established a scientific committee with 5 members (2 of whom also acted as coordinators) and a panel including 21 members, all of whom are considered national experts in MS. The Delphi process involved several phases: 1) an exhaustive literature review; 2) selection of dimensions and drafting of items; 3) selection of panellists and invitation to participate; 4) first round of assessment of items; 5) review and editing of items for which consensus was not established, based on the feedback received; 6) second round of assessment of items for which consensus was not established; 7) final assessment of items for which consensus was not established. Phases 4 and 6 were conducted by the expert panel, with the scientific committee being responsible for the remaining phases.
For each item, panellists rated their level of agreement using a 9-point Likert-type scale (scored from 1, “completely disagree,” to 9, “completely agree”), and were invited to add comments. Based on the scores given, items were categorised as rejected (scores of 1–3), undetermined (4–6), or accepted (7–9); consensus was considered to have been reached if ≥ 66.6% of the expert panel agreed, as in other studies following the Delphi method.13,14 Descriptive analysis of the results was performed with the IBM SPSS statistics software, version 22.
ResultsPresented below are the results derived from expert consensus established through the Delphi process. The supplementary material includes a diagram depicting the phases of the Delphi process (Fig. S1), items for which consensus was established in each dimension (Tables S2–S9, S11), and the list of bibliographic references reviewed during the drafting of items (Revisión de la literatura). Table S1 presents the DMTs currently approved in Spain, the indications included on the summary of product characteristics, and conditions for funding (therapeutic position statements).
A high level of consensus was established. Of 148 items considered, consensus was reached for 110; 102 were accepted in rounds 1 or 2, with a mean (standard deviation) level of consensus of 86.8% (10.3%). Consensus was greater than 80% for 73.5% of items. In round 2, consensus was not reached for 34 items; these were assessed by the scientific committee (consensus was reached for 8 and not reached for 26). The percentage of agreement is not reported for the 8 items accepted by the scientific committee (Tables S6 and S8).
Early diagnosisThe 2017 McDonald criteria10 enable earlier diagnosis of MS, mainly as a result of the inclusion of oligoclonal bands (OCB) in the cerebrospinal fluid (CSF) as an alternative to clinical or magnetic resonance Imaging (MRI) evidence demonstrating dissemination in time. Due to the lack of conclusive data on diagnostic accuracy, those criteria did not include the optic nerve among the regions that can be considered to demonstrate dissemination in space. Despite this, given that it is one of the most frequently affected regions at disease onset, and in the light of recently reported data on sensitivity, specificity, and diagnosis,15 increasing numbers of authors suggest that it should be included.16–19 Recently described brain MRI findings20,21 may also help to confirm the diagnosis of MS.17 Other paraclinical studies, such as spinal cord MRI, optical coherence tomography (OCT), visual evoked potentials (VEP), and immunoglobulin G (IgG) index have also been shown to be relevant in diagnosis.22,23 Thus, our consensus positions are as follows:
- •
Diagnostic accuracy in MS increases with the inclusion of the optic nerve among the regions considered for establishing dissemination in space. If optic neuropathy is suspected, the visual system should also be evaluated with OCT and VEP.
- •
Central vein sign and presence of lesions with iron rims may serve as radiological markers in the differential diagnosis of patients with inconclusive clinical and radiological characteristics.
- •
Spinal cord MRI is recommended, and enables detection of lesions that may guide therapeutic decision-making.
- •
Paraclinical assessment should also include determination of OCBs and intrathecal IgG synthesis index.
In patients with radiologically isolated syndrome (RIS), such findings as spinal cord lesions, central vein sign, OCBs in the CSF, and certain demographic factors indicate greater likelihood of a clinical event. In these cases, we recommend:
- •
scheduling a brain MRI study and clinical assessment in the next 6–12 months; and
- •
performing such complementary examinations as cognitive assessment, ophthalmological and urological examination, VEP, and CSF analysis (if not previously performed).
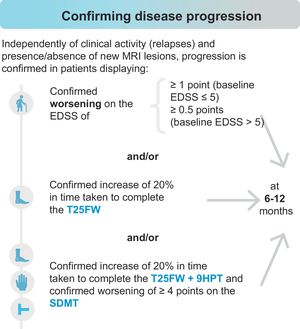
In turn, early identification of progression to SPMS or PPMS is highly challenging, as both phenotypes lie on a continuous spectrum. The panellists believe that conversion to a progressive form may be confirmed upon detection of worsening in measures of disability (Expanded Disability Status Scale [EDSS]) and/or functional examination of the lower limbs (Timed 25-Foot Walk test [T25FW]), and/or combined functional examination of the lower and upper limbs (T25FW and Nine-Hole Peg Test [9HPT]) plus cognitive assessment (Symbol Digit Modalities Test [SDMT]), independently of clinical activity (relapses) and the presence or absence of new MRI lesions, as shown in Fig. 1. To confirm progression to SPMS using the EDSS, additional measures of disability (T25FW, 9HPT, and SDMT) should also be used. Worsening on the 9HPT or SDMT independently is insufficient to establish progression. Although there was consensus that worsening of 20% on the T25FW only can be used to diagnose progression (66.7%), the statement that functional worsening should be confirmed with assessment both of the lower and the upper limbs (T25FW and 9HPT) and of information processing speed (SDMT) achieved full consensus (100%).
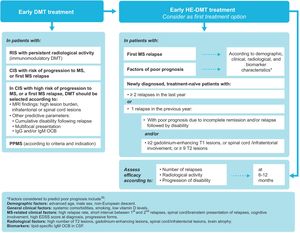
Early treatment with disease-modifying therapiesClinically isolated syndrome (CIS) is defined as a single episode of neurological symptoms suggestive of MS. Although not all patients with CIS go on to develop MS,24 the majority do, and up to 60% of patients with CIS have a confirmed diagnosis of MS within 4 years.25 Several studies have shown early treatment with DMTs to be beneficial in this population.26–28 In the Barcelona inception cohort, with a minimum follow-up period of 10 years, patients with CIS who started DMT early (median of 4 months after the first attack) presented lower risk of progressing to EDSS scores of 3 points than those who started this treatment later (median of 36 months).26 Factors predicting poorer prognosis in CIS include high MRI lesion burden,26,29 particularly in infratentorial areas.24 In fact, a prospective study of 1015 patients with CIS concluded that the number of lesions on brain MRI is a high-impact prognostic factor, whereas presence of IgG OCBs is a medium-impact factor and demographic and topographical characteristics are low-impact factors.30 Furthermore, the presence of spinal cord lesions31 or of at least 2 gadolinium-enhancing spinal cord lesions in CIS32 is associated with a significantly greater risk of progression. In the light of the benefits of early treatment with DMTs, and depending on the patient’s diagnosis, we make the following recommendations (Fig. 2):
- •
In patients with RIS presenting persistent radiological activity, immunomodulatory DMTs should be considered.
- •
In patients with an initial MS relapse or with CIS and risk of progressing to MS:
- o
DMTs should be offered, even if lesion load is low, once other possible diagnoses are ruled out.
- o
DMT should be started if lesion load is high and the patient presents infratentorial/spinal cord lesions or factors of poor prognosis.
- o
DMTs should be selected according to predictive parameters such as MRI findings, cumulative disability after the attack, multifocal presentation, and presence of OCBs.
- o
- •
Patients with a recent diagnosis of MS should be offered DMT as soon as possible to control disease activity and progression. Treatment with HE-DMT may be started, in accordance with the characteristics of the treatment and the patient’s clinical and radiological characteristics, lifestyle, and preferences.
- •
It is reasonable to start DMT as early as possible in patients with PPMS, in accordance with the relevant criteria and treatment indications, although the level of evidence for this recommendation is low.
Early onset of disease-modifying treatment.
* Factors of poor prognosis (not all factors have the same specific weight in decision-making) include: demographic characteristics (advanced age, male sex, non-European origin); general clinical characteristics (systemic comorbidities, smoking, low vitamin D levels); clinical data related to MS (high relapse rate; short interval between first and second relapses; brainstem, cerebellar, or spinal cord involvement at onset; poor recovery after first relapse; high EDSS score at diagnosis; polysymptomatic onset; cognitive deficits; progressive forms); radiological data (high number of lesions or lesion volume on T2-weighted sequences, presence of gadolinium-enhancing lesions in the spinal cord or infratentorial areas, global brain atrophy or grey matter atrophy); biomarkers (IgM OCBs in the CSF).39 CIS: clinically isolated syndrome; CSF: cerebrospinal fluid; DMT: disease-modifying therapy; MRI: magnetic resonance imaging; MS: multiple sclerosis; OCB: oligoclonal bands; PPMS: primary progressive MS; RIS: radiologically isolated syndrome.
Due to the availability of a high number of DMTs and the lack of precise biomarkers to establish their safety and effectiveness in each individual, selecting a tailored treatment can be highly challenging. A treatment escalation approach has traditionally been followed in which patients are initially treated with moderate- and low-risk DMTs (classically referred to as “first-line” treatments), with a subsequent switch to HE-DMTs with greater risk of severe adverse events (“second-line” treatments) if the patient presented disease activity. Although we currently lack results from clinical trials directly comparing treatment escalation against the use of HE-DMTs as the initial treatment, some observational studies and subgroup analyses from clinical trials have been published. They have shown, consistently and with an acceptable level of evidence, that patients receiving early HE-DMT treatment presented less disease activity,33,34 less disability,34,35 and lower risk of progressing to SPMS,36 at least in the first 5 years of treatment, than patients receiving these treatments later.
The ultimate aim of the current treatments for MS is to achieve the best possible control of disease activity (measured by the no evidence of disease activity [NEDA-3] criteria) and the best possible quality of life. Real-life studies have found that patients treated early with HE-DMTs are approximately twice as likely to achieve NEDA as those treated with moderate-efficacy DMTs.37 Therefore, and given the fact that persistent clinical or subclinical activity may cause irreversible neurological damage, enabling the activation of molecular pathways that favour progression, we may consider HE-DMTs as the first option, having assessed the patient and the risks and benefits of treatment. We particularly recommend starting HE-DMT treatment (Fig. 2) in the following groups:
- •
patients with demographic, clinical, and radiological factors of poor prognosis; and
- •
newly diagnosed, treatment-naïve patients with ≥ 2 relapses in the last year.
The following groups may also be eligible for initial treatment with HE-DMTs:
- •
patients with a first MS relapse, depending on the patient and disease characteristics, with no need to delay treatment or follow an escalation approach; and
- •
newly-diagnosed, treatment-naïve patients with a single relapse in the last year but with clinical (incomplete remission and/or relapse followed by disability) and/or radiological evidence of poor prognosis (≥ 2 gadolinium-enhancing lesions on T1-weighted sequences, spinal cord/infratentorial involvement, or ≥ 9 lesions on T2-weighted sequences).
Despite the availability of data showing that patients with greater disease activity,33 younger patients,38 and patients with shorter disease duration38 are likely to benefit the most from early HE-DMT treatment, more evidence is needed to recommend early treatment onset in young, newly diagnosed, treatment-naïve patients, independently of the number of previous relapses or radiological activity.
Generally, the selection of an initial treatment after diagnosis of MS should not follow the classical “lines of therapy”; rather, the main consideration should be the presence or absence of factors of poor prognosis (epidemiological, clinical, radiological, or other biomarkers) for the appearance of new relapses or progression of disability (94.7% of panellists agreed with this statement). Both therapeutic inertia (maintaining the same treatment despite presence of clinical or radiological activity) and the so-called treatment cycling (lateral escalation with oral and injectable DMTs of moderate efficacy) delay onset of HE-DMT treatment and result in a missed therapeutic opportunity, as persistent clinical or subclinical disease activity may cause irreversible neurological damage. In any case, the decision to start treatment will always be made jointly with the patient, taking into account his/her priorities and preferences.
To tailor treatment and control the disease to the greatest extent possible, the effectiveness of DMTs should be evaluated periodically. In patients receiving early treatment with HE-DMTs, we recommend evaluating effectiveness according to the number of relapses and/or radiological activity at 6–12 months, and progression of disability at 6–12 months.
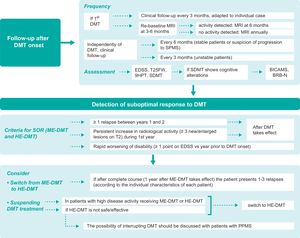
In-person and remote follow-upGiven the current lack of sufficiently accurate biomarkers for predicting the suitability of the DMT selected in each case, thorough follow-up plays an essential role after treatment onset. The therapeutic lag of DMTs is estimated at 12–30 weeks for relapses and 30–70 weeks for disability progression.40 The MAGNIMS-CMSC-NAIMS consensus guidelines recommend performing a re-baseline MRI study at 3–6 months after DMT onset to detect new lesions that may appear before treatment begins to take effect, with follow-up studies annually.19 In addition to clinical and radiological follow-up, cognitive assessment provides information on the patient’s current and future situation, and may serve as an early marker of disease.41,42 Regarding in-person follow-up (Fig. 3), we issue the following recommendations:
- •
Follow-up consultations should be held every 3 months after onset of the first DMT. However, the frequency of visits should be decided on a case-by-case basis, according to the characteristics of the patient and treatment.
- •
Independently of the DMT selected (moderate- or high-efficacy), follow-up consultations should be held at least every 6 months in clinically and radiologically stable patients, and every 3 months (where possible) in unstable patients. If progression to SPMS is suspected, follow-up consultations should be held at least every 6 months.
- •
A “re-baseline” MRI study should be performed 3–6 months after DMT onset and annually thereafter. If radiological activity is detected in the absence of clinical activity, an additional MRI study should be performed after 6 months. Although this study is advisable, it may be difficult to perform in clinical practice, even at reference centres, for logistical, organisational, technological, or care-related reasons.
- •
The recommended clinical assessments to perform at follow-up consultations are the EDSS, T25FW, 9HPT, and SDMT. To screen for cognitive involvement, validated tests other than the SDMT may also be used; cognitive assessment should be conducted annually, using the same test each time. Short- or intermediate-duration neuropsychological test batteries such as the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) or Brief Repeatable Battery of Neuropsychological Tests (BRB-N), respectively, may also be used if screening tests detect cognitive alterations.
Follow-up during treatment and after detection of suboptimal treatment response.
9HPT: Nine-Hole Peg Test; BICAMS: Brief International Cognitive Assessment for Multiple Sclerosis; BRB-N: Brief Repeatable Battery of Neuropsychological Tests; DMT: disease-modifying treatment; EDSS: Expanded Disability Status Scale; HE-DMT: high-efficacy DMT; ME-DMT: moderate-efficacy DMT; MRI: magnetic resonance imaging; MS: multiple sclerosis; PPMS: primary progressive MS; SDMT: Symbol Digit Modalities Test; SOR: suboptimal response; SPMS: secondary-progressive MS; T25FW: Timed 25-Foot Walk test.
Telemedicine has become increasingly important since the beginning of the SARS-CoV-2 pandemic,43 and is an alternative to consider in patients with difficulty attending in-person appointments due to distance or disability.44 Our expert panel agreed that, in the light of this experience, telemedicine may complement in-person patient follow-up and replace certain in-person appointments with patients whose disease is stable and who do not have visual, auditory, or cognitive difficulties. The implementation of telemedicine in clinical practice depends both on technological development and on the resources available; therefore, when the necessary resources are available, we recommend that:
- •
Validated applications on electronic devices (eg, smartphones) should be used between consultations for patients to report their health status and for remote cognitive assessment.
- •
Consultations should incorporate digital tools that help raise suspicion of disease progression.
- •
Resources should be used to enable patients to monitor their adherence to treatment, with notifications to remind them when treatment should be administered.
In the coming years, artificial intelligence programs are expected to facilitate precision medicine, helping to optimise diagnosis, follow-up, and therapeutic decision-making. The use of digital tools may also promote patient commitment by providing patients with accessible, real-time information on their health status and helping them to manage their disease.
Detection of suboptimal response and optimisation of treatmentThe appearance of new relapses, new MRI lesions, or worsening of disability, confirmed following an interval after DMT onset, suggests disease activity and, therefore, suboptimal treatment response. However, it is difficult to establish the exact number of lesions or relapses or the specific degree of cumulative disability that should define suboptimal response; several proposals have been made on this subject.4,6,7,19,45–48 According to the results of the Delphi consensus process, the same criteria should generally be used to identify suboptimal response in patients receiving high- or moderate-efficacy DMTs:
- •
presence of one or more relapses between years 1 and 2 after DMT onset;
- •
presence of persistent radiological activity (≥ 3 new/enlarged lesions on T2-weighted sequence) in the first year after DMT onset; and
- •
rapid increase in progression of disability (≥ 1 points on the EDSS compared to scores in the year prior to DMT onset).
Treatment should be switched if a suboptimal response is detected. The new DMT should be selected according to the type of DMT used previously and the disease activity observed. Generally, 1–3 relapses are considered sufficient to switch from a moderate- to a high-efficacy DMT after a full course of treatment (1 year after the DMT takes effect). This wide range is explained by the fact that, when relapses occur, the following variables must be considered: location/semiology, functional impact, sequelae, and time since onset of treatment with moderate-efficacy DMT.
Stable patients receiving HE-DMT treatment, under clinical and radiological follow-up, and presenting no safety/tolerability problems should continue with their current treatment; de-escalation should not be considered. In turn, non-responders may be identified during the first year of treatment, not before 6–12 months, after onset of DMT. Withdrawal or suspension of medication should be considered in the following cases:
- •
In patients with highly active disease in whom DMT is withdrawn or suspended in the event of suboptimal response or problems with tolerability/safety, a new HE-DMT should be started as soon as possible, taking into account disease activity before and during treatment, the pharmacokinetics and biological activity of the previous DMT, and the risk of rebound.
- •
If the HE-DMT used is ineffective or unsafe, treatment should be switched to a different HE-DMT.
- •
In patients with PPMS in whom the DMT is not effective/safe and for whom no alternative treatment is available (to date, the only approved DMT for PPMS is ocrelizumab), the possibility of interrupting treatment should be discussed with the patient.
Fig. 3 shows the main consensus positions regarding patient follow-up after detection of suboptimal response to DMT treatment.
Patient perspectivesThe patient’s perspective, determined with patient-reported outcome measures (PROM) and patient-reported experience measures (PREM), is a key consideration at all stages of disease management.12 To the greatest extent possible, patients should be assessed with tools specifically validated for MS, with a view to ensuring standardised use of these tools between centres, enabling results to be compared. We recommend assessment with these tools at least once per year, although each case should be considered on an individual basis. We recommend assessing the following domains, and give examples of validated tools that may be used:
- •
physical and psychological impact of MS (eg, Multiple Sclerosis Impact Scale [MSIS-29]);
- •
symptoms of anxiety and depression (eg, Beck Depression Inventory, Hospital Anxiety and Depression Scale);
- •
fatigue (eg, Fatigue Severity Scale, Modified Fatigue Impact Scale);
- •
quality of life (eg, Multiple Sclerosis International Quality of Life questionnaire [MusiQoL], Multiple Sclerosis Quality of Life [MSQOL]);
- •
spasticity (eg, Multiple Sclerosis Spasticity Scale [MSSS-88], Numeric Rating Scale for Spasticity [NRS-S]); and
- •
satisfaction with treatment, since treatment onset.
Furthermore, without the need for specific instruments, we recommend:
- •
encouraging patients to report problems with sexual function and sleep alterations; and
- •
assessing treatment preferences before onset of treatment.
The availability of biomarkers capable of predicting disease progression, evaluating treatment response, and monitoring patients’ status is fundamental in decision-making and management of MS. Although numerous studies have been performed, few of these biomarkers have been validated, and fewer still are used in clinical practice.49 To be incorporated in clinical practice, a biomarker must be highly accurate (in terms of sensitivity and specificity), easy to measure and analyse, and cost-effective.
Loss of brain or spinal cord volume on MRI is associated with progression of disability and cognitive impairment, and is an accurate biomarker of disease progression and early treatment response50; however, the complexity of the study and its analysis, as well as the difficulty of translating group outcomes to the individual level, have constituted limitations for its use in clinical practice.50 Evaluation with such other imaging techniques as OCT has also been proposed as a biomarker of disability and cognitive impairment.51 OCT is considered viable but not useful in everyday practice for predicting disease progression.
With respect to molecular biomarkers, some studies report that levels of chitinase-3–like 1 protein (CHI3L1) in the CSF52,53; miRNA levels in the CSF, plasma, or serum45,54; and serum or CSF neurofilament light chain (NFL) levels52,55 predict prognosis and treatment response, although they have not been validated in the clinical context. Presence of immunoglobulin M (IgM) OCBs,56,57 and especially lipid-specific bands, is predictive of an aggressive disease course; therefore, this biomarker may be useful in identifying candidates for early HE-DMT treatment.58 We consider lipid-specific IgM OCBs to be a useful, viable prognostic biomarker of MS in everyday clinical practice.
Generally, the majority of potential biomarkers are not sufficiently viable or useful at present for their use to be recommended in clinical practice. There is a need for further research to validate and standardise these biomarkers, and increased resources to enable their use in decision-making for individual patients.
PregnancyGiven the high incidence of MS in women of childbearing age, therapeutic decision-making must often take into account the possibility of pregnancy. Patients who plan to become pregnant must be informed about the possible risks to mother and fetus and should be involved in the development of the treatment plan. Despite a growing body of evidence on the safety of DMT in pregnant patients, the number of drugs considered safe during pregnancy remains limited.4,59 The decision to continue treatment depends on disease severity and the DMT in question.59,60 Regarding DMT and pregnancy, we issue the following recommendations:
- •
In patients not receiving DMT and who have presented a relapse the previous year, treatment should be started and pregnancy delayed until the patient has been stable for 12 months.
- •
In clinically and radiologically unstable patients who plan to become pregnant, treatment should be optimised and pregnancy delayed for at least 12 months. If the patient is receiving DMT and has presented a relapse over the last 12 months, pregnancy should be delayed.
- •
In the event of unplanned pregnancy, the risks and benefits of each DMT should be evaluated according to the summary of product characteristics (Table S10).
- •
In patients not intending to breastfeed and who discontinued DMT use during pregnancy, treatment should be resumed as soon as possible after delivery, taking into account the patient’s condition.
The results of a study of over 1.4 million pregnancies61 showed that non-contrast MRI monitoring of MS activity during the first trimester is safe, whereas use of gadolinium increased the risk of some diseases and of fetal or neonatal death.61 However, other studies have found that MRI has a teratogenic effect,62 and that radiological findings are not essential in the treatment plan63; therefore, we recommend avoiding routine MRI studies during pregnancy. In the event that MRI is deemed necessary, the study should be performed after the first trimester; furthermore, new/enlarged T2 lesions should be sufficient to detect disease activity, avoiding the administration of gadolinium.
VaccinationThe different action mechanisms of DMTs include the reduction of lymphocyte counts, which increases the risk of infection, with a potential impact on MS activity.64,65 One strategy to prevent infections, especially in patients receiving HE-DMT, is vaccination.65 Most studies have found that inactivated vaccines do not increase the risk of developing MS or of relapses,65,66 whereas there is evidence that live attenuated vaccines may increase this risk,67 which has resulted in a certain controversy regarding their use in patients with MS.
The COVID-19 pandemic has demonstrated the continued existence of anti-vaccination movements. In a context in which information is transmitted immediately, regardless of its veracity, it is particularly important to trust in scientific evidence. Studies have demonstrated the safety of vaccines against COVID-19 in patients with MS, although serological follow-up is recommended in those receiving anti-CD20 monoclonal antibodies or SP1 receptor modulators.68 We recommend that patients with MS be vaccinated as indicated by healthcare authorities, taking into account the following:
- •
After MS is diagnosed, the local vaccination schedule should be completed. Before starting immunosuppressive treatment, titres of antibodies against relevant pathogens should be determined and vaccination should be proposed to the patient.
- •
In patients scheduled to begin immunosuppressive treatment, vaccination should be completed at least 4 weeks before treatment onset for live attenuated vaccines and at least 2 weeks before onset for inactivated vaccines.
- •
Live attenuated vaccines should be avoided in patients currently or recently treated with immunosuppressive DMTs or oral immunomodulatory DMTs. Inactivated vaccines are considered to be safe.
- •
In the event of a relapse, vaccination should be delayed until resolution of the relapse and cessation of clinical activity, if possible.
- •
Where possible, serological response should be monitored one to 2 months after the last dose.
- •
Patients receiving immunosuppressive or immunomodulatory DMTs can be administered vaccines against SARS-CoV-2, as the associated benefits outweigh the risks.
This consensus statement, developed using the Delphi method, provides recommendations intended to optimise the management of MS in clinical practice in Spain. Recommendations are based on recent scientific evidence and the resources currently available in Spanish hospitals. Early diagnosis and treatment with DMTs are essential. We should stress the need to abandon the classical terminology of “lines of therapy,” as the drugs previously considered to be “second-line” are high-efficacy treatments that may be considered as a first option, according to the characteristics of the patient and their disease.
FundingThis study received funding from Novartis Farmacéutica S.A. The authors retained editorial control of the manuscript at all times and approved the final version.
Conflicts of interestJosé E. Meca-Lallana has received fees for consulting, training, or research, and has participated in clinical trials and other research projects for Alexion, Biogen, Bristol-Meyers-Squibb, Janssen, Merck, Novartis, Roche, and Sanofi.
Sergio Martínez Yélamos: Sergio Martínez Yélamos’ employer (Hospital Universitario de Bellvitge/Institud d’Investigació Biomèdica de Bellvitge) has in the last 3 years, exclusively for the purpose of funding research activities, received funds for consulting, partnerships, and donations from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Janssen, Merck, Novartis, Roche, and Sanofi.
Sergio Martínez Yélamos has in the last 3 years, exclusively for attendance at conferences, received funds from Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Roche, and Sanofi.
Sara Eichau has received lecture honoraria and consulting fees from Biogen, Novartis, Merck, Bayer, Sanofi Genzyme, Roche, Janssen, Bristol-Meyers, and Teva.
Miguel Ángel Llaneza has received fees for consulting, training, or research, and has participated in clinical trials and other research projects for Almirall, Bayer, Biogen, BMS, Janssen, Merck, Novartis, Sanofi-Genzyme, Roche, Teva, and Viatris.
Joaquín Peña Martinez has received fees for lectures, consulting, and conference attendance from Biogen, Bristol-Myers Squibb, Janssen, Merck, Novartis, Roche, and Sanofi-Genzyme.
Virginia Meca Lallana has received fees for lectures, scientific activities, and conference attendance from Almirall, Bayer, Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Roche, Sandoz, Sanofi-Genzyme, Terumo, and Teva.
Ana María Alonso Torres has received lecture honoraria and consulting fees from Almirall, Biogen, BMS, Janssen, Merck, Novartis, Roche, Sandoz, and Sanofi.
Ester Moral Torres has received fees for consulting, lectures, and scientific activities and funds for conference attendance from Actelion, Almirall, Bayer, Biogen-Idec, Bristol-Myers Squibb, Merck, Teva, Novartis, Roche, and Sanofi-Genzyme.
Jordi Río has received lecture honoraria and fees for participating on advisory boards from Biogen-Idec, Merck, Novartis, Teva, Janssen, Bristol Myers Squibb, and Sanofi-Aventis.
Carmen Calles has received lecture honoraria and fees for participating in courses, consulting, and monographs from Teva, Biogen, Sanofi, Merck, Bristol-Myers, Roche, Celgene, Novartis, Sandoz, and Janssen.
Adrián Ares Luque has received consulting fees, lecture honoraria, and funds to attend conferences and other medical meetings from Bayer, Biogen, Bristol, Janssen, Merck, Novartis, Roche, Sanofi, and Teva.
Lluís Ramió-Torrentà has received consulting fees, lecture honoraria, and funds to attend conferences from Biogen, BMS, Janssen, Merck, Novartis, Roche, and Sanofi-Genzyme.
María Eugenia Marzo Sola has no conflicts of interest to declare.
José María Prieto is a consultant for Bayer Pharmaceuticals, Biogen Spain S.L., Bristol-Myers Squibb, Daiichi Sankyo España, Genzyme Corporation, Janssen España, Merck Serono, Novartis Pharmaceuticals Corporation, Sanofi, Sandoz Iberia, Teva Pharmaceutical Industries, Roche Pharma, Almirall Prodesfarma S.A., and Celgene España S.L; has participated in/moderated meetings and/or symposia organised by Almirall Prodesfarma S.A., Bayer Pharmaceuticals, Biogen Spain S.L., Bristol-Myers Squibb, Genzyme Corporation, Janssen España, Merck Serono, Novartis Pharmaceuticals Corporation, Sanofi, Teva Pharmaceutical Industries, and Roche Pharma; and has received funding for research projects from Almirall Prodesfarma S.A., Biogen Spain S.L., Novartis Pharmaceutical Corporation, Teva Pharmaceutical Industries, and Sanofi.
María Luisa Martínez Ginés has received lecture honoraria and consulting fees from Merck, Biogen, Novartis, Sanofi-Genzyme, Almirall, BMS, Janssen, Roche, and Viatris.
Rafael Arroyo has received lecture honoraria and consulting fees from Biogen, Novartis, Merck, Teva, Roche, Sanofi-Genzyme, Bristol-Myers Squibb, and Janssen.
María Ángeles Otano Martinez has received lecture honoraria and consulting fees from Biogen Idec, Sanofi-Genzyme, Novartis, Merck, and Janssen.
Luis Brieva Ruiz has received funding for research projects by his group, fees for lectures and training, and funds to attend conferences from Bayer, Biogen, Roche, Merck, Novartis, Allmirall, BMS, Janssen, and Sanofi.
Montserrat Gómez Gutiérrez has received fees for presentations and consulting from Novartis, Biogen, Merck, Sanofi, Janssen, Roche, and Bial.
Alfredo Rodríguez-Antigüedad Zarranz has participated in scientific consulting or spoken at scientific meetings organised by Merck, Biogen, Novartis, Roche, Sanofi, Tevam and BMS.
Victoria Galán Sánchez-Seco has received lecture honoraria from Biogen Idec, Sanofi-Genzyme, Novartis, Roche, and Teva.
Lucienne Costa-Frossard has received lecture honoraria and consulting fees from Biogen, Novartis, Merck, Teva, Roche, Sanofi-Genzyme, Bristol-Myers Squibb, and Janssen.
Miguel Ángel Hernández Pérez has received lecture honoraria and consulting fees from Biogen, Bristol- Myer, Sanofi, Novartis, Roche, Merck, and Janssen.
Lamberto Landete Pascual has received fees for consulting and participation in scientific and training activities from Almirall, Bayer, Biogen, Bristol-Myers, Sanofi-Genzyme, Merck, Novartis, UCB Pharma, Roche, and Teva.
Monserrat González Platas has received lecture honoraria and consulting fees from Biogen, Novartis, Roche, Sanofi-Genzyme, Bristol-Myers Squibb, and Janssen.
Celia Oreja-Guevara has received lecture honoraria and consulting fees from Biogen Idec, Sanofi-Genzyme, Novartis, Roche, Merck and Teva.
AcknowledgementsLaura Prieto del Val (Dynamic Science S.L.U., Evidenze Clinical Research) collaborated as a scientific consultant and medical writer in the drafting of this manuscript and in the drafting of items. We are also grateful for the valuable comments and contributions of Ricardo Ginestal, José Ramón Ara, Ana Belén Caminero, Georgina Arrambide, and Óscar Fernández.