Numerous regions of the brain, such as the medial frontal cortex, orbitofrontal cortex, insula, and amygdala, participate in the autonomic control of cardiovascular functions such as heart rate. The degenerative process in frontotemporal dementia (FTD) involves the listed anatomical structures and may therefore produce dysautonomic cardiovascular symptoms.
AimTo observe whether or not non-cardiogenic bradycardia was more frequent in a group of patients with FTD than in subjects with mild cognitive impairment or dementia of a different aetiology.
Patients and methodOnce patients with primary cardiac arrhythmia were excluded, we registered the heart rates of 258 patients with cognitive symptoms (36 with FTD, 22 with Alzheimer disease, 23 with vascular dementia, 10 with other dementias, and 167 with non-dementia cognitive impairment).
ResultsBradycardia (<60 beats/minute) was significantly more frequent in patients with FTD. This difference remained significant after excluding subjects undergoing treatment with a potentially bradycardic effect. Bradycardia was more prevalent in behavioural FTD cases than in cases of the aphasic variant, and we detected a trend towards higher frequency among patients with more pronounced right hemisphere atrophy. Moreover, mean systolic blood pressure in FTD patients was lower than in other participants, and systolic hypotension (<120 and <100mmHg) was more prevalent.
ConclusionBradycardia was more frequent in the FTD sample than in other patients with cognitive symptoms. Further investigations will be necessary before we may consider bradycardia to be a sign supporting diagnosis of FTD or its behavioural variant.
Diversas áreas cerebrales, como la corteza orbitofrontal y frontomedial, la ínsula y la amígdala, intervienen en el control del sistema nervioso autónomo sobre funciones cardiovasculares como la frecuencia cardíaca. El proceso degenerativo de la demencia frontotemporal (DFT) involucra estas estructuras anatómicas y, por tanto, podría producir síntomas cardiovasculares disautonómicos.
ObjetivoObservar si la bradicardia de origen cerebral es más frecuente en pacientes con DFT que en enfermos con deterioro cognitivo ligero o demencia de otra etiología.
Pacientes y métodoUna vez excluidos los pacientes con arritmia de origen cardíaco, se registró la frecuencia cardíaca de 258 pacientes con síntomas cognitivos (36 con DFT, 22 con enfermedad de Alzheimer, 23 con demencia vascular, 10 con otras demencias y 167 con deterioro cognitivo sin demencia).
ResultadosLa bradicardia (<60ppm) fue estadísticamente más frecuente en los pacientes con DFT. La diferencia se mantuvo significativa tras excluir a los que estaban en tratamiento con potencial efecto bradicardizante. La bradicardia fue más prevalente en la DFT conductual que en la DFT afásica, y hubo tendencia al predominio en los pacientes con mayor atrofia en el hemisferio derecho. La presión arterial sistólica de los pacientes con DFT fue inferior a la de los otros sujetos del estudio, y fue mayor la prevalencia de hipotensión sistólica (<120 y <100mmHg).
ConclusiónSe ha observado mayor frecuencia de bradicardia en los pacientes con DFT que en otros pacientes con síntomas cognitivos. Antes de considerar este dato semiológico como un signo de apoyo al diagnóstico de DFT, será necesario realizar nuevas observaciones.
Diverse areas of the cerebral cortex and certain subcortical subnuclei participate in the neural circuits that influence cardiovascular activity.1 Among other functions, they exert a regulatory effect on the response of the autonomic nervous system to cognitive activities and emotional changes. One of the manifestations of this modulation is the change in heart rate that accompanies processes such as mental arithmetic2,3 and other tasks requiring concentration,4 as well as stressful situations5–7 and those involving danger,8 and changes in mood or emotion.9,10
Cortical areas intervening in cardiovascular modulation include the orbitofrontal cortex11,12 and frontal medial cortex4,6–9,11,13 (including the anterior cingulate8,13–15), the insula,16–20 and cerebral amygdala.13 Specific neurological disorders that damage or excite these areas of the brain are accompanied by tachycardia, bradycardia, changes in cardiac reactivity, and other symptoms of autonomic nervous system dysfunction. Such is the case in ictal bradycardia syndrome, observed in some patients with temporal lobe or frontal lobe epilepsy, which presents at times with asystole.21,22 Other examples are changes in autonomic nervous system control over the heart in cerebrovascular events located in the insula.19
Frontotemporal dementia (FTD) is characterised by an underlying degenerative process involving many of the anatomical areas mentioned above. Active in cerebral control over the autonomic nervous system, these areas include the prefrontal and temporal cortex, insula, and amygdala.23–25 For this reason, it seems reasonable to conclude that the presence of cardiovascular dysfunction might be more common in these patients than in patients with dementia or cognitive alterations in which the anatomical location of the lesion is totally or mainly excluded from the regions listed above. The article presented here describes using simple observation of the presence or absence of bradycardia in patients with cognitive symptoms to determine if there are any differences between patients diagnosed with FTD and those with other diagnoses.
Patients and methodsWe retrospectively examined the medical histories of 650 consecutive patients who were examined by a neurologist after noticing cognitive symptoms. This study excluded patients with no clear diagnosis (patients whose diagnosis was uncertain, who declined to undergo necessary tests, or whose tests were pending at the time). The study also excluded patients without mild cognitive impairment or dementia (patients whose neuropsychological examination yielded normal results; many of these cases were diagnosed with a neurological, psychiatric, or systemic disorder with reactive symptoms that motivated the cognitive neurology consult). From the remaining recruits, we selected patients whose heart rate had been recorded, later eliminating those with pacemakers or irregular heartbeats (most of whom had atrial fibrillation). Pulse and arterial blood pressure were recorded simultaneously with an Omron M6 upper arm monitor. In addition to providing a record of observed heart rate, the analysis groups patients with heart rates below 60bpm to compare them to the other participants.
This study recorded patients’ sex, age, neurological diagnosis, and any treatments at the time when heart rate was measured. To facilitate data analysis, some diagnoses were grouped with others. The final categories were FTD, Alzheimer disease, vascular dementia, other dementia, and no dementia. The 2001 McKhann et al. criteria were used to establish the clinical diagnosis of FTD.26 All patients with FTD showed neuroimaging changes compatible with the suspected diagnosis. We also recorded whether the patient's dominant symptoms were behavioural, aphasic, or both.
Patients were also grouped according to whether or not they were taking medications with a potentially bradycardic effect.
We also list the arterial blood pressure readings which were measured at the same time as heart rate, but blood pressure is not the main focus of the study and we did not examine all of the factors that might have affected these measurements.
Regions of the cerebral cortex that exhibited atrophy in neuroimaging scans were also recorded for all patients with FTD.
In line with the working hypothesis, we used statistical analysis to compare heart rate and presence of bradycardia between patients with FTD and the other diagnostic groups. The analysis examines the potential bradycardic effects exerted by certain drugs.
Heart rate and blood pressure (systolic and diastolic) were compared between patients with and without FTD. The t-test was used for groups with a normal distribution; the Mann–Whitney U test was used when subgroups being compared contained few elements. The same comparisons for presence or absence of bradycardia or hypotension were made using the chi-square test or Fisher's exact test. This procedure was also used to compare results from patients with aphasic and behavioural FTD. Lastly, we recorded whether bradycardia had a higher incidence rate among FTD patients with impairment in a specific lobe or hemisphere. We used the Kruskal–Wallis non-parametric test for this purpose, given that subgroups exhibited different sizes and variance.
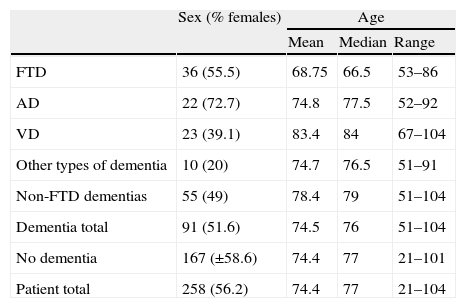
ResultsThe sample included 258 patients with cognitive symptoms: 91 with dementia and the remaining 167 with other types of cognitive impairment (grades 2 or 3 on Reisberg's Global Deterioration Scale).27 Thirty-six of the patients with dementia met criteria for FTD26; 22 were diagnosed with Alzheimer disease, 23 with vascular dementia, and 10 with dementia due to other causes. Table 1 displays selected demographic data from individuals in the sample. Most patients had attended a general neurology consultation before making an appointment with the cognitive neurology unit because their clinical profiles were not typical of Alzheimer disease. This explains why the diagnoses in our sample do not reflect the typical distribution in the population with cognitive impairment, in which Alzheimer disease is the predominant cause.
Sex and age of patients included in the study.
| Sex (% females) | Age | |||
| Mean | Median | Range | ||
| FTD | 36 (55.5) | 68.75 | 66.5 | 53–86 |
| AD | 22 (72.7) | 74.8 | 77.5 | 52–92 |
| VD | 23 (39.1) | 83.4 | 84 | 67–104 |
| Other types of dementia | 10 (20) | 74.7 | 76.5 | 51–91 |
| Non-FTD dementias | 55 (49) | 78.4 | 79 | 51–104 |
| Dementia total | 91 (51.6) | 74.5 | 76 | 51–104 |
| No dementia | 167 (±58.6) | 74.4 | 77 | 21–101 |
| Patient total | 258 (56.2) | 74.4 | 77 | 21–104 |
FTD: frontotemporal dementia; VD: vascular dementia; AD: Alzheimer disease.
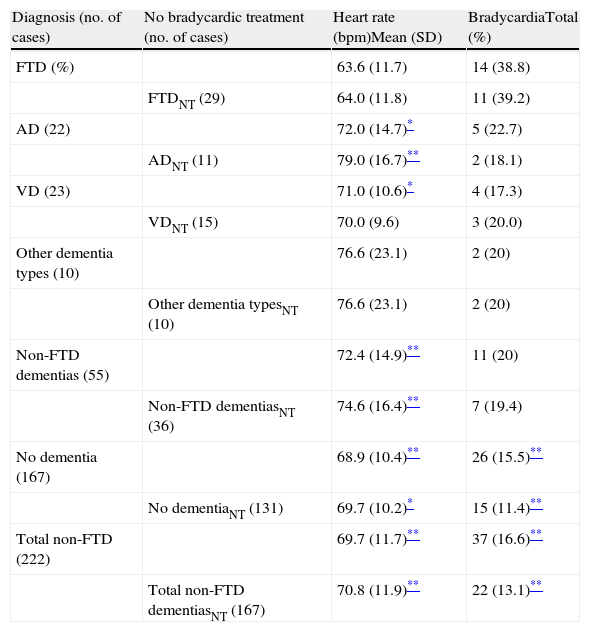
Heart rates in patients, who are grouped by diagnosis, and the percentage of patients with bradycardia (defined as heart rate <60bpm), both displayed inter-group differences as shown in Table 2. Heart rate was lower and the percentage of patients with bradycardia was higher in the FTD group than in other participants, although the difference was not statistically significant in some small subgroups.
Data on heart rate and bradycardic treatment in the different groups included in the study.
| Diagnosis (no. of cases) | No bradycardic treatment (no. of cases) | Heart rate (bpm)Mean (SD) | BradycardiaTotal (%) |
| FTD (%) | 63.6 (11.7) | 14 (38.8) | |
| FTDNT (29) | 64.0 (11.8) | 11 (39.2) | |
| AD (22) | 72.0 (14.7)* | 5 (22.7) | |
| ADNT (11) | 79.0 (16.7)** | 2 (18.1) | |
| VD (23) | 71.0 (10.6)* | 4 (17.3) | |
| VDNT (15) | 70.0 (9.6) | 3 (20.0) | |
| Other dementia types (10) | 76.6 (23.1) | 2 (20) | |
| Other dementia typesNT (10) | 76.6 (23.1) | 2 (20) | |
| Non-FTD dementias (55) | 72.4 (14.9)** | 11 (20) | |
| Non-FTD dementiasNT (36) | 74.6 (16.4)** | 7 (19.4) | |
| No dementia (167) | 68.9 (10.4)** | 26 (15.5)** | |
| No dementiaNT (131) | 69.7 (10.2)* | 15 (11.4)** | |
| Total non-FTD (222) | 69.7 (11.7)** | 37 (16.6)** | |
| Total non-FTD dementiasNT (167) | 70.8 (11.9)** | 22 (13.1)** |
FTD: frontotemporal dementia; VD: vascular dementia; AD: Alzheimer disease; NT: no bradycardic treatment.
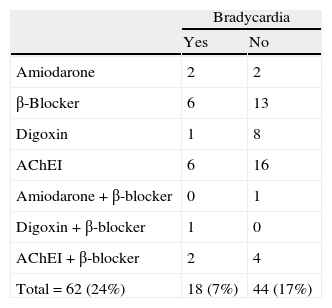
To control for the bias that might arise from a patient taking a drug with a bradycardic effect, patients on these drugs were analysed separately from the rest. Of 62 patients taking bradycardic drugs (24% of the total), 18 had bradycardia (7%) and the remaining 44 did not (17%). Table 3 presents a list of recorded drugs and presence or absence of bradycardia in the patients taking them. As we see in Table 2, bradycardia was not only more common in the overall FTD group (n=36), but also in the subgroup that excluded patients whose bradycardia was affected by a bradycardic drug (n=29).
Patients with normal heart rate or bradycardia and on bradycardic treatment.
| Bradycardia | ||
| Yes | No | |
| Amiodarone | 2 | 2 |
| β-Blocker | 6 | 13 |
| Digoxin | 1 | 8 |
| AChEI | 6 | 16 |
| Amiodarone+β-blocker | 0 | 1 |
| Digoxin+β-blocker | 1 | 0 |
| AChEI+β-blocker | 2 | 4 |
| Total=62 (24%) | 18 (7%) | 44 (17%) |
AChEI: acetylcholinesterase inhibitors.
The percentage out of the 258 patients in the study is shown in brackets.
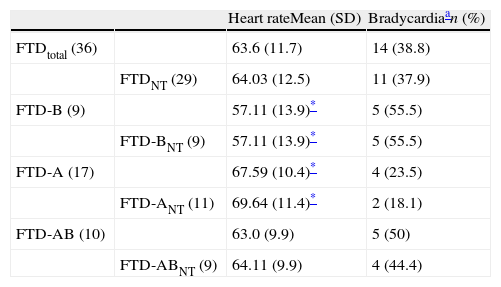
Patients with FTD and bradycardia were categorised according to predominant symptoms (behavioural, aphasic, or mixed), and their distribution is shown in Table 4. We observe lower heart rate and a higher incidence of bradycardia in patients with behavioural or mixed forms than in those with aphasic FTD. Only the difference between mean frequencies in the behavioural and the aphasic groups are statistically significant.
Heart rate and presence of bradycardia in patients with FTD, indicating predominant symptoms.
| Heart rateMean (SD) | Bradycardiaan (%) | ||
| FTDtotal (36) | 63.6 (11.7) | 14 (38.8) | |
| FTDNT (29) | 64.03 (12.5) | 11 (37.9) | |
| FTD-B (9) | 57.11 (13.9)* | 5 (55.5) | |
| FTD-BNT (9) | 57.11 (13.9)* | 5 (55.5) | |
| FTD-A (17) | 67.59 (10.4)* | 4 (23.5) | |
| FTD-ANT (11) | 69.64 (11.4)* | 2 (18.1) | |
| FTD-AB (10) | 63.0 (9.9) | 5 (50) | |
| FTD-ABNT (9) | 64.11 (9.9) | 4 (44.4) |
A: aphasic symptoms are predominant; B: behavioural symptoms are predominant; AB: aphasic and behavioural symptoms, neither predominant; FTD: frontotemporal dementia; NT: no bradycardic treatment.
All patients in the sample with a diagnosis of FTD (36) underwent structural neuroimaging tests (29 MRI; 7 CT). Fifteen of these patients also had functional neuroimaging tests (12 SPECT and 3 PET). Observations on the topography of the atrophied area were based on structural neuroimaging findings, with support from functional neuroimaging studies where available. We should clarify the findings reported in Tables 5a and 5b. The occipital lobe was excluded because there were no FTD cases with apparent damage to that lobe. The amygdala and insula, which are presumably involved in controlling heart rate, are not included. This is because volumetric measurement techniques were not applied to grey matter in the neuroimaging tests that were carried out; furthermore, in some cases, resulting sections were not optimal for studying those structures. For the above reasons, atrophy could not be reliably evaluated in those areas except where it was pronounced. In the case of the amygdala, a minor loss of volume may be difficult to assess depending on the associated degree of hippocampal atrophy. This is also true of the insula if the images are inadequate, depending on the level of atrophy in the frontal and temporal opercula and adjacent subcortical structures.
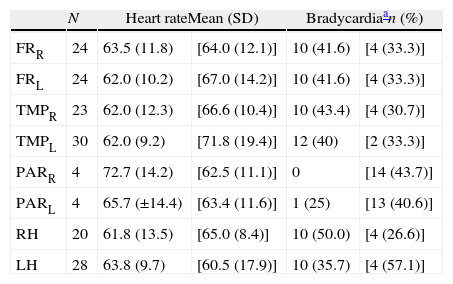
Topography of neuroimaging changes detected in the 36 patients with FTD.
| N | Heart rateMean (SD) | Bradycardiaan (%) | |||
| FRR | 24 | 63.5 (11.8) | [64.0 (12.1)] | 10 (41.6) | [4 (33.3)] |
| FRL | 24 | 62.0 (10.2) | [67.0 (14.2)] | 10 (41.6) | [4 (33.3)] |
| TMPR | 23 | 62.0 (12.3) | [66.6 (10.4)] | 10 (43.4) | [4 (30.7)] |
| TMPL | 30 | 62.0 (9.2) | [71.8 (19.4)] | 12 (40) | [2 (33.3)] |
| PARR | 4 | 72.7 (14.2) | [62.5 (11.1)] | 0 | [14 (43.7)] |
| PARL | 4 | 65.7 (±14.4) | [63.4 (11.6)] | 1 (25) | [13 (40.6)] |
| RH | 20 | 61.8 (13.5) | [65.0 (8.4)] | 10 (50.0) | [4 (26.6)] |
| LH | 28 | 63.8 (9.7) | [60.5 (17.9)] | 10 (35.7) | [4 (57.1)] |
R: right; FTD: frontotemporal dementia; FR: cortex of the frontal lobe; RH: right hemisphere; LH: left hemisphere; L: left; N: number of patients with atrophy in the localisation indicated at the beginning of the line; PAR: cortex of the parietal lobe; TMP: cortex of the temporal lobe.
Values from individuals with this lobe preserved are indicated between square brackets.
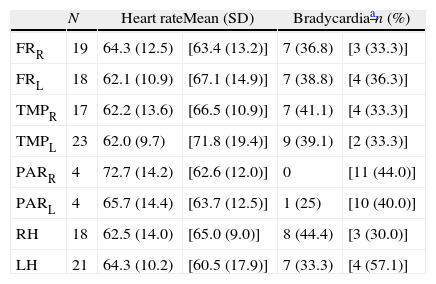
Topography of neuroimaging changes detected in the 29 patients with FTD and no bradycardic treatment.
| N | Heart rateMean (SD) | Bradycardiaan (%) | |||
| FRR | 19 | 64.3 (12.5) | [63.4 (13.2)] | 7 (36.8) | [3 (33.3)] |
| FRL | 18 | 62.1 (10.9) | [67.1 (14.9)] | 7 (38.8) | [4 (36.3)] |
| TMPR | 17 | 62.2 (13.6) | [66.5 (10.9)] | 7 (41.1) | [4 (33.3)] |
| TMPL | 23 | 62.0 (9.7) | [71.8 (19.4)] | 9 (39.1) | [2 (33.3)] |
| PARR | 4 | 72.7 (14.2) | [62.6 (12.0)] | 0 | [11 (44.0)] |
| PARL | 4 | 65.7 (14.4) | [63.7 (12.5)] | 1 (25) | [10 (40.0)] |
| RH | 18 | 62.5 (14.0) | [65.0 (9.0)] | 8 (44.4) | [3 (30.0)] |
| LH | 21 | 64.3 (10.2) | [60.5 (17.9)] | 7 (33.3) | [4 (57.1)] |
R: right; FTD: frontotemporal dementia; FR: cortex of the frontal lobe; RH: right hemisphere; LH: left hemisphere; L: left; N: number of patients with atrophy in the localisation indicated at the beginning of the line; PAR: parietal cortex; TMP: temporal cortex.
Values from individuals with this lobe preserved are indicated between square brackets.
Concerning the topography of neuroimaging study abnormalities and the presence or absence of bradycardia, the statistical analysis found no significant differences between distinct affected lobes of the brain (Tables 5a and 5b). We only observe a slightly lower heart rate and higher percentage of bradycardia among patients with changes in the right hemisphere.
DiscussionDeparting from the theory that FTD affects brain structures related to autonomic nervous system control over heart function, this study examined a group of FTD patients and found a higher prevalence of bradycardia and systolic arterial hypertension than in subjects with cognitive impairment of other causes.
As was anticipated, mean age in the FTD patient group was lower than in the rest of the patients (Table 1) because onset of this disease frequently occurs in individuals younger than 65 years.28
Although samples were small, we should mention that the FTD group exhibited no sex-related differences, but that females were predominant in the Alzheimer group and males were dominant in the vascular dementia group (Table 1). This concurs with epidemiological data published on this subject.26,29,30
The prevalence rate of bradycardia (<60bpm) in the total elderly population has not been determined, but we know it is not uncommon. The condition is asymptomatic in most cases, especially if heart rate remains over 50bpm. This finding may be related to cardiac changes or changes in cardioregulatory and vasoregulatory hormonal and electrolytic systems. Bradycardia may also be linked to autonomic nervous system changes, and it is sometimes caused by various types of organic lesions and often present as an adverse drug effect. Bradycardia cases are not rare among dementia patients, who are frequently elderly and on polytherapy. Considering probable margins of error, some 20% of the participants in this study were likely to exhibit a heart rate below 60bpm. However, if the presence of bradycardia were found to be significantly more common among patients sharing a specific diagnosis, the finding would be more interesting and potentially valuable as a clinical sign supporting that diagnosis. In fact, the study does find higher prevalence of bradycardia among patients with FTD compared to other diagnostic groups. The difference is hypothetical, given that sample sizes in this analysis are insufficient to determine whether or not this situation is present in the total population of dementia patients. Nevertheless, it does serve as a starting point for future observations that might confirm or refute this preliminary finding.
Twenty-six of the 258 patients in the study mentioned having had symptoms potentially related to bradycardia, such as falls or brief losses of consciousness with no established cause (3 patients with FTD, 3 with AD, 3 with vascular dementia, 3 with other types of dementia, and 14 without dementia. The consultation revealed bradycardia in the 3 patients with AD, 1 patient with another type of dementia, and 2 subjects without dementia). These data support that bradycardia is rarely accompanied by symptoms (the manifestations described above only affected 6 of the 51 patients found to have bradycardia, and some symptoms may not have been related to the bradycardia itself). At the same time, they lead us to suspect that bradycardia in some patients may only be symptomatic at times, and therefore not detected in the consultation (20 patients with a history of symptoms had a normal heart rate during the consultation). Two patients with bradycardia were later fitted with cardiac pacemakers.
Cerebral changes that may produce bradycardia vary, and they include intracranial hypertension, convulsions, tumours, or vascular lesions that affect specific areas of the brain.31 It is reasonable to believe that many patients diagnosed with FTD present bradycardia at some point. In all of the processes described above, bradycardia stems from excessive stimulation of cerebral structures linked to the parasympathetic nervous system, or inhibition of/injury to such structures. Chief among these structures are the medial prefrontal cortex, basal frontal cortex, insula, and amygdala; in FTD, these regions of the brain undergo degenerative changes even in the initial stages of the disease.
Nevertheless, FTD disease progression exhibits considerable inter-individual variability. Some patients show initial changes in prefrontal regions, while in others, temporal changes are initially predominant; changes may be quite symmetrical or markedly asymmetrical in both cases, at least during the early stages. This is how doctors profile phenotypes of the behavioural variant of FTD, the form with onset marked by progressive nonfluent aphasia, semantic dementia, and other less-frequent variants. Current research shows that the circuits for parasympathetic cardiovascular control are predominant in the left hemisphere, while circuits for sympathetic control predominate in the right hemisphere.32,33 Depending on the disease phenotype in question, the patient will show an increased propensity towards tachycardia or bradycardia, or else maintain the balance between the two systems. However, changes in cardiovascular reactivity which stem from cerebral control are always likely to exist, even if they are subtle. These factors explain why bradycardia does not appear in many patients with FTD, but if studies were to show higher incidence rates in these patients than in patients with other types of dementia, this finding could be considered a clinically valuable sign.
In many patients with non-frontotemporal dementia, some of the listed anatomical structures will also undergo changes, but this does not occur in all cases, or the process is less intense than in FTD. While FTD exerts an early effect on the fronto-insular and fronto-amygdalar circuit, with the involvement of the anterior cingulate, Alzheimer disease primarily affects the posterior limbic pathways and has the greatest impact on the hippocampus, with damage extending to the posterior cingulate cortex and external temporoparietal area.23 AD damage also invades frontal insular areas,34 but this is less pronounced than in FTD, at least during early stages. Vascular dementia, in turn, is characterised by the heterogeneous topography of its lesions. Diseased tissue may be limited to or predominant in posterior or anterior cortical or subcortical territories; impairment may be focal, multifocal, or diffuse. In this way, some cases may exhibit signs of a cardiovascular control disorder, as in the insular strokes mentioned above.19 In other patients, cardiovascular control will remain normal35,36 or be affected by an associated heart disease.
Autonomic dysfunction is part of a classic array of symptoms in some types of dementia, such as Lewy body dementia, dementia associated with Parkinson's disease, and dementia in some patients with multiple-system atrophy.36,37 The mere presence of bradycardia of cerebral origin, or of other types of autonomic dysfunction, is insufficient to establish a defined aetiological diagnosis. Only when it presents with other clinical signs can it aid the doctor in finding diagnostic criteria compatible with the patient's clinical status. If the hypothesis proposed by this study were confirmed, FTD would be added to the list of nosological entities whose diagnosis would be suggested or supported by the presence of bradycardia.
It is difficult to draw conclusions about the behavioural and aphasic variants of FTD (Table 4) because distinguishing between these populations decreases the sample size. Language changes or behavioural changes (social, eating, and sexual habits) are often initially dominant in patients with FTD.26 Our patient cohort consisted of 9 with the behavioural variety and 17 with the aphasic variety of FTD. The remaining 10 patients were in a more advanced stage and already displayed both types of changes when they came in for consultation. Their medical histories were insufficient to determine which type of symptoms was initially dominant. It is believed that patients with progressive aphasia are conscious of the language problem and are first seen by neurologists. In contrast, many patients whose initial symptoms are behavioural are seen by psychiatrists or general practitioners for extended periods of time before being referred for a neurology consult. It is therefore possible that most of these 10 patients with a long history of the disease and mixed symptoms experienced an onset with behavioural symptoms. If this is true, it is in line with data from the literature, which considers the behavioural variety to be more common. Keeping in mind that the sample sizes are small, results showed that heart rates were lower and prevalence of bradycardia was higher in patient groups with behavioural or mixed FTD than in the aphasic FTD group (Table 4). The similar prevalence rates for bradycardia in these 2 subgroups seem to support the hypothesis that patients presenting both types of symptoms first experienced behavioural changes, and that an increased risk of bradycardia accompanies the behavioural variant. As a general rule, language disorders mostly depend on decreased left hemisphere function, and behavioural changes are more common when there are structural changes in the right hemisphere.38 If we accept this statement, the result is congruent with the hypothesis that the right hemisphere contains more neuronal circuits controlling sympathetic activity related to cardiovascular function; damage to the right hemisphere (atrophy, in this case) will elicit bradycardia.
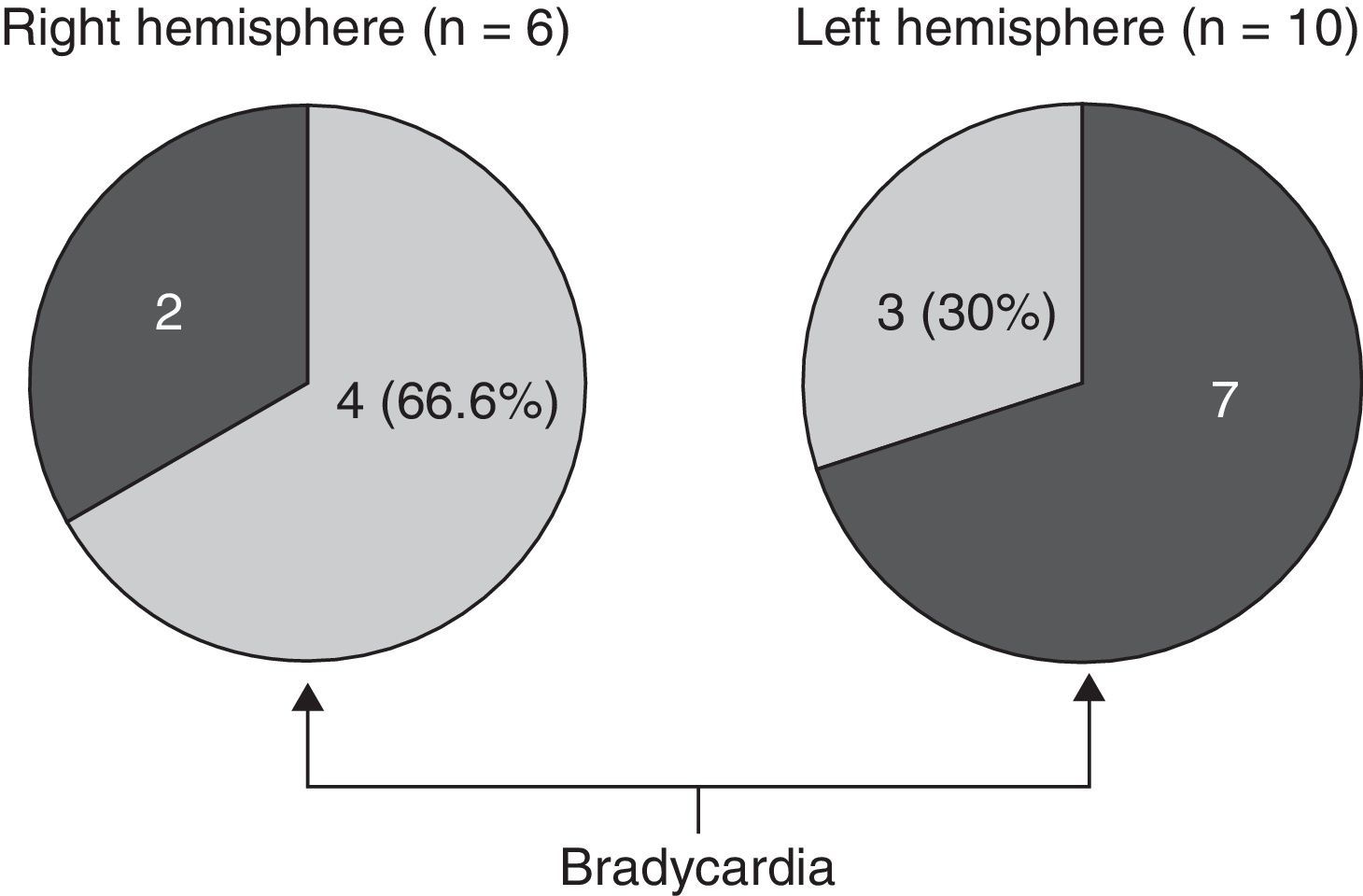
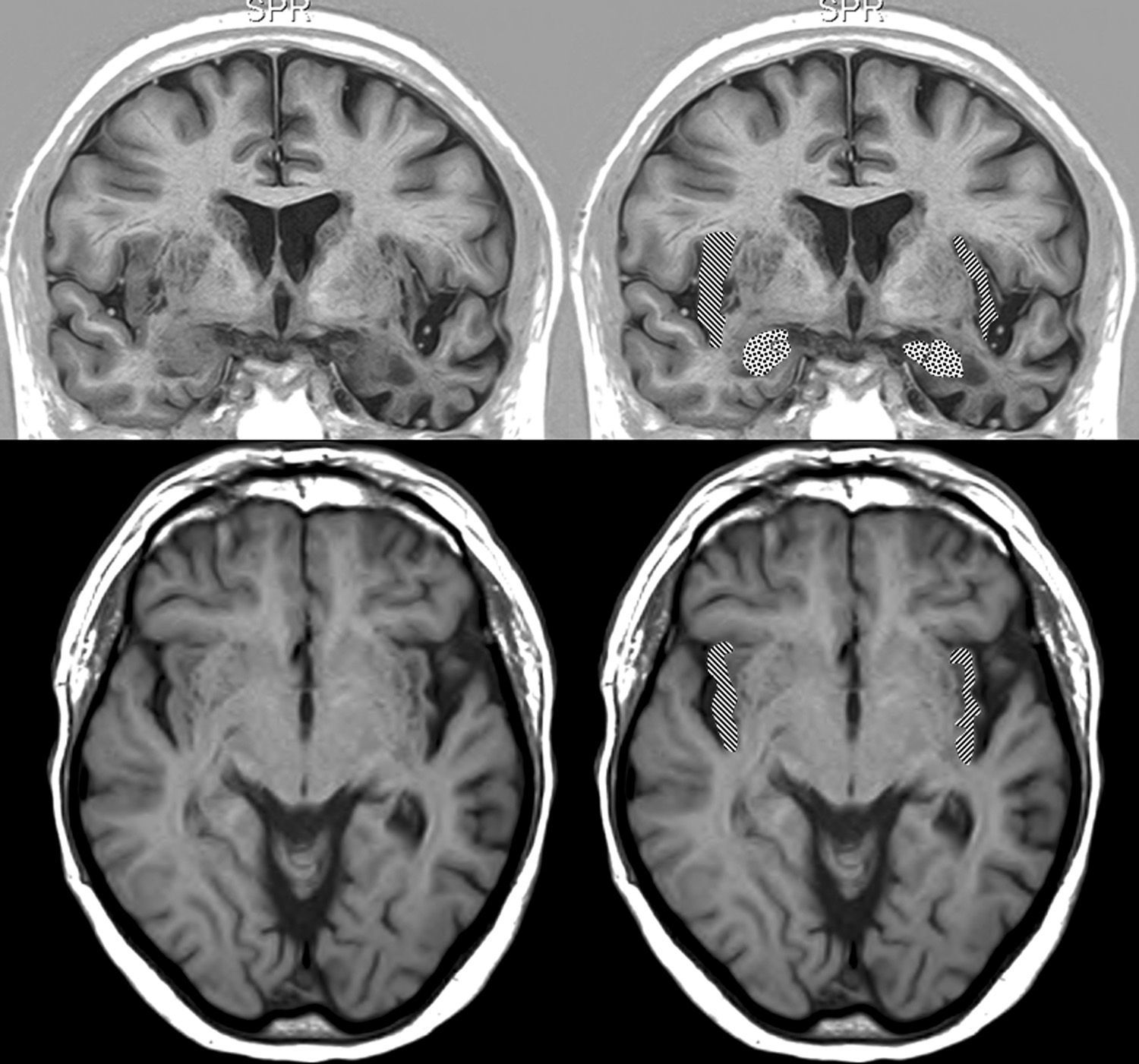
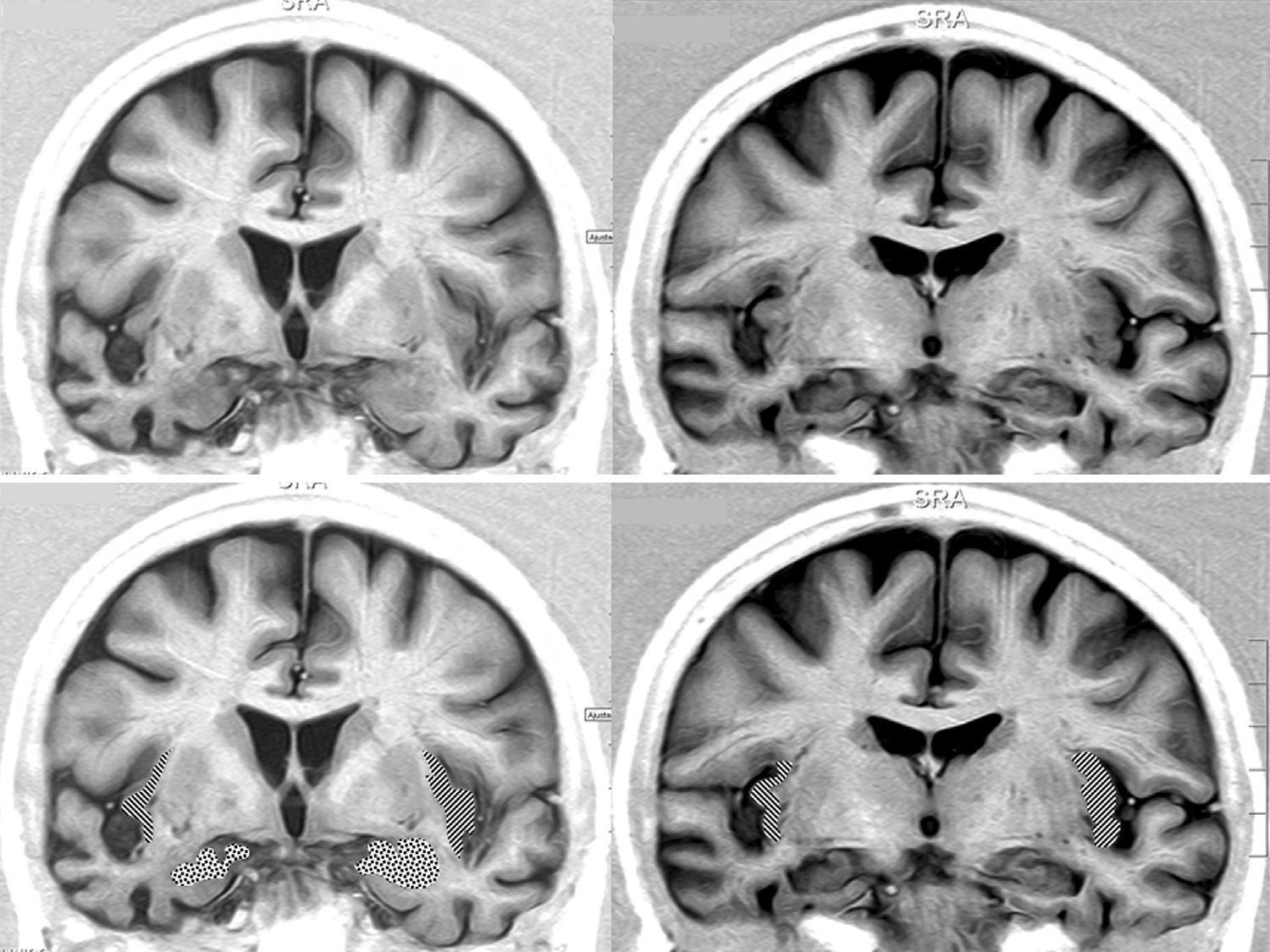
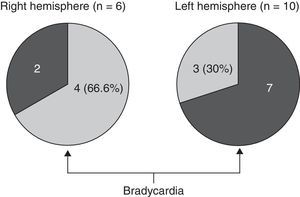
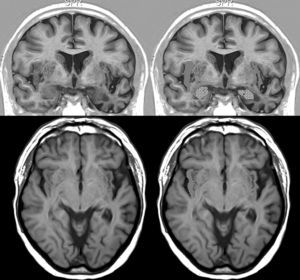
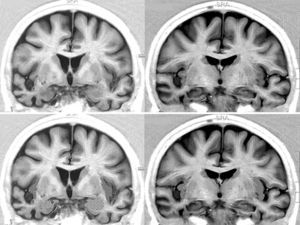
We opted to consider lesion topography only in those patients with FTD. Patients without dementia exhibit lesions that may exert a destructive or inhibitory effect on function, while other lesions cause irritation and may therefore stimulate activity in that area. On the other hand, various types of degenerative dementia have different effects on subcortical and brainstem nuclei related to the adrenergic and cholinergic systems intervening in the autonomic nervous system. These effects are generally not seen in neuroimaging studies. The sample size decreases when only those patients with FTD are selected, but so does the heterogeneity that may skew the value of the result. Additionally, we should be mindful that FTD is a degenerative disease that affects both hemispheres of the brain, although unilateral impairment may be dominant in early stages of the disease. This study found no significant associations between presence of bradycardia and the lobe of the brain with structural changes. Nevertheless, we did observe lower heart rate and more frequent bradycardia in patients with damage to the right hemisphere, while the opposite was true in patients with left hemisphere changes (Tables 5a and 5b). If we select patients with FTD in whom atrophy was clearly predominantly unilateral, we observe a higher proportion of patients whose bradycardia is not drug-induced and who exhibit marked right hemisphere atrophy (Fig. 1). These 16 patients were right-handed. The difference was not statistically significant, but this was probably due to the low number of patients in each of the subgroups. These results only allow us to point out the increased tendency towards bradycardia in patients with more marked impairment of the right hemisphere. This is coherent with the theory that this hemisphere contains cortical areas that modulate the sympathetic nervous system, which in turn affects the heart.32,33 In any case, it should be noted that bradycardia may appear in patients with more pronounced impairment of either the right or left hemisphere, and that some studies of patients with ictal epilepsy could not establish any specific laterality associated with the appearance of bradycardia.39,40Figs. 2 and 3 show 2 representative imaging scans from patients in this study with FTD and bradycardia. Atrophy of the temporal lobe and insula demonstrates clear left laterality in Fig. 2 and right laterality in Fig. 1. Both cases seem to exhibit more pronounced atrophy in the internal anteroinferior part of the insular cortex.
Brain MRI in a patient with FTD and bradycardia. Male patient aged 67 with FTD-semantic dementia in an intermediate stage and a heart rate of 56bpm. Marked atrophy of the left temporal lobe. Above: coronal inversion recovery image; below, axial FLAIR sequence. On the right side, the striped area marks the insular cortex and the dotted area marks the amygdala.
Brain MRI in a patient with FTD and bradycardia. Female patient aged 65 with a 3-year history of FTD-behavioural variant. Readings of 39 and 51bpm in 2 consecutive consultations. Coronal inversion recovery sequences. Note the marked atrophy of the right temporal lobe. In the lower section, the dotted area marks the amygdala and the striped area marks the insular cortex.
If future studies were to confirm a higher tendency towards bradycardia among patients with FTD, we would have a new diagnostic sign in addition to a finding to consider when making treatment decisions. For example, cholinesterase inhibitors might not be indicated for these patients; in 2011, these drugs were advised against by the British Association for Psychopharmacology41 because of their potential bradycardic effect.42 While stressing that samples in this study were small, we should mention that at the time of the first consultation, 7 patients with FTD were taking cholinesterase inhibitors and 3 had bradycardia (42.8%), whereas bradycardia was only present in 28.5% of the other patients in this study (6 of 21).
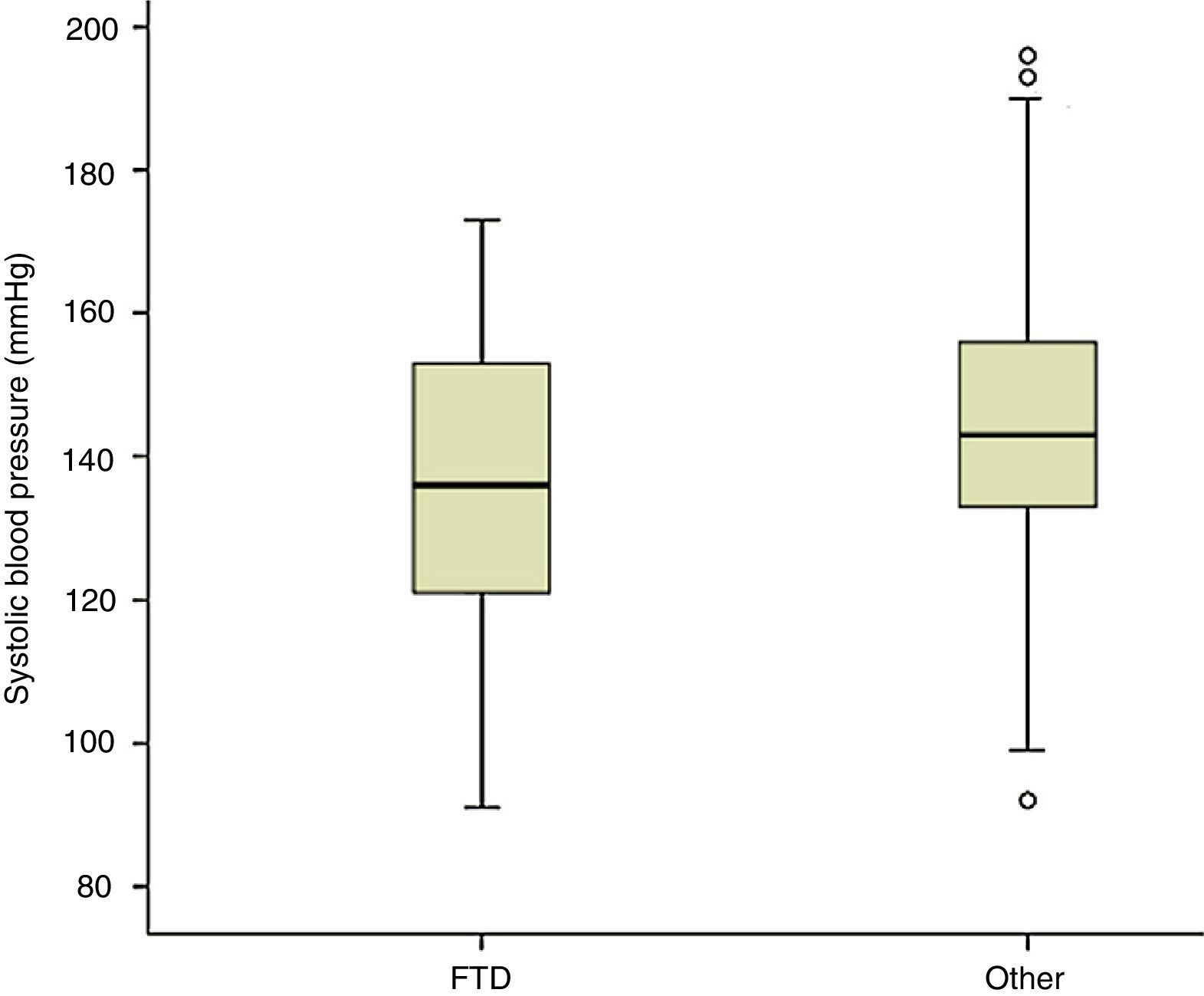
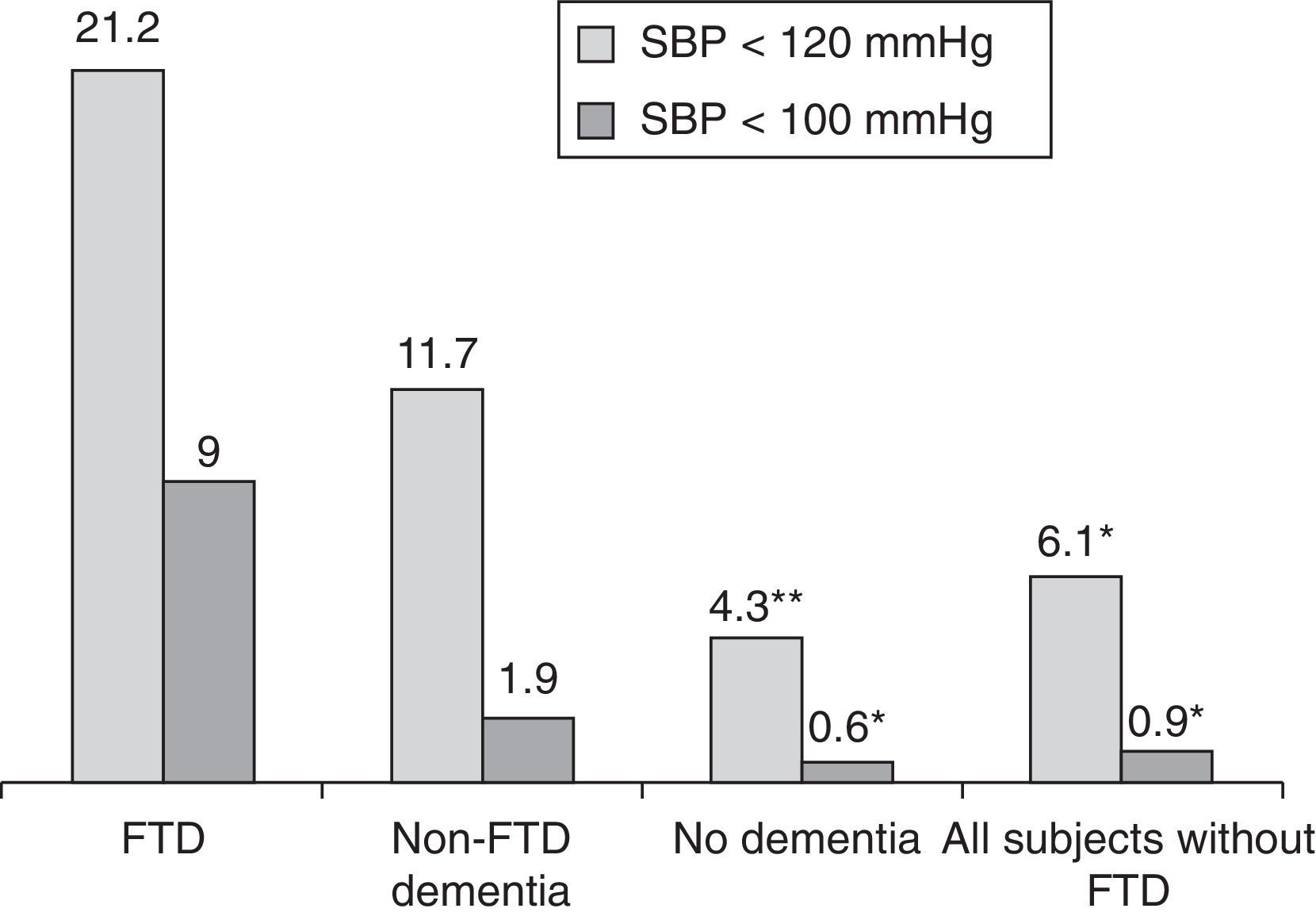
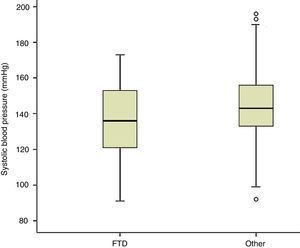
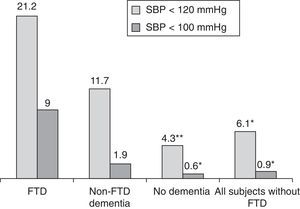
Measuring arterial blood pressure was not one of this study's main objectives. Multiple factors are at work in blood pressure changes, and recording and analysing these factors should be undertaken in a separate study. Nevertheless, results from blood pressure measurements taken along with heart rate show lower systolic readings among patients with FTD than in the rest of the participants (P<.01; Fig. 4). If we initially and arbitrarily define systolic hypotension as a systolic pressure of less than 120mmHg, that category includes 21.2% of the patients in the FTD group, compared to 6.1% of the other subjects (11.7% of patients with other types of dementia and 4.3% of patients without dementia) (Fig. 5). Patients with a systolic reading below 100 account for 9% of the FTD group and 0.9% of the rest of the patients (Fig. 5). These data could justify planning a prospective study to assess whether this change can be extrapolated to all FTD patients. If this were the case, the study would subsequently determine if the difference was due to the same mechanisms suspected of being linked to a higher incidence of bradycardia in this group, that is, if systolic hypotension might be caused by atrophy in the temporal and/or frontal lobe structures active in the autonomic modulation of cardiovascular functions.
In summary, measuring heart rate in patients with cognitive symptoms reveals higher prevalence of bradycardia among patients with FTD. This finding was more common in, but not limited to, patients whose symptoms were predominantly behavioural rather than aphasic. These results issue from the analysis of sporadic observations in small samples, and therefore call for a larger study. If these findings are confirmed, presence of bradycardia may support a suspected diagnosis of FTD in the aetiological differential diagnosis of patients with cognitive symptoms. It would also be a reason for doctors to proceed with caution when prescribing these patients drugs known to cause bradycardia.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Robles Bayón A, Gude Sampedro F, Torregrosa Quesada J.M. Bradicardia en la demencia frontotemporal. Neurología. 2014;29:76–85.