There is increasing evidence supporting that neuromyelitis optica (NMO) is an inflammatory humoral mediated disorder associated with NMO-IgG/AQP-4 antibodies. However, little is known about the subsets of B cells and T cells that contribute to the pathogenesis or therapy response.
ObjectivesTo describe the clinical and immunological changes associated with intravenous immunoglobulins (IV-Igs) plus rituximab (RTX) in a patient with a severe acute attack of NMO and intrathecal synthesis of NMO-IgG/AQP-4, who previously did not respond to intravenous methylprednisolone and plasma exchange.
MethodsWe sequentially analysed the levels of NMO-IgG/AQP-4 by immunohistochemistry, and B and T cells subsets by multiparametric flow-cytometry, in the CSF and peripheral blood (PB), before and after IV-Igs plus RTX therapy.
ResultsIn the CSF before treatment, and compared to PB, there was a higher percentage of CD4+ T cells and a lower percentage of CD8+ T cells and CD19+ B cells. After therapy, the percentage of CD4+ T cells remained high, and that of CD8+ T cells increased. The observed decrease in the percentage of CD19+ B cells was lower than in the PB. When the CSF was compared, it was found that the percentage of effector-memory and effector CD8+ T cells had increased after therapy, and that of IgM memory B cells and switched-memory B cells decreased. The observed changes paralleled the decrease of NMO-IgG/AQP-4 results to negative and the clinical improvement.
ConclusionsOur findings confirm that, besides intrathecal humoral immune response against AQP4, B and T cell subsets are involved in the modulation of inflammation within and outside the central nervous system.
La neuromielitis óptica (NMO) es una enfermedad predominantemente humoral mediada por anticuerpos IgG-NMO/AQP-4. Sin embargo, no se conoce bien la contribución de las diferentes subpoblaciones de células B y T en su patogenia o en la respuesta a los tratamientos.
ObjetivosDescribir los cambios clínicos e inmunológicos asociados al tratamiento con inmunoglobulinas intravenosas (Ig-IV) y rituximab (RTX) en una paciente con un brote grave de NMO y síntesis intratecal de IgG-NMO/AQP-4 que no había respondido a metilprednisolona y recambio plasmático.
MétodosSe analizaron, de forma secuencial en el LCR y en la sangre periférica (SP), las subpoblaciones linfocitarias mediante citometría de flujo multiparamétrica y los IgG-NMO/AQP-4, antes y después del tratamiento con Ig-IV y RTX.
ResultadosEn el LCR antes del tratamiento, y comparado con la SP, predominaban las células T CD4+ y estaban menos representadas las T CD8+ y las B CD19+. Tras el tratamiento, el porcentaje de células T CD4+ se mantuvo alto, el de T CD8+ aumentó y el de B CD19+ disminuyó, aunque menos que en la SP. Al comparar los LCR se vio que tras la terapia el porcentaje de células T CD8+ memoria efectoras y efectoras había aumentado, y el de células B memoria IgM y el de células B con cambio de isotipos, disminuido. Los cambios observados fueron paralelos a la negativización de los IgG-NMO/AQP-4 y a la mejoría clínica.
ConclusionesNuestros hallazgos confirman que, además de una respuesta inmunitaria humoral intratecal durante el brote de NMO, subpoblaciones específicas de células B y T participan en la modulación de la inflamación dentro y fuera del sistema nervioso central.
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the CNS affecting the optic nerve and the spinal cord. The course of the disease is usually marked by relapses, and the rapid disability it produces is associated with high rates of early mortality.1,2 Most patients with NMO present autoantibodies, NMO-IgG/AQP-4, which bind to a water channel, aquaporin-4 (AQP-4), a component mainly expressed in astrocyte endfeet.1–5 Findings from several studies support the pathogenic role of NMO-IgG/AQP-4, involving selective loss of AQP-4 in CNS lesions,6 a correlation between AQP4 antibody titres and disease activity,7,8 and induction of NMO-like histopathology in animal models by passive transfer of antibodies from humans with NMO.1 B cells and humoral immune response are believed to play a prominent role in NMO since patients with this disease show increased CSF and serum levels of cytokines promoting B-cell activation and antibody production, including BAFF and interleukin-6.9,10 This is important because, after crossing the blood-brain barrier, memory B cells are re-stimulated in the CNS due to their affinity for the antigen. They then mature, undergo clonal expansion, and differentiate into antibody-secreting plasma cells.11
There is no current consensus on treatment for patients with severe NMO relapses who do not respond to conventional treatments, which include cycles of intravenous methylprednisolone (IVMP) and plasma exchange (PE). We present the case of a patient with a severe NMO relapse and intrathecal synthesis of NMO-IgG/AQP-4 who did not respond to treatment with IVMP and PE. Nevertheless, our patient responded well to treatment with intravenous immunoglobulins (IVIg) and rituximab (RTX). We also describe the changes in B and T cell subsets and NMO-IgG/AQP4 antibody titres in CSF and peripheral blood (PB) after treatment.
Clinical caseOur patient, a 17-year-old female, was evaluated in another centre on 28 September 2009 after presenting subacute vision loss in the right eye progressing to complete blindness. Results from brain and spinal cord MRI scans were normal. We ruled out potential infectious and autoimmune causes of her clinical symptoms. The patient recovered visual acuity in 3 weeks after treatment with IVMP 1g/day for 7 days followed by progressively lower doses of oral prednisone. One month later, she presented vomiting and painful dysaesthesia in the feet, followed by progressive symptoms of transverse myelitis with quadriplegia and a C2 sensory level, in addition to left eye pain. The patient was admitted to the ICU on 27 November 2009 due to respiratory complications, hypotonic voice, and severe disability (level 9.0) according to Kurtzke's Expanded Disability Status Scale (EDSS).12 We performed a complete blood count, liver function test, hepatitis serology test, tuberculin skin test, microbiology test, electrocardiogram, echocardiogram, and tests for electrolytes, blood urea nitrogen, creatinine, autoimmune disease antibodies, and tumour markers. Results from all tests were normal. Results from the brain and spinal cord MRI scans were normal except for an area of gadolinium uptake in the left optic nerve and a large gadolinium-enhanced oedematous lesion extending from C1 to T10 (Fig. 1). A CSF test showed 53 lymphocytes/mm3, a protein level of 62mg/dL, and an IgG index of 0.84 (reference value<0.70). Immunohistochemistry13,14 revealed presence of NMO-IgG/AQP-4 in serum (titre 1:2000) and CSF (titre 1:128), and intrathecal synthesis of NMO-IgG/AQP-4. Our patient was treated with IVMP 1g/day for 5 days with no signs of improvement, followed by a dose of 500mg/day for 5 days and PE for 5 consecutive days. Two days after the last PE session, respiratory symptoms improved somewhat and the patient was able to move the fingers of her left hand slightly. An additional lumbar puncture (9 December 2009) yielded 43 lymphocytes/mm3, a protein level of 28mg/dL, and an IgG index of 1.48. NMO-IgG/AQP-4 findings were still positive in CSF (titre 1:2), but negative in serum. Our patient was treated with IVIg and RTX due to serum hypogammaglobulinaemia (which may have been linked to previous PE treatment) and her critical condition. IVIg has been shown to be effective in some cases of NMO.15 Treatment started on 11 December 2009 at a dose of 400mg/kg for 5 consecutive days; 72hours after the last dose she was administered RTX 375mg/m2 per week for 2 weeks. On 29 December 2009 another lumbar puncture was performed, showing 15 lymphocytes/mm3, a protein level of 31mg/dL, and an IgG index of 0.48. NMO-IgG/AQP-4 findings were negative in both serum and CSF. Results from another brain MRI scan, including an optic nerve study, were normal. A spinal cord MRI scan displayed continuous spinal cord thinning from C1 to T10 with no gadolinium uptake. The patient had improved and was able to walk short distances with crutches (EDSS level 6.5). She experienced marked and progressive improvements over a 2-month period; 4 months later, results from the neurological examination were normal. A follow-up spinal cord MRI test showed no hyperintensities in fat suppression sequences (STIR) and a more pronounced spinal cord thinning (Fig. 2). Since then, the patient has received 2 RTX cycles per year to prevent relapses. She has experienced no further episodes and neurological examinations yield normal results; serum remains positive for NMO-IgG/AQP-4, although at a low titre (August 2013).
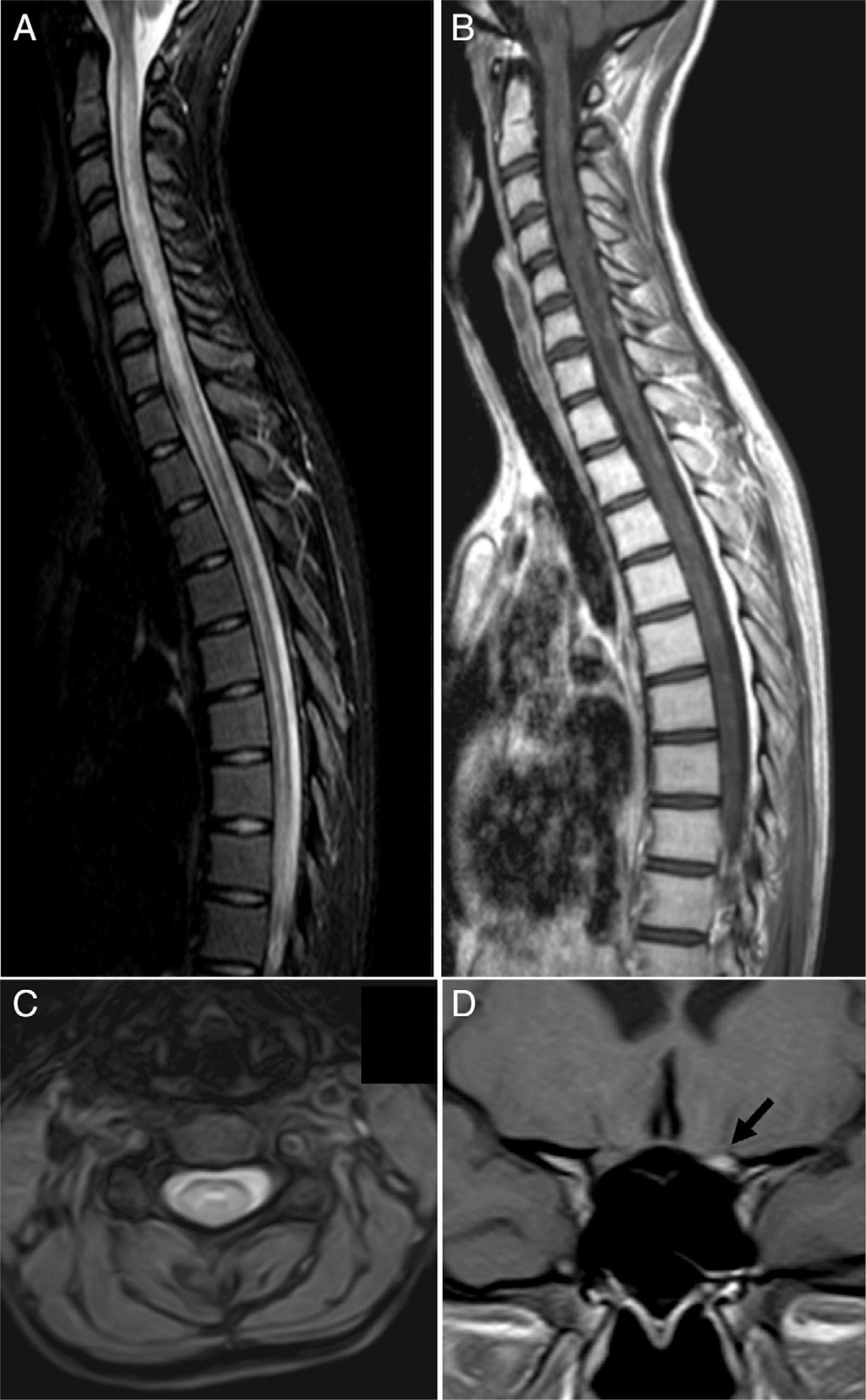
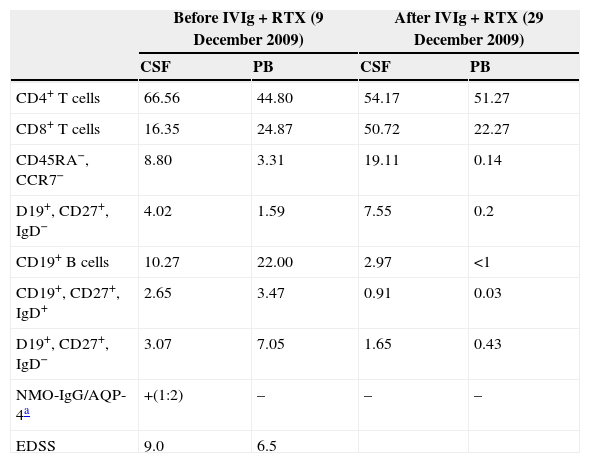
MRI scan at the time of admission. (A) Spinal cord MRI, sagittal fat suppression sequence (STIR), showing increased diffuse signal intensity extending along the spinal cord. (B) Sagittal T1-weighted sequence displaying patchy contrast enhancement along the spinal cord and hypointensities suggesting necrotic lesions in the central region of the spinal cord. (C) Axial T2-weighted sequence showing the central location of the lesion. (D) Coronal T1-weighted sequence revealing marked gadolinium enhancement in the left optic nerve (arrow).
We collected CSF and PB samples simultaneously to analyse NMO-IgG/AQP-4 levels using immunohistochemical methods. B and T cell subsets were analysed using multiparametric flow-cytometry, as described in previous studies.16–18
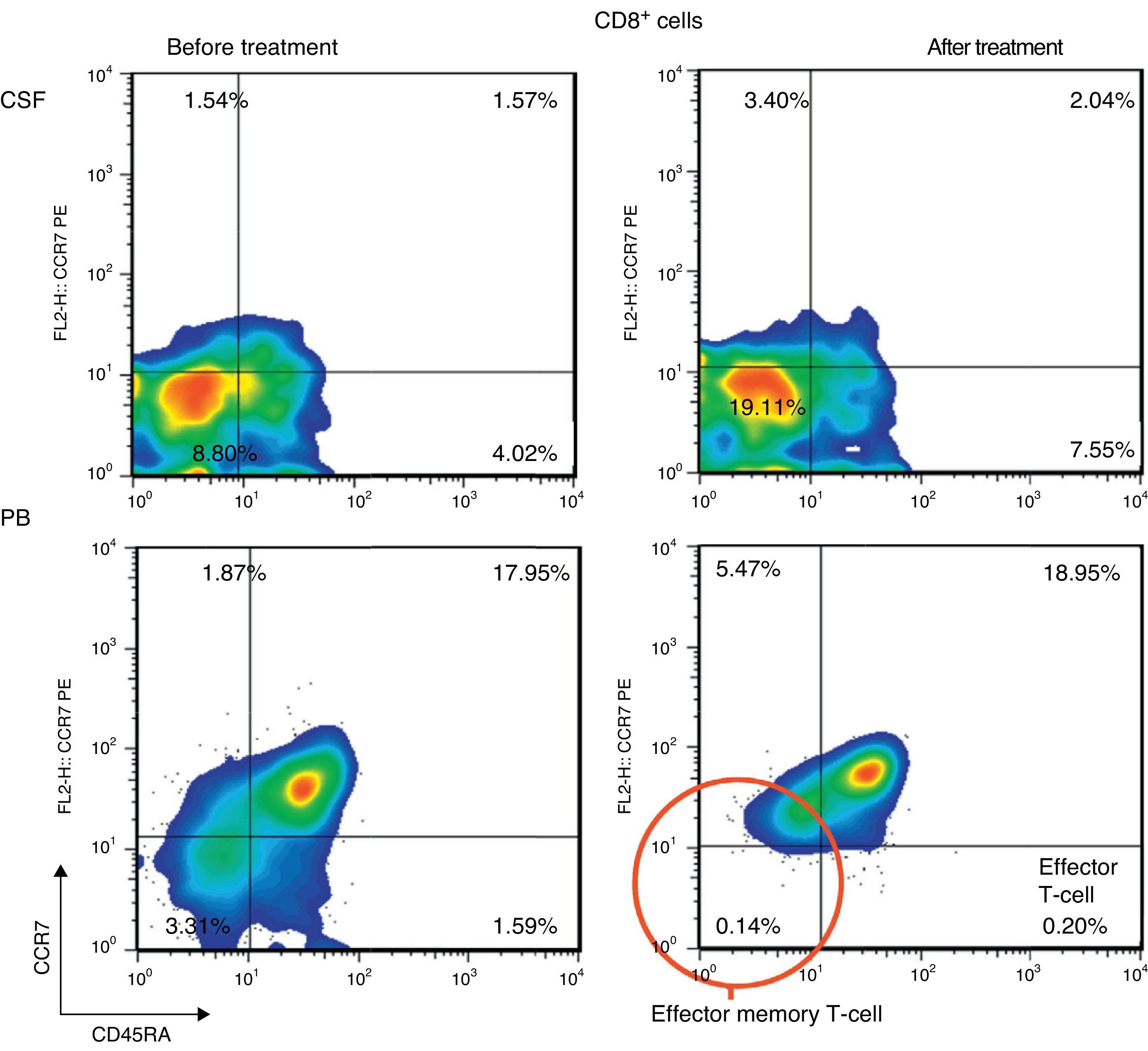
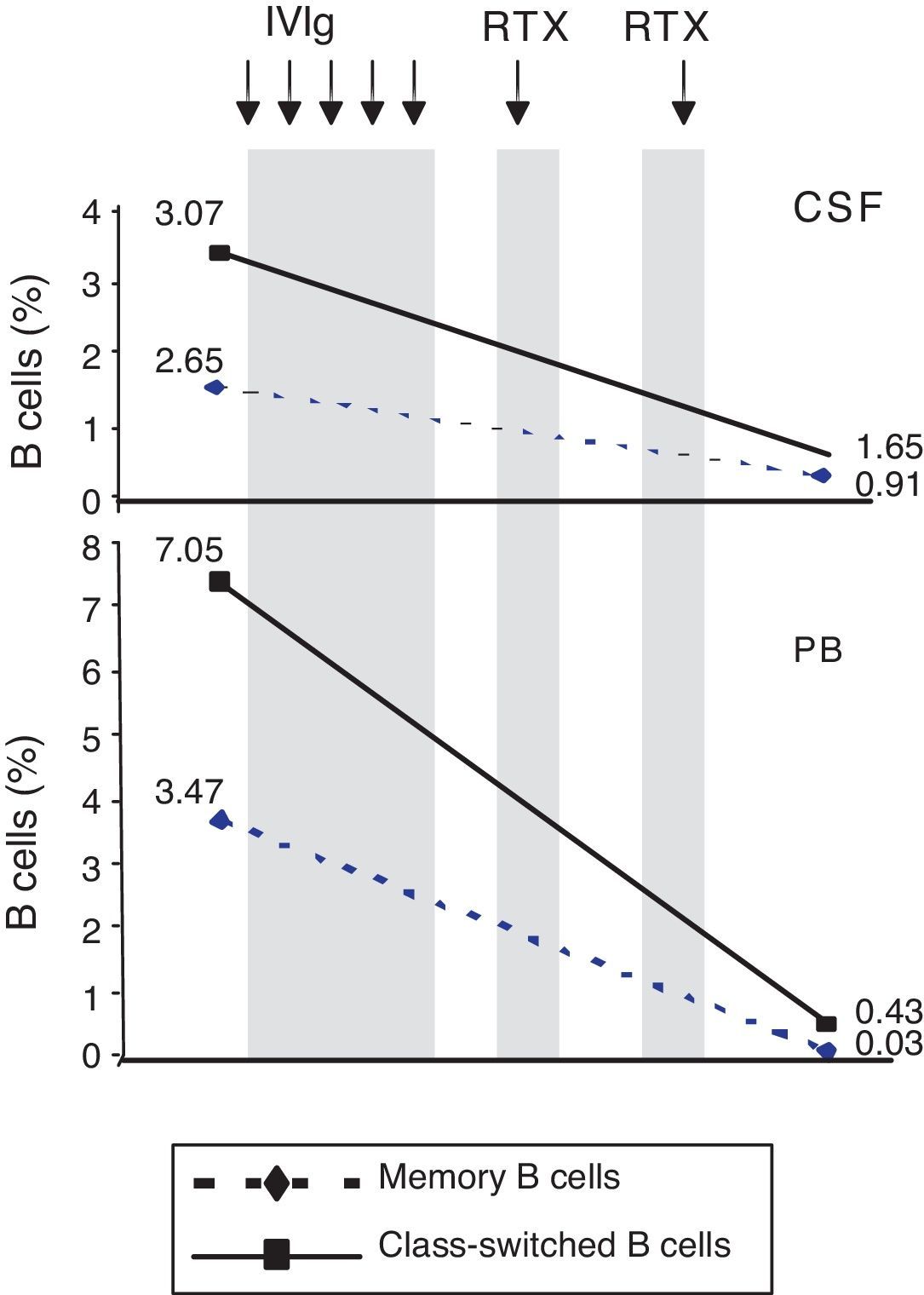
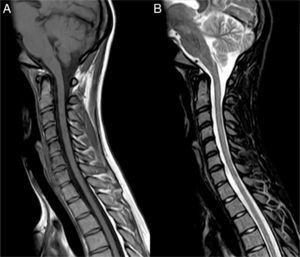
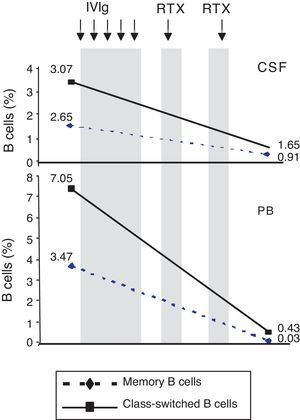
ResultsPercentage of B and T cell subsetsBefore IVIg and RTX treatment: levels of CD4+ T cells were higher in CSF than in PB (66.56% vs 44.80%), whereas levels of CD8+ T cells and CD19+ B cells were lower (16.35% vs 24.87%, and 10.27% vs 22%, respectively). The percentages of memory B cells (CD19+, CD27+, IgD+; 2.65% vs 3.47%) and class-switched B cells (CD19+, CD27+, IgD−; 3.07% vs 7.05%) were also lower in CSF. On the other hand, the percentages of CD8+ effector memory T cells (CD45RA−, CCR7 −; 8.80% vs 3.31%) and effector T cells (CD45RA+, CCR7−) were higher in CSF than in PB (Table 1). CSF also showed higher percentages of CD4+ regulatory T cells (CD4+, CD25hi+, and CD4+, CD25+, Foxp3+; 2.25% vs 0.40%, and 3.25% vs. 0.12%, respectively) and regulatory/suppressor T cells (CD8+, CD25+, CD28−; 5.56% vs 2.40%). NMO-IgG antibodies were found in CSF (titre 1:2), but not in serum (Table 1).
Changes in the percentages of T and B cells and in NMO-IgG/AQP-4 titres in CSF and PB before and after treatment with intravenous immunoglobulins and rituximab.
| Before IVIg+RTX (9 December 2009) | After IVIg+RTX (29 December 2009) | |||
|---|---|---|---|---|
| CSF | PB | CSF | PB | |
| CD4+ T cells | 66.56 | 44.80 | 54.17 | 51.27 |
| CD8+ T cells | 16.35 | 24.87 | 50.72 | 22.27 |
| CD45RA−, CCR7− | 8.80 | 3.31 | 19.11 | 0.14 |
| D19+, CD27+, IgD− | 4.02 | 1.59 | 7.55 | 0.2 |
| CD19+ B cells | 10.27 | 22.00 | 2.97 | <1 |
| CD19+, CD27+, IgD+ | 2.65 | 3.47 | 0.91 | 0.03 |
| D19+, CD27+, IgD− | 3.07 | 7.05 | 1.65 | 0.43 |
| NMO-IgG/AQP-4a | +(1:2) | – | – | – |
| EDSS | 9.0 | 6.5 | ||
Data are expressed as percentages of the total lymphocyte count.
EDSS: expanded disability status scale; IVIg: intravenous immunoglobulins; CSF: cerebrospinal fluid; PB: peripheral blood; RTX: rituximab.
After IVIg and RTX treatment: the percentage of CD4+ T cells was higher in CSF than in PB (54.17% vs 51.27%); CD8+ levels had increased (50.72% vs 22.27%) whereas CD19+ levels had decreased (2.97% vs <1%) (Table 1). Comparing results from CSF samples taken before and after treatment showed decreased percentages of both memory B cells (98% of IgM; 2.65% vs 0.91%) and class-switched B cells (3.07% vs 1.65%). On the other hand, the percentage of CD8+ effector memory T cells (CD45RA−, CCR7−; 8.80% to 19.11%) and effector T cells (CD45RA+, CCR7−; 4.02% vs 7.55%) had increased after treatment (Table 1, Figs. 3 and 4). We also compared the results of PB samples taken before and after treatment and found lower percentages of memory B cells (3.47% vs 0.03%) and class-switched B cells (7.05% vs 0.43%) after treatment. Furthermore, unlike in CSF samples, there were also reductions in percentages of CD8+ effector memory T cells (CD45RA−, CCR7−; 3.31% vs 0.14%) and effector T cells (CD45RA+, CCR7−; 1.59% vs 0.20%) (Table 1, Figs. 3 and 4). NMO-IgG/AQP-4 findings were negative in both serum and CSF (Table 1).
Standard treatment for NMO relapses usually requires high doses of IVMP. When patients do not respond as expected, PE has been shown to be a safe alternative in 50% to 75% of all cases.1,19–21 However, no randomised controlled trials have been conducted to evaluate preventive treatments for NMO relapses. Current treatment options are based on small observational studies using such immunosuppressants as azathioprine, mitoxantrone, mycophenolate mofetil, and more recently, rituximab.1
In this study, we present the case of an adolescent girl who experienced a severe NMO relapse shortly after NMO onset. She presented intrathecal synthesis of NMO-IgG/AQP-4 and did not respond to treatment with IVMP and PE. We decided to treat the patient with IVIg and RTX due to lack of improvement and the presence of NMO-IgG/AQP-4 in CSF despite previous PE treatment. IVIg inhibits B cell-mediated antigen presentation and T cell proliferation.22 RTX is a monoclonal anti-CD20 B cell antibody that targets the CD20 marker and induces depletion of CD20+ B cells, including pre-B cells, naive B cells, and memory B cells.9 Both drugs have been shown to be effective for preventing NMO relapses.1,9,15,23
Before our patient underwent treatment with IVIg and RTX, CSF samples showed a high percentage of B cells and recruitment of memory B cells (predominantly IgM) and class-switched B cells. This finding, in conjunction with intrathecal synthesis of NMO-IgG/AQP-4, may confirm the role played by humoral immune response in NMO.1,24 We now know that recruitment of class-switched B cells is largely responsible for accumulation of memory B cells in CSF during neuroinflammation.25 Furthermore, memory B cells can rapidly differentiate into antibody-secreting cells after antigen recognition and contribution by T cells.26 We observed higher percentages of CD8+ effector memory T cells and effector T cells in CSF than in PB. This finding has never been described in the literature. The reason for this recruitment is not clearly understood. It may be due to chemokine secretion by NMO-specific B cells in the CNS, which leads to recruitment from PB, activation, and proliferation of those cells. Alternatively, those cells may be CD8+ regulatory/suppressor T cells, which may help resolve the inflammatory process.25–27 In any case, their presence indicates that T cells are involved in the pathogenesis of NMO.28
After treatment with IVIg and RTX, as was expected, we found that CD19+ B cells, memory B cells, and class-switched B cell subtypes were considerably depleted. However, percentages were still higher in CSF than in PB. We must be mindful that the analysis was performed after the patient had received 2 doses of RTX, and that complete depletion is usually seen after 4 doses.29,30 We were surprised to find an increase in the percentage of CD8+ T cells in CSF, since these cell populations normally dwindle in multiple sclerosis.31 However, transient increases have been described in other autoimmune diseases.32 We did not evaluate whether CD8+ effector memory and effector T cells were cytotoxic or regulatory cells. As a result, we could not assess their role in this case. According to some studies, NMO-IgG/AQP-4 titres may decrease after treatment with RTX9,33 and memory B cell depletion can affect the production of anti-AQP-4 antibodies by short-lived plasma cells. In our patient, however, antibodies were completely depleted while memory B cells were still present, which highlights the complex connection between B and T cell subsets. In fact, CD19+ B cell or CD27+ memory cell re-emergence in PB (which usually takes place at 6-9 months) may indicate the need to administer an additional cycle of RTX.9,33 In any case, immunological changes in our patient were parallel to rapid and marked improvement of symptoms. However, the potential impact of previous treatment with IVMP and PE on these changes is difficult to determine.
In conclusion, our findings confirm that an intrathecal humoral immune response against AQP-4 takes place during NMO relapses. This immune response is antigen-induced and dependent on B and T cell subsets. A better understanding of NMO pathophysiology is necessary to develop more precise therapeutic strategies for this entity.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: de Andrés C, Teijeiro R, Saiz A, Fernández P, Sánchez-Ramón S. Cambios en las Subpoblaciones de Linfocitos B y T en el Título de Anticuerpos Anti-Acuaporina-4 tras el Tratamiento de un Brote Agudo con Inmunoglobulinas y Rituximab. Neurología. 2015;30:276–282.