Low histamine metabolism has been suggested to play a role in the pathogenesis of allergy and migraine. We investigated the possible association between 2 single-nucleotide polymorphisms (SNP), C314T HNMT and C2029G DAO, and the presence and severity of migraine and migraine-related disability.
Materials and methodsWe studied the frequency of C314T HNMT and C2029G DAO allelic variants in 162 mothers of children with allergies (80 with migraine and 82 without) using a TaqMan-based qPCR Assay and a case–control model. We conducted a logistic regression analysis to examine the association between migraine and the allelic and haplotype variants.
ResultsMutant C2029G DAO SNP was found significantly more frequently in the group of women with migraine than in controls (OR, 1.6; 95% CI, 1.1–2.1). No significant differences were found in frequencies of genotypes or alleles in the case of C314T HNMT SNP. Both mutated alleles were associated with migraine-related disability. Coexistence of alleles for both SNPs (haplotypes) showed a strong association with migraine. Haplotypes containing both mutated alleles (either heterozygous or homozygous) were very strongly associated with MIDAS grade IV migraine (OR, 45.0; 95% CI, 5.2–358). This suggests that mutant alleles of C314T for histamine N-methyltransferase (HNMT) and C2029G for diamine oxidase (DAO) polymorphisms may interact in a way that increases the risk and impact of migraine.
ConclusionsWe suggest a synergistic association between HNMT and DAO functional polymorphisms and migraine; this hypothesis must be further confirmed by larger studies. However, the characteristics and ethnic differences between analysed populations should be considered when interpreting the results.
Se ha sugerido que una degradación disminuida de histamina puede contribuir en la patogénesis de migraña y alergia. Este trabajo investiga una posible asociación entre 2 polimorfismos de un solo nucleótido (SNP) de 2 enzimas que degradan histamina, C314T para la histamina N-metil-transferasa (HNMT) y C2029G para diaminoxidasa (DAO), con la presencia, discapacidad y severidad de la migraña.
Material y métodosSe reclutó a 162 madres de niños alérgicos (80 con migraña y 82 sin migraña) determinando las variantes alélicas por PRC tiempo real usando un modelo de casos y controles. Mediante regresión logística se determinaron las OR para los genotipos y haplotipos.
ResultadosEl alelo mutado G para DAO fue significativamente más frecuente en el grupo de mujeres migrañosas que en los controles (OR = 1,6; IC del 95% = 1,1-2,1). No encontramos diferencias significativas para el alelo mutado T de la HNMT. Ambos alelos mutados estuvieron asociados a la discapacidad causada por la migraña. La coexistencia de ambas mutaciones (haplotipos) mostró una fuerte asociación con migraña. Los haplotipos que tenían ambos alelos mutados (ya sea como homocigotos o heterocigotos) estuvieron fuertemente asociados a la discapacidad por migraña grado iv (OR = 45,0, IC del 95% = 5,2-358). Esto sugiere que los alelos mutados T para HNMT y G para DAO pueden interactuar incrementando el riesgo y el impacto de la migraña.
ConclusionesSe sugiere una asociación sinérgica de polimorfismos de HNMT y DAO con migraña el cual debe ser confirmado en futuros estudios. La interpretación debe tomar en cuenta las características étnicas de la población estudiada.
Migraine is considered a recurrent neurovascular headache disorder with varying clinical phenotypes.1,2 The most common types of migraine, migraine with and without aura, are characterised by presence of recurrent headache lasting 4 to 72hours, which is exacerbated by physical exercise and associated with nausea, photophobia, phonophobia, and neurological symptoms.3 Prevalence of migraine is estimated at 4% before puberty and increases to 25% in women of childbearing age. The disorder has considerable personal, social, and economic impact.4 The exact causes and physiopathological mechanisms of migraine are still to be determined.2
According to several studies, some rare and common forms of migraine have a genetic basis.5,6 For example, several genes with an involvement in familiar hemiplegic migraine have been identified.5 In contrast, the most prevalent types of migraine (migraine with and without aura) are polygenic and the influence of genetic factors is difficult to determine.7 According to a study by Ducros et al.,5 nearly half of the studied families with familial hemiplegic migraine displayed mutations in the calcium-channel gene CACNA1A, located on chromosome 19, whereas a locus was mapped on chromosome 1 in the other half of the families; the role of these loci in typical migraine is still to be understood. Genetic approaches to migraine with and without aura include linkage analysis and genome-wide association studies.5,6,8 These techniques have identified susceptibility loci for migraine on chromosomes 19, 4, and X.5
Some neurotransmitters have been associated with migraine, the most frequently studied being serotonin and dopamine.9–11 Three genes related to the serotonergic system have been associated with migraine with and without aura: the HTR2B and MAO A receptor genes have been associated with susceptibility for migraine without aura, whereas the dopa-decarboxylase receptor gene has been associated with susceptibility for migraine with aura.10 Furthermore, there is evidence that several single-nucleotide polymorphisms (SNP) in genes of the dopaminergic system play a role in the pathogenesis of migraine. Three SNPs have been found to be associated with migraine with aura: rs2097629 in the dopamine beta-hydroxylase gene, rs7131056 in DRD2, and rs40184 in SLC6A3.11
Histamine is a neurotransmitter and neuromodulator that appears to have a significant involvement in migraine pathogenesis.12,13 Migraine attacks may be triggered by the ingestion of histamine-rich foods14; in fact, headache is a common feature of histaminosis.15 In migraine patients, headache may be histamine-dose-dependent.16,17 Histamine is degraded by 2 enzymes: histamine N-methyltransferase (HNMT; EC 2.1.1.8) and diamine oxidase (DAO) or amiloride-binding protein 1 (ABP1; EC 1.4.3.6). HNMT is involved in the degradation of intracellular histamine18 whereas DAO is secreted and participates in the degradation of extracellular histamine.18,19
The genes coding for HNMT and DAO are polymorphic. The HNMT gene is located at 2q22.1 and has 8 SNPs; only one of these is non-synonymous and is located in exon 4, causing the amino acid substitution Thr105Ile (rs11558538).20 This gene has been associated with decreased HNMT activity.21 Three non-synonymous SNPs have been mapped to the DAO gene, located at 7q34-36. SNP rs1049793 has been found to decrease the protein's activity by altering its form through amino acid substitution His645Asp.22 In light of the above, our study focuses on these 2 SNPs, the HNMT Thr105Ile and DAO His645Asp polymorphisms, which we will refer to as C314T and C2029G, respectively, to reflect nucleotide substitutions.
These SNPs have been studied in the context of certain histamine-related conditions, mainly allergic diseases. An association between asthma and HNMT Thr105Ile was described by Yan et al.,23 although this has not been observed in subsequent studies.24,25 Likewise, no association between that SNP and such other histamine-related conditions as ulcerative colitis26 and gastric ulcer27 has been found. However, more recent studies have reported a positive correlation between this polymorphism and presence of asthma.28,29 Furthermore, no association has been found between the C2029G DAO polymorphism and increased risk of histamine-related conditions; this polymorphism has, however, been linked to severity of ulcerative colitis symptoms26 and clinical manifestations of asthma and allergic rhinitis.30 Only 2 studies have addressed the role of these 2 polymorphisms on migraine pathogenesis, reporting no significant association.31,32 Our study evaluated the association between the HNMT C314T and DAO C2029G SNPs, and presence, severity, and associated disability of migraine in a sample of Mexican mothers of children with allergies.
Material and methodsWe conducted a case-control study and analysed data using a logistic regression model. Our study included 162 unrelated adult women aged 20 to 55 years (mean age, 34.2±7.1 years). They all were mothers of allergic children cared for at the paediatric allergology service of the Secretariat of Health of Torreón, in Coahuila, Mexico, between March 2013 and May 2014. Eighty women met the diagnostic criteria for migraine and not for any other type of headache; these women were included in the case group. Migraine was diagnosed by a specialist with the help of a neurologist, using a validated questionnaire and following the diagnostic criteria of the International Headache Society.3 The control group included 82 women who had not experienced headache for at least one year prior to inclusion in the study.
Cases reported having experienced episodes of pain for a mean of 7.3±4.7 years; 20.9% had a family history of migraine and 58.3% had migraine with aura. The Migraine Disability Assessment (MIDAS) test was used to determine migraine severity and level of disability in the case group.33 None of the cases were receiving preventive treatment for migraine; they only took analgesics during headache episodes. Controls were unrelated healthy Mexican women who did not experience migraine or other type of headache.
The selected women were invited to participate in the study and signed informed consent forms prior to inclusion. Our study complies with the principles of the Declaration of Helsinki. The study was approved by the research ethics committee of the School of Medicine at Universidad Autónoma de Coahuila, Mexico. The women included and their children participated in another genetic association study conducted by our research group to analyse allergies.
DNA was obtained from peripheral blood (5mL) using the “salting-out” procedure.34 Polymorphisms were analysed using real-time polymerase chain reaction (PCR). Genotyping was conducted using TaqMan technology (Applied Biosystems®) to detect the following SNPs: DAO rs1049793 (C_7599774_10), a non-synonymous SNP causing the His664Asp amino acid substitution, and HNMT rs11558538 (C_11650812_20), a non-synonymous SNP causing the Thr105Ile amino acid substitution.
Polymorphisms were analysed with an Applied Biosystems® 7300 Real-Time PCR System using a DNA concentration of 8ng. Cycling conditions were as follows: initial denaturation at 95°C for 10minutes, then 40 cycles at 95°C for 15seconds, and 60°C for 90seconds. Of the 324 samples collected (162 for HNMT and 162 for DAO), 320 were successfully genotyped for the 2 SNPs analysed in this study (160 for HNMT and 160 for DAO).
We estimated the proportion of patients with the HNMT and DAO SNPs in each group. Differences were analysed with the Chi-square test or the Fisher exact test, as appropriate. We estimated the magnitude of the association between migraine and the HNMT and DAO allelic variants using odds ratios (OR) with 95% confidence intervals (95% CI).
To evaluate whether the HNMT and DAO SNPs had a synergistic effect on migraine severity or disability, subjects were classified as SNP carriers or non-carriers, considering several haplotypes. We conducted a logistic regression analysis to assess whether these haplotypes were correlated with migraine severity and disability. Associations were expressed using OR with 95% CI. Two-tailed P-values ≤ .05 were considered statistically significant. Statistical analysis was performed using STATA 11.1® statistical software. Hardy-Weinberg equilibrium was tested using Arlequin software v2000 (CMPG Zoological Institute, University of Bern, Bern, Switzerland).
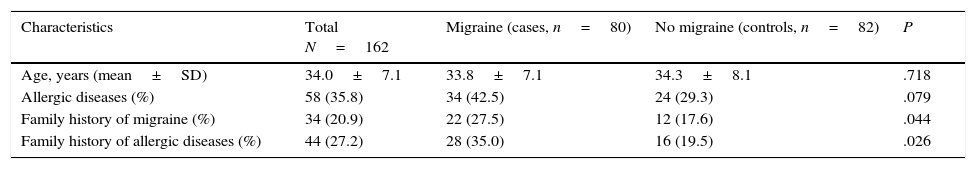
ResultsA total of 162 mothers of allergic children were included in our study. Mean age was 34±7.1 years. Eighty had migraine according to the diagnostic criteria of the International Headache Society and 82 were controls. Table 1 summarises the main clinical characteristics of our sample. Presence of a family history of allergies and migraine was significantly more frequent in cases than in controls (P<.05).
Population characteristics.
| Characteristics | Total N=162 | Migraine (cases, n=80) | No migraine (controls, n=82) | P |
|---|---|---|---|---|
| Age, years (mean±SD) | 34.0±7.1 | 33.8±7.1 | 34.3±8.1 | .718 |
| Allergic diseases (%) | 58 (35.8) | 34 (42.5) | 24 (29.3) | .079 |
| Family history of migraine (%) | 34 (20.9) | 22 (27.5) | 12 (17.6) | .044 |
| Family history of allergic diseases (%) | 44 (27.2) | 28 (35.0) | 16 (19.5) | .026 |
All participants were mothers of allergic children. A family history of allergic diseases and migraine was reported by participants themselves in all cases. All participants were consecutively selected and unrelated. Data were compared using the Chi-square test.
In the control group, the genotype distribution of the SNPs studied followed the Hardy-Weinberg equilibrium. In the case group, the genotype distribution of the HNMT SNP also followed the Hardy-Weinberg equilibrium, while that of the DAO polymorphism did not. A significant difference was observed in the genotype distribution of this SNP between mothers with migraine and a healthy Hispanic population (P=.001).35
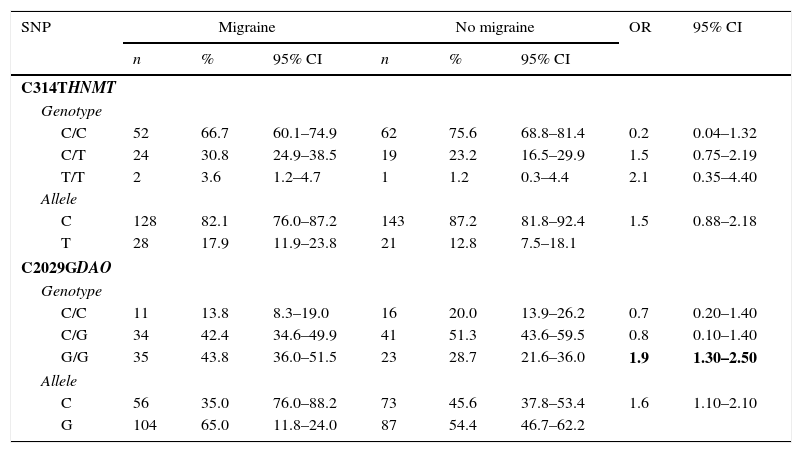
Table 2 shows genotype distributions and allele frequencies for HNMT and DAO in both groups. According to the comparison of HNMT allele frequencies, the mutant T allele of the C314T polymorphism was not significantly more frequent in cases than in controls (P=.189) (Table 2).
Genotype and allele frequency distribution for the C314T HNMT and C2029G DAO SNPs in cases and controls.
| SNP | Migraine | No migraine | OR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | |||
| C314THNMT | ||||||||
| Genotype | ||||||||
| C/C | 52 | 66.7 | 60.1–74.9 | 62 | 75.6 | 68.8–81.4 | 0.2 | 0.04–1.32 |
| C/T | 24 | 30.8 | 24.9–38.5 | 19 | 23.2 | 16.5–29.9 | 1.5 | 0.75–2.19 |
| T/T | 2 | 3.6 | 1.2–4.7 | 1 | 1.2 | 0.3–4.4 | 2.1 | 0.35–4.40 |
| Allele | ||||||||
| C | 128 | 82.1 | 76.0–87.2 | 143 | 87.2 | 81.8–92.4 | 1.5 | 0.88–2.18 |
| T | 28 | 17.9 | 11.9–23.8 | 21 | 12.8 | 7.5–18.1 | ||
| C2029GDAO | ||||||||
| Genotype | ||||||||
| C/C | 11 | 13.8 | 8.3–19.0 | 16 | 20.0 | 13.9–26.2 | 0.7 | 0.20–1.40 |
| C/G | 34 | 42.4 | 34.6–49.9 | 41 | 51.3 | 43.6–59.5 | 0.8 | 0.10–1.40 |
| G/G | 35 | 43.8 | 36.0–51.5 | 23 | 28.7 | 21.6–36.0 | 1.9 | 1.30–2.50 |
| Allele | ||||||||
| C | 56 | 35.0 | 76.0–88.2 | 73 | 45.6 | 37.8–53.4 | 1.6 | 1.10–2.10 |
| G | 104 | 65.0 | 11.8–24.0 | 87 | 54.4 | 46.7–62.2 | ||
OR values were obtained using logistic regression. Statistically significant values are shown in bold.
However, the mutant G allele of the C2029G DAO polymorphism was significantly more frequent in patients with migraine (P=.044). The OR of migraine for carriers of the mutant G allele of DAO compared to wild-type homozygous women was 1.6 (95% CI, 1.1–2.1). Likewise, G/G homozygous women were at greater risk of migraine than wild-type homozygous women (OR, 1.9; 95% CI, 1.3–2.5) (Table 2).
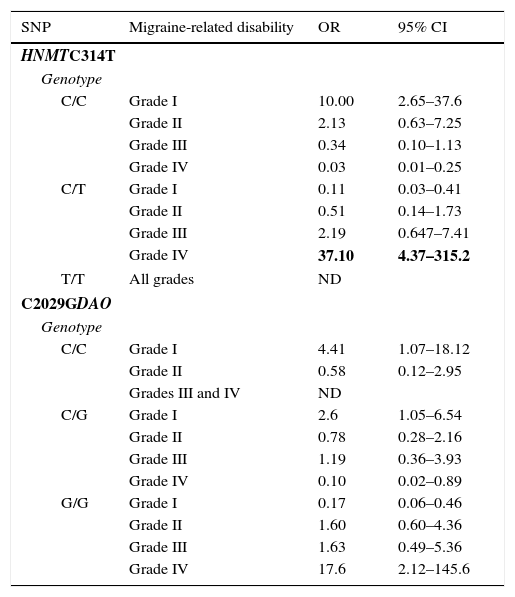
The women were classified as carriers and non-carriers of each of the mutations (whether homozygous or heterozygous) to gather data on the effects of each polymorphism on the phenotypes and clinical characteristics of the study population. The HNMT and DAO polymorphisms showed a similar distribution when the population was subdivided according to presence of aura and mean duration of migraine attacks. Both polymorphisms were positively correlated with high MIDAS scores. Presence of the C allele for the HNMT polymorphism was associated with a lower degree of disability due to migraine (OR, 10.0; 95% CI, 2.65–37.6). In contrast, presence of the T allele (in heterozygous individuals) was associated with higher degree of disability (OR, 37.1; 95% CI, 4.37–315.2) (Table 3). The small number of homozygous mutant individuals prevented us from analysing the frequency of migraine-related disability in this subset.
Association between migraine-related disability and HNMT C314T and DAO C2029G SNP genotypes.
| SNP | Migraine-related disability | OR | 95% CI |
|---|---|---|---|
| HNMTC314T | |||
| Genotype | |||
| C/C | Grade I | 10.00 | 2.65–37.6 |
| Grade II | 2.13 | 0.63–7.25 | |
| Grade III | 0.34 | 0.10–1.13 | |
| Grade IV | 0.03 | 0.01–0.25 | |
| C/T | Grade I | 0.11 | 0.03–0.41 |
| Grade II | 0.51 | 0.14–1.73 | |
| Grade III | 2.19 | 0.647–7.41 | |
| Grade IV | 37.10 | 4.37–315.2 | |
| T/T | All grades | ND | |
| C2029GDAO | |||
| Genotype | |||
| C/C | Grade I | 4.41 | 1.07–18.12 |
| Grade II | 0.58 | 0.12–2.95 | |
| Grades III and IV | ND | ||
| C/G | Grade I | 2.6 | 1.05–6.54 |
| Grade II | 0.78 | 0.28–2.16 | |
| Grade III | 1.19 | 0.36–3.93 | |
| Grade IV | 0.10 | 0.02–0.89 | |
| G/G | Grade I | 0.17 | 0.06–0.46 |
| Grade II | 1.60 | 0.60–4.36 | |
| Grade III | 1.63 | 0.49–5.36 | |
| Grade IV | 17.6 | 2.12–145.6 | |
OR values were obtained using logistic regression.
ND: not determined/analysed due to the small number of patients in this category.
Presence of the C allele for the DAO polymorphism was associated with a lower level of disability (OR, 4.41; 95% CI, 1.07–18.12). Likewise, homozygous C/C individuals were observed to display a lower level of disability (OR, 2.6; 95% CI, 1.05–6.54). In contrast, presence of the G allele was a risk factor for severe disability. Homozygous G/G individuals were at a greater risk of severe disability (OR, 17.6; 95% CI, 2.12–145.6) (Table 3).
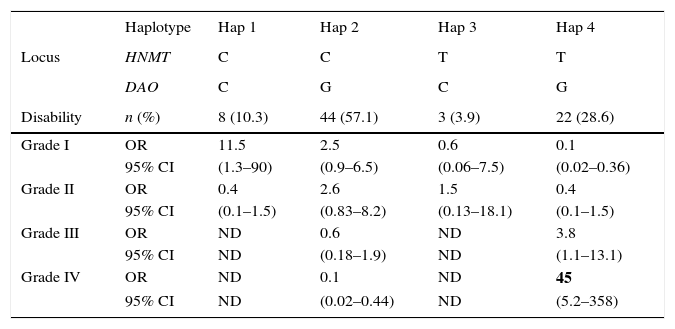
A logistic regression analysis of the haplotypes revealed a stronger association between these polymorphisms and migraine. We considered 4 haplotypes (Table 4). Haplotype 1: wild-type alleles in both loci. Haplotype 2: a single mutant allele in the DAO locus. Haplotype 3: a single mutant allele in the HNMT locus. Haplotype 4: mutant alleles in both loci (whether homozygous or heterozygous). We found significant differences in haplotype frequency between women with and without migraine (P=.038). In women with migraine, we observed a strong association between haplotype 1 and grade I disability (OR, 11.5; 95% CI, 1.3–90) and an even stronger association between haplotype 4 and grade IV disability (OR, 45.0; 95% CI, 5.2–558). We could not estimate the OR for grade III and IV disability given haplotypes 1 and 3 since there were no individuals in those categories (Table 4).
Association between haplotypes and migraine-related disability.
| Haplotype | Hap 1 | Hap 2 | Hap 3 | Hap 4 | |
|---|---|---|---|---|---|
| Locus | HNMT | C | C | T | T |
| DAO | C | G | C | G | |
| Disability | n (%) | 8 (10.3) | 44 (57.1) | 3 (3.9) | 22 (28.6) |
| Grade I | OR | 11.5 | 2.5 | 0.6 | 0.1 |
| 95% CI | (1.3–90) | (0.9–6.5) | (0.06–7.5) | (0.02–0.36) | |
| Grade II | OR | 0.4 | 2.6 | 1.5 | 0.4 |
| 95% CI | (0.1–1.5) | (0.83–8.2) | (0.13–18.1) | (0.1–1.5) | |
| Grade III | OR | ND | 0.6 | ND | 3.8 |
| 95% CI | ND | (0.18–1.9) | ND | (1.1–13.1) | |
| Grade IV | OR | ND | 0.1 | ND | 45 |
| 95% CI | ND | (0.02–0.44) | ND | (5.2–358) |
For HNMT, C is the wild-type allele and T is the mutant allele. For DAO, C is the wild-type allele and G is the mutant allele. Haplotype 1 has only wild-type alleles in both loci, haplotype 2 has a mutant allele in the DAO locus, haplotype 3 has a mutant allele in the HNMT locus, and haplotype 4 has mutant alleles in both loci (whether homozygous or heterozygous). Migraine-related disability was calculated using the MIDAS test. OR values were obtained using logistic regression.
ND: not determined (no participants were included in these categories).
Our study included a sample of mothers of allergic children. The prevalence of allergic diseases in these women (35.8%) was higher than in the general population, suggesting a genetic component to these conditions. The frequency of migraine was higher in our sample (49.4%) than in other female populations,4 which points to an association between allergic diseases and migraine.36,37 In fact, we found a greater prevalence of allergic diseases in women with migraine (42.9%) than in those without (29.3%) (Table 1). Significant differences were also observed in presence of family history of allergic diseases between women with and without migraine (35% vs 19.5%; P=.026), which suggests that allergic diseases have a genetic basis and are associated with migraine, as previous studies have pointed out.1,38
It has been suggested that histamine plays a role in migraine pathogenesis, given that the condition is more frequent in patients with allergic diseases36,37 and plasma histamine levels are significantly higher in patients with migraine.39,40 Several studies have reported an increase in spontaneous histamine release from leukocytes in patients with migraine compared to controls39,41; furthermore, experimental models have suggested that dural mast cell activation may play a role in migraine pathogenesis.42
Our sample displayed an extremely high prevalence of the mutant G allele of the C2029G DAO polymorphism (59.4%). However, this allele has also been found to be very frequent in the Hispanic population (43.2%). The higher prevalence of the G allele observed in our study may be due to a selection bias, given that all the women included were mothers of allergic children and may therefore be carriers of an allele also linked to allergic disease. Furthermore, we found a significant difference in prevalence of the G allele between women with and without allergic diseases (data not shown). Genotype distribution of the DAO polymorphism was in Hardy-Weinberg equilibrium in women without migraine; however, a deviation from Hardy-Weinberg equilibrium was observed in the subset of women with migraine, which suggests that the 2 populations are different.
The frequency of the mutant T allele in the HNMT locus was 15.3% in our sample, compared to 10.4% in the healthy Hispanic population.43 The genotype distribution of this polymorphism was in Hardy-Weinberg equilibrium in the groups with and without migraine. In addition to this, no significant differences in genotype distribution were found between patients and controls; however, the small size of our sample and the low frequency of the mutant allele may have contributed to these findings. Our results did not change after adjusting for age, disease duration, presence of aura, or consumption of histamine-rich foods. However, the logistic regression analysis revealed a positive correlation between presence of the mutant T allele and presence of migraine-related disability. The homozygous wild-type C/C genotype was associated with grade I migraine-related disability (OR, 10.0; 95% CI, 2.65–37.6) and was found to have a powerful protective effect against grade IV disability (OR, 0.03; 95% CI, 0.01–0.25). Likewise, presence of the T allele in heterozygous women was a risk factor for grade IV disability (OR, 37.1; 95% CI, 4.37–315.2).
Regarding the DAO polymorphism, the frequency of the mutant G allele was significantly higher in women with migraine than in controls (65% vs 54.4%; P=.044) (Table 2). According to the logistic regression analysis, the C/C genotype was associated with grade I disability (OR, 4.4; 95% CI, 1.07–18.12); G/G homozygous individuals were at greater risk of grade IV disability (OR, 17.6; P=.008). The association between these SNPs and symptom severity has previously been reported for other histamine-related conditions.26,30 Severity of migraine, rated on a 0–10 scale on MIDAS question B, was moderately correlated (r=0.426) with migraine-related disability (first 5 MIDAS items); however, no association was found between alleles/genotypes and severity of migraine. Menon et al.44 recently observed an association between the A118G polymorphism of the μ-opioid receptor gene and severity of migraine using MIDAS question B exclusively; however, our study only found an association between the SNPs studied and the grade of migraine-related disability.
Co-presence of alleles for both SNPs (haplotypes) showed a strong association with presence of migraine. Haplotype distribution was significantly different in women with and without migraine. Haplotype 1 (wild-type alleles in both loci) was strongly associated with grade I disability (OR, 11.5; 95% CI, 1.3–90), whereas haplotype 4 (mutant alleles in both loci) was strongly associated with grade IV disability (OR, 45.0; 95% CI, 5.2–358). The above suggests that mutant C314T HNMT and C2029G DAO alleles may interact, increasing patients’ degree of migraine-related disability.
The main limitation of our study was its small sample size. We feel, however, that there is sufficient evidence to support the hypothesis of a synergistic effect of both SNPs on migraine, at least in mothers of allergic children, given that type II errors are much more likely to occur than type I errors in studies with small sample sizes. Likewise, our results should be interpreted with caution due to the possibility of selection bias. However, this type of bias could also result in a greater frequency of the risk alleles in our control group; despite this consideration, our study revealed significant differences in genotype distribution between cases and controls.
In conclusion, we present a synergistic association between the C314T HNMT and the C2029G DAO polymorphisms and the presence of migraine and migraine-related disability; further studies with greater sample sizes are necessary to confirm this association. The characteristics of the populations studied and the ethnic differences between them should be considered when analysing the results. Furthermore, interactions with other HNMT and DAO SNPs and other SNPs affecting histamine metabolism (histidine decarboxylase, histamine receptors, etc.) may have an impact on migraine, as recently seen in a study reporting an association between the PTX3 gene rs3816527 polymorphism with susceptibility to migraine in male patients.45
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Meza-Velázquez R, López-Márquez F, Espinosa-Padilla S, Rivera-Guillen M, Ávila-Hernández J, Rosales-González M. Asociación de polimorfismos de diaminoxidasa e histamina N metiltransferasa con la presencia, discapacidad y severidad de migraña en un grupo de madres mexicanas de niños alérgicos. Neurología. 2017;32:500–507.