Multiple sclerosis (MS) is a chronic, demyelinating, autoimmune disease of the central nervous system causing neuroinflammation. Experimental autoimmune encephalitis (EAE) is a model of the disease. MS is classically treated with interferon beta (IFN-β) and glatiramer acetate (GA). Melatonin (MLT) has been reported to modulate immune system responses. The aim of the present study is to analyse the effects of MLT administration in comparison with the first-line treatments for MS (IFN-β and GA).
MethodsEAE was induced in male Sprague-Dawley rats; the animals subsequently received either IFN-β, GA, or MLT. Cerebrospinal fluid (CSF) samples were analysed by multiplex assay to determine the levels of proinflammatory cytokines. The neurological evaluation of EAE was also recorded.
ResultsAll immunised animals developed EAE. We evaluated the first relapse-remission cycle, observing that IFN-β and GA had better results than MLT in the clinical evaluation. Neither EAE nor any of the treatments administered modified CSF IL-1β and IL-12p70 concentrations. However, IFN-β and MLT did decrease CSF TNF-α concentrations.
ConclusionFurther studies are needed to evaluate the molecular mechanisms involved in the behaviour of MLT in EAE, and to quantify other cytokines in different biological media in order for MLT to be considered an anti-inflammatory agent capable of regulating MS.
La esclerosis múltiple (EM) es una enfermedad crónica desmielinizante autoinmune del sistema nervioso central (SNC) que produce neuroinflamación, un modelo es la encefalitis autoinmune experimental (EAE). La EM ha sido tratada con interferón beta (IFN-beta) y acetato de glatiramero (AG). Se ha descrito que la melatonina (MLT) modula la respuesta del sistema inmune. El objetivo de este estudio fue observar el efecto de la administración de melatonina contra los tratamientos de primera línea utilizados en la EM (IFN-β y AG).
MétodosA ratas macho Sprague Dawley se les indujo EAE y se administraron IFN-β, AG o MLT. Se colectó líquido cefalorraquídeo y se midieron citocinas proinflamatorias por multiplex, además del registro de la evaluación neurológica de la EAE.
ResultadosTodos los animales inmunizados establecieron la EAE. Se evaluó el primer ciclo de recaída-remisión, observando que IFN-β y AG tienen mejores resultados que MLT en la evaluación clínica. La concentración en el LCR tanto de IL-1β e IL-12p70 no se vio modificada por el modelo o por los tratamientos administrados. EL TNF-α se vio disminuido en el LCR por el IFN-β y MLT bajo el modelo de MS.
ConclusionesEs necesario realizar estudios posteriores para evaluar los mecanismos moleculares involucrados en el comportamiento de la MLT en la EAE, así como la cuantificación de otras citocinas en diferentes matrices biológicas para poder considerar a la MLT como un agente antiinflamatorio regulador de la EM.
Multiple sclerosis (MS) is a chronic, demyelinating, autoimmune disease of the central nervous system (CNS), characterised by demyelination, axonal damage, and neuronal loss.1–4 MS represents the main cause of neurobiological disability in young adults and has psychological, financial, social, and health consequences.3 Prevalence of MS in Mexico amounts to 15 cases per 100 000 population, with women more frequently affected.5
Various studies establish experimental autoimmune encephalomyelitis (EAE) as an adequate model for studying numerous aspects of MS. EAE is an experimentally induced autoimmune disorder of the CNS,6 characterised by disruption of the blood-brain barrier (BBB), perivascular infiltration of lymphocytes into the CNS, microglial activation, and lower limb paralysis.6–8 Proinflammatory molecules secreted by infiltrated macrophages, T cells, and activated glial cells interact with the CNS to determine the inflammatory process that leads to myelin and oligodendrocyte destruction.6 The clinical course of EAE is characterised by weight loss, ascending paralysis, and spontaneous recovery.8
First-line treatments for MS include interferon beta (IFN-®) and glatiramer acetate (GA).9 IFN-® is a cytokine with antiviral activity and strong anti-inflammatory effects.10,11 It has been shown to be effective for treating relapsing-remitting multiple sclerosis (RRMS), reducing relapse frequency, severity of the disease, and the development of brain lesions, as well as delaying disease progression.12 GA is a random synthetic copolymer based on the composition of myelin basic protein,13–15 which reduces the rate of RRMS relapses16; its action mechanism is not yet understood.17
Melatonin (MLT) or N-acetyl-5-methoxytryptamine is a neuromodulator synthesized in the pineal gland. MLT receptors are expressed on the membranes of CD4 + T cells, CD8 + T cells, B cells, and monocytes.18,19 Molecular mechanisms responsible for the pleiotropic effects of MLT involve binding to high-affinity G-protein-coupled receptors at the membrane level, and/or interaction with intracellular targets that modulate signal transduction pathways, redox-modulated processes, or free radical scavenging.20
MLT has been associated with amelioration of autoimmune diseases, including EAE8; use of the protein may therefore provide an alternative solution in MS treatment, preventing the pathophysiological development of MS and its model, EAE. No study has compared the clinical courses associated with immunomodulatory therapies (IFN-® and GA) for MS and with MLT in the EAE model. Therefore, we aimed to analyse the effect of administering MLT as compared with first-line treatments for MS (IFN-® and GA) in the development of EAE.
Material and methodsAll experiments were conducted at the Western Biomedical Research Centre of the Mexican Institute of Social Security.
AnimalsWe used a sample of 128 adult male Sprague-Dawley rats of 60 days postnatal age (Harlam Laboratories SA de CV [Mexico]), which were kept under standard vivarium conditions, with a 12-h light/dark cycle and ad libitum access to food and water.
Experimental procedures and care of the experimental animals observed the official Mexican guidelines (NOM-062-ZOO-1999). The research project was approved by the local research and healthcare ethics committee of the Mexican Institute of Social Security (registry number 2013-1305-3).
Induction of experimental autoimmune encephalitisEAE was induced by administering 150 µL of a homogenate containing 25 µL of pig spinal cord homogenate, 25 µL of pig brain homogenate, and 100 µL of Freund’s Complete Adjuvant (Sigma & Aldrich). Homogenate was administered subcutaneously in one hind leg. The tissues used in the pig spinal cord and brain homogenates were obtained from healthy animals that had undergone no previous intervention. The solution was homogenised in phosphate buffer (100 mM of sodium phosphate dibasic [JT Baker], 50 mM of sucrose [JT Baker], and 0.5 mM of EDTA [JT Baker]; pH 7.4), as follows: 1 g of brain tissue in 1 mL of phosphate buffer was placed in a homogeniser until tissue fully disintegrated; the mixture was centrifuged at 1000g for 15 minutes; the supernatant was recovered and centrifuged again at 14 000 rpm for 20 minutes, and the supernatant was recovered again and frozen until needed. This recovered supernatant is referred to as brain homogenate. For the pig spinal cord homogenate, frozen tissue was weighed and homogenised with phosphate buffer at a ratio of 1:10 (tissue:buffer); it was homogenised until the tissue completely disintegrated, and later centrifuged at 1000g for 5 minutes, the supernatant was recovered and frozen.
Neurological assessment of experimental autoimmune encephalitisDisease severity was assessed from day 0 to day 12 after EAE induction using the clinical scale described by Fang et al.,7 which establishes that grade 0 = normal rat, grade 1 = partial loss of tail tonicity, grade 2 = loss of tail tonicity, grade 3 = unsteady gait and mild paralysis, grade 4 = lower limb paralysis, and grade 5 = moribund or death. The EAE model is considered successful when the score exceeds 2.
Experimental groups and treatmentAnimals were distributed into 8 groups of 16 animals each, as follows: 1) control group, with no treatment or manipulation; 2) EAE group, with EAE and no treatment; 3) IFN-® group, without EAE and treated with IFN-® 1b (dosed at 8000 U, every third day, intramuscularly); 4) GA group, without EAE and treated with GA (dosed at 0.50 mg/kg/day, subcutaneously); 5) MLT group, without EAE and treated with MLT (dosed at 10 mg/kg/day, intraperitoneally); 6) EAE–IFN-® group, with EAE and treated with IFN-® 1b (dosed at 8000 U every third day, intramuscularly); 7) EAE-GA group, with EAE and treated with GA (dosed at 0.50 mg/kg/day, subcutaneously); and 8) EAE-MLT group, with EAE and treated with MLT (dosed at 10 mg/kg/day, intraperitoneally).
IFN-® 1b (Uri®eta®) was reconstituted with 0.9% saline solution. GA (Copaxone®) was directly administered in its commercial format. MLT (Sigma) was dissolved in absolute alcohol in absence of light, and later added to 0.9% saline solution. All treatments were administered from day 0 to day 12 after induction.
Cerebrospinal fluid collectionCerebrospinal fluid was collected at day 12 after induction. Rats were anaesthetised by administering 50 µg/kg body weight of dexmedetomidine and 80 mg/kg body weight of ketamine, both intramuscularly. Once rats were anaesthetised, the upper part of the back of the neck was shaved. Animals were then immobilised in a stereotactic apparatus, with the head fixed at 90° ventrally, separating the upper cervical vertebrae. Iodine solution was used for asepsis of the area. Subsequently, 100 µL of articane-epinephrine (1:100 000) was administered subcutaneously at the puncture site.
We marked a 22 G needle at 1 cm from the tip of the bevel to serve as visual reference. We located the occipital crest and the cranial part of the atlas; at the mid-point between these structures, the 22 G needle was inserted to a depth of 3 or 4 mm with the bevel angled 30° above the horizontal plane; the angle was subsequently corrected to 0° and the needle was inserted a further 3 or 4 mm. CSF began flowing due to positive intracranial pressure.
A 1 mL syringe with a 25 G needle was used to aspirate the CSF contained in the cone of the needle used for the puncture, which remained in place until all the CSF that had flown from the cisterna magna had been aspirated. The CSF recovered (50 µL) was transferred to an Eppendorf tube immediately after extraction, and frozen at −80 °C.
Cytokine quantificationTo determine CSF IL-1®, IL-12p70, and TNF-〈 levels, we used the Milliplex map kit (cat. No. RECYTMAG-65 K; Merck, USA) according to the supplier’s instructions. We added the assay buffer to every well of the plate and subsequently decanted it. We then added the standard solution, control, samples, and beads to the corresponding wells and allowed them to incubate for 2 hours at room temperature with agitation; plates were subsequently washed and the detection antibody and streptavidin-phycoerythrin added. Finally, the content was agitated, the plate washed, and the reading fluid added. We used a Luminex 200 device (Texas, USA), recording 50 events per bead. The concentration of each cytokine in CSF was determined from standard curves prepared in the plate. Data were acquired with the xPONENT program (Luminex, USA) and analysed using the Milliplex Analyst 5.1 software; data were reported as pg/mL.
Statistical analysisThe statistical analysis was performed with the IBM® SPSS® Statistics (version 21; USA) and GraphPad Prism (version 5.0; USA) software. Data are expressed as mean ± standard error of the mean. Inter-group comparisons were analysed using the Kruskal-Wallis and Mann-Whitney U tests. P values ≤ .05 were considered statistically significant.
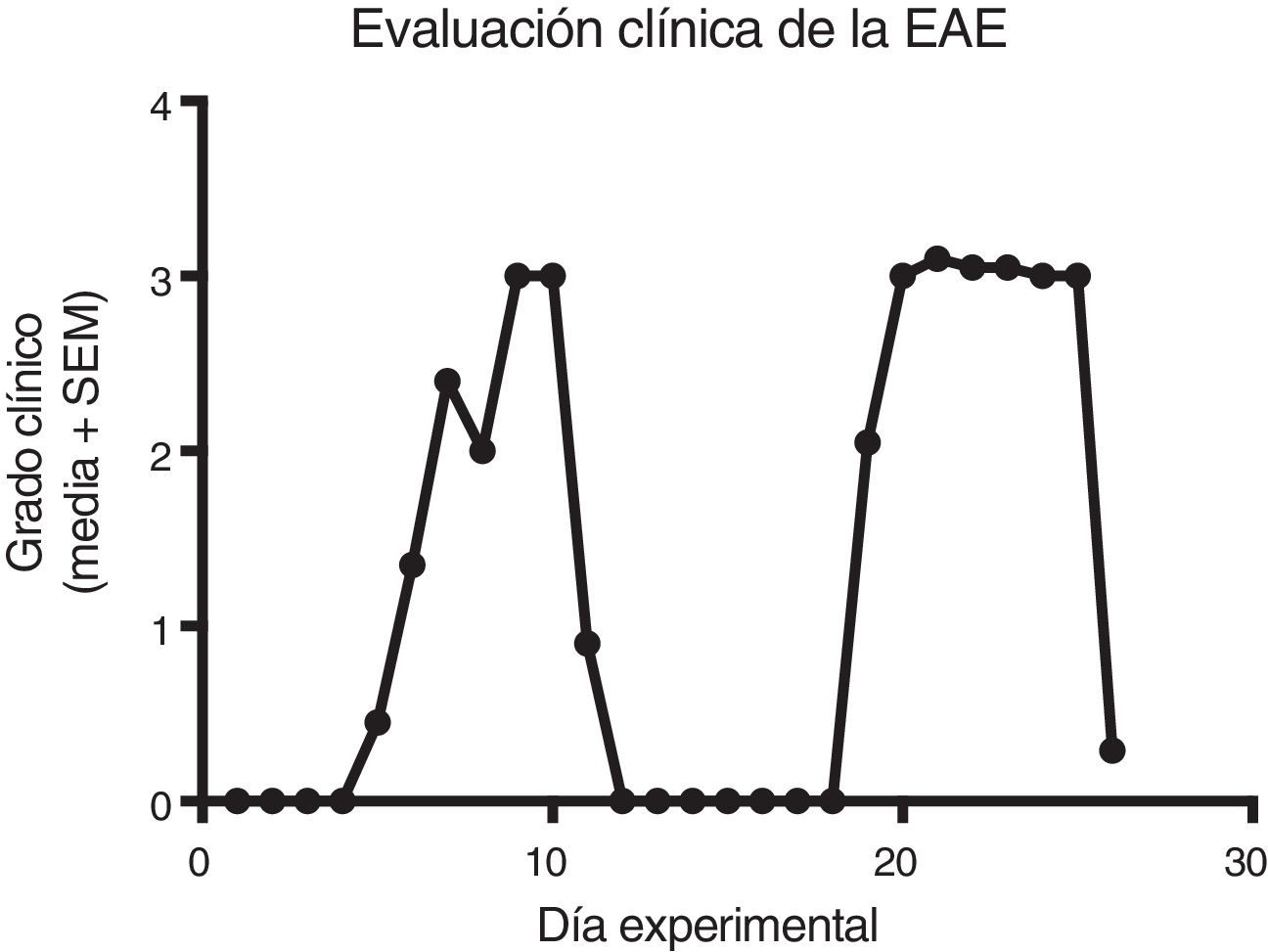
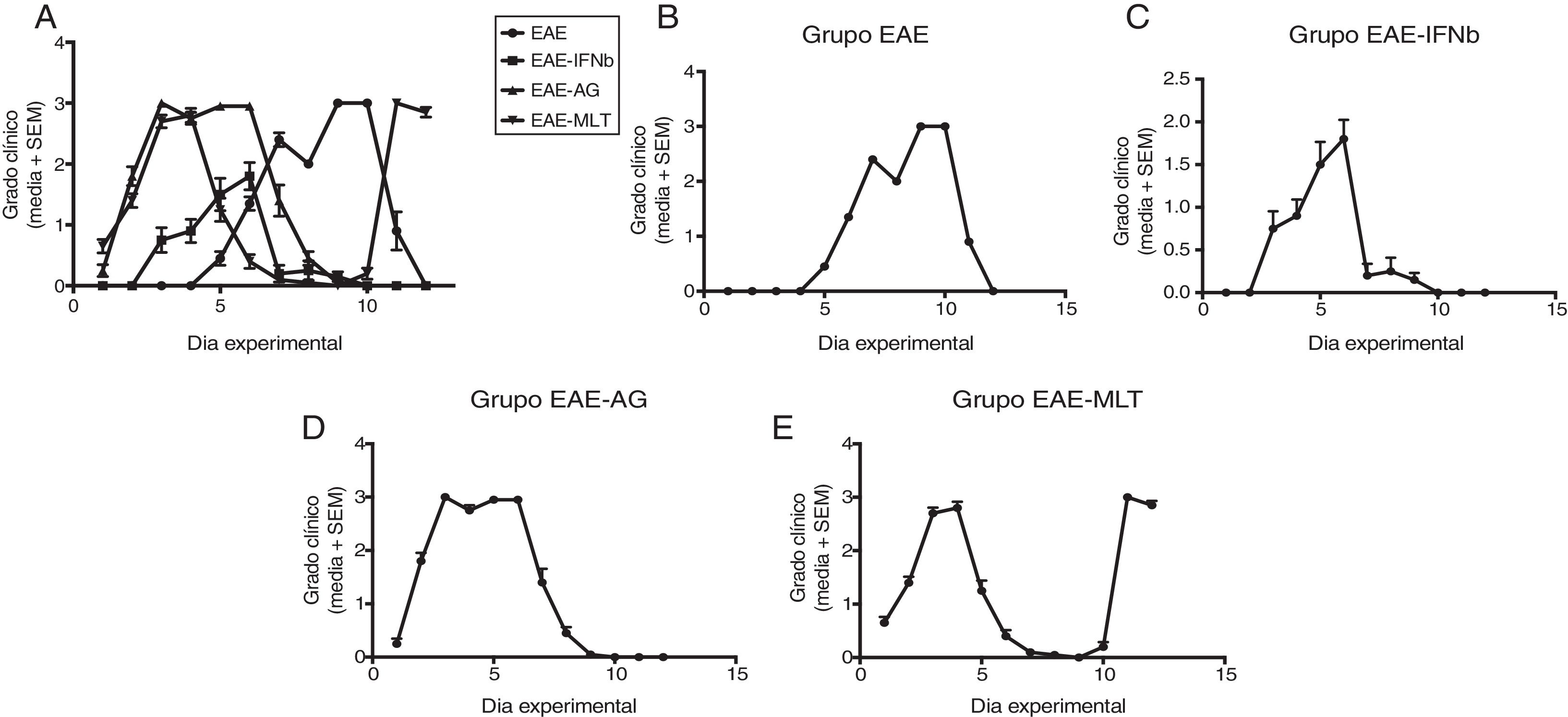
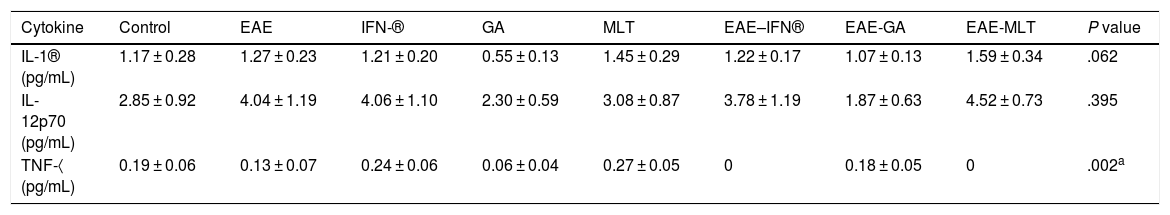
ResultsClinical diagnosis and assessment of experimental autoimmune encephalitisWe implemented and standardised the relapsing-remitting EAE model by immunising male Sprague-Dawley rats of 60 days postnatal age with the experimental homogenate together with Freund’s Complete Adjuvant; animals reached a maximum of grade 3 on the EAE clinical assessment scale. The relapsing-remitting EAE model is the most appropriate for assessing treatments since it recreates the most frequent type of MS, relapsing-remitting MS; the treatments analysed in this study are the ones used to treat this type of MS (Fig. 1). We assessed only the first relapse-remission cycle, spanning 12 experimental days. All immunised animals displayed EAE, achieving at least grade 2 of the disease, which was considered successful (Fig. 2A–E).
Clinical degree of EAE (mean ± SEM) observed in the EAE, EAE–IFN-®, EAE-GA, and EAE-MLT groups over the 12-day experimental period. A) Comparison of the clinical assessment of EAE in experimental groups. B) Clinical assessment of the EAE group. C) Clinical assessment of the EAE–IFN-® group. D) Clinical assessment of the EAE-GA group. E) Clinical assessment of the EAE-MLT experimental group.
EAE: rats with experimental autoimmune encephalitis and no treatment; EAE-GA: rats with EAE treated with glatiramer acetate; EAE–IFN-®: rats with EAE treated with interferon beta; EAE-MLT: rats with EAE treated with melatonin; SEM: standard error of the mean.
EAE appeared sooner in the animals treated with IFN-®, GA, and MLT than in the group with no treatment. IFN-® reduced the severity and duration of clinical signs and symptoms, whereas treatment with GA established a plateau at clinical grade 3 for several days, with subsequent recovery. However, treatment with MLT improved symptoms more rapidly than GA, achieving total recovery of the animals, although a relapse was observed by the end of the experimental period (Fig. 2E).
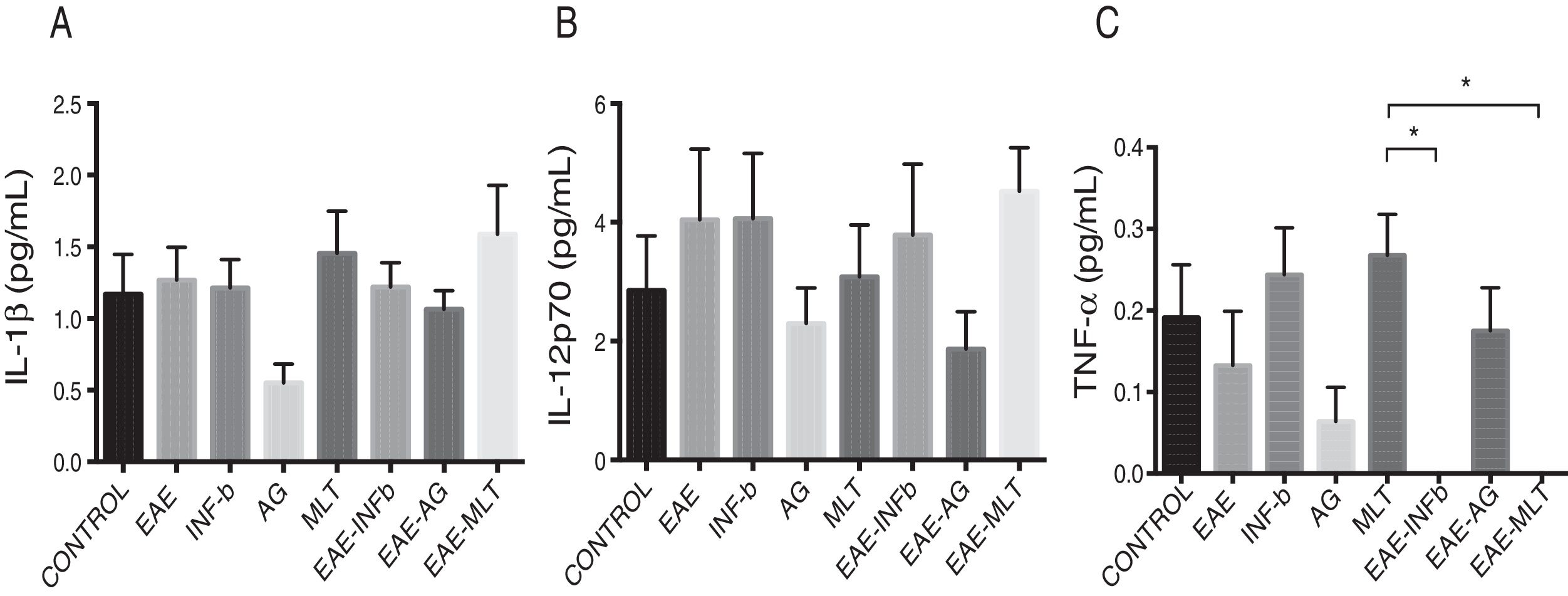
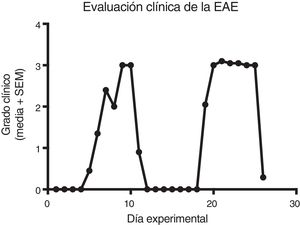
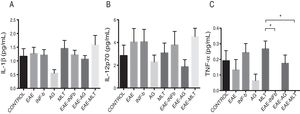
Quantification of proinflammatory cytokines in the cerebrospinal fluidCSF IL-1® concentration in the different experimental groups ranged between 0.55 ± 0.13 and 1.59 ± 0.34 pg/mL; differences between groups were not statistically significant (P = .0618, 95% CI). IL-12p70 concentration ranged from 1.87 ± 0.63 to 4.52 ± 0.73 pg/mL; however, these differences were not statistically significant (P = .3948, 95% CI). Therefore, CSF concentrations of IL-1® and IL-12p70 were not modified by the model or by the treatments that the animals were receiving. TNF-〈 concentration ranged from 0.0 ± 0.0 to 0.27 ± 0.05 pg/mL; this difference was statistically significant, with a P value < .0015 (95% CI). Therefore, EAE and the treatments administered affected TNF-〈 levels to different degrees, even below the lower limit of detection of the device used, with a significant effect between treatment with IFN-〈 and MLT in EAE rats (Table 1 and Fig. 3A–C). We should mention that CSF concentrations of these cytokines are lower than the concentrations reported in the serum by other studies.
Mean CSF concentration of IL-1®, IL-12p70, and TNF-〈 in each experimental group.
| Cytokine | Control | EAE | IFN-® | GA | MLT | EAE–IFN® | EAE-GA | EAE-MLT | P value |
|---|---|---|---|---|---|---|---|---|---|
| IL-1® (pg/mL) | 1.17 ± 0.28 | 1.27 ± 0.23 | 1.21 ± 0.20 | 0.55 ± 0.13 | 1.45 ± 0.29 | 1.22 ± 0.17 | 1.07 ± 0.13 | 1.59 ± 0.34 | .062 |
| IL-12p70 (pg/mL) | 2.85 ± 0.92 | 4.04 ± 1.19 | 4.06 ± 1.10 | 2.30 ± 0.59 | 3.08 ± 0.87 | 3.78 ± 1.19 | 1.87 ± 0.63 | 4.52 ± 0.73 | .395 |
| TNF-〈 (pg/mL) | 0.19 ± 0.06 | 0.13 ± 0.07 | 0.24 ± 0.06 | 0.06 ± 0.04 | 0.27 ± 0.05 | 0 | 0.18 ± 0.05 | 0 | .002a |
Values are expressed as mean ± standard error of the mean. CSF: cerebrospinal fluid; EAE: rats with experimental autoimmune encephalitis and no treatment; EAE-GA: rats with EAE treated with glatiramer acetate; EAE–IFN-®: rats with EAE treated with interferon beta; EAE-MLT: rats with EAE treated with melatonin; GA: rats treated with glatiramer acetate only; IFN-®: rats without EAE treated with interferon beta; IL: interleukin; MLT: rats without EAE treated with melatonin; SEM: standard error of the mean; TNF-〈: rats without EAE treated with tumour necrosis factor alpha.
CSF IL-1®, IL-12p70, and TNF-〈 concentration (mean ± SEM) in each experimental group; control, EAE, IFN-®, GA, MLT, EAE–IFN-®, EAE-GA, and EAE-MLT. A) CSF IL-1® concentration in the different groups. B) CSF IL-12p70 concentration in the different groups. C) CSF TNF-〈 concentration in the different groups.
CSF: cerebrospinal fluid; EAE: rats with experimental autoimmune encephalitis receiving no treatment; EAE-GA: rats with EAE treated with glatiramer acetate; EAE–IFN-®: rats with EAE treated with interferon beta; EAE-MLT: rats with EAE treated with melatonin; GA: rats without EAE treated with glatiramer acetate; IFN-®: rats without EAE treated with interferon beta; IL: interleukin; MLT: rats without EAE treated with melatonin; SEM: standard error of the mean; TNF-〈: rats without EAE treated with tumour necrosis factor alpha.
*Kruskal-Wallis test and Dunn post-hoc test.
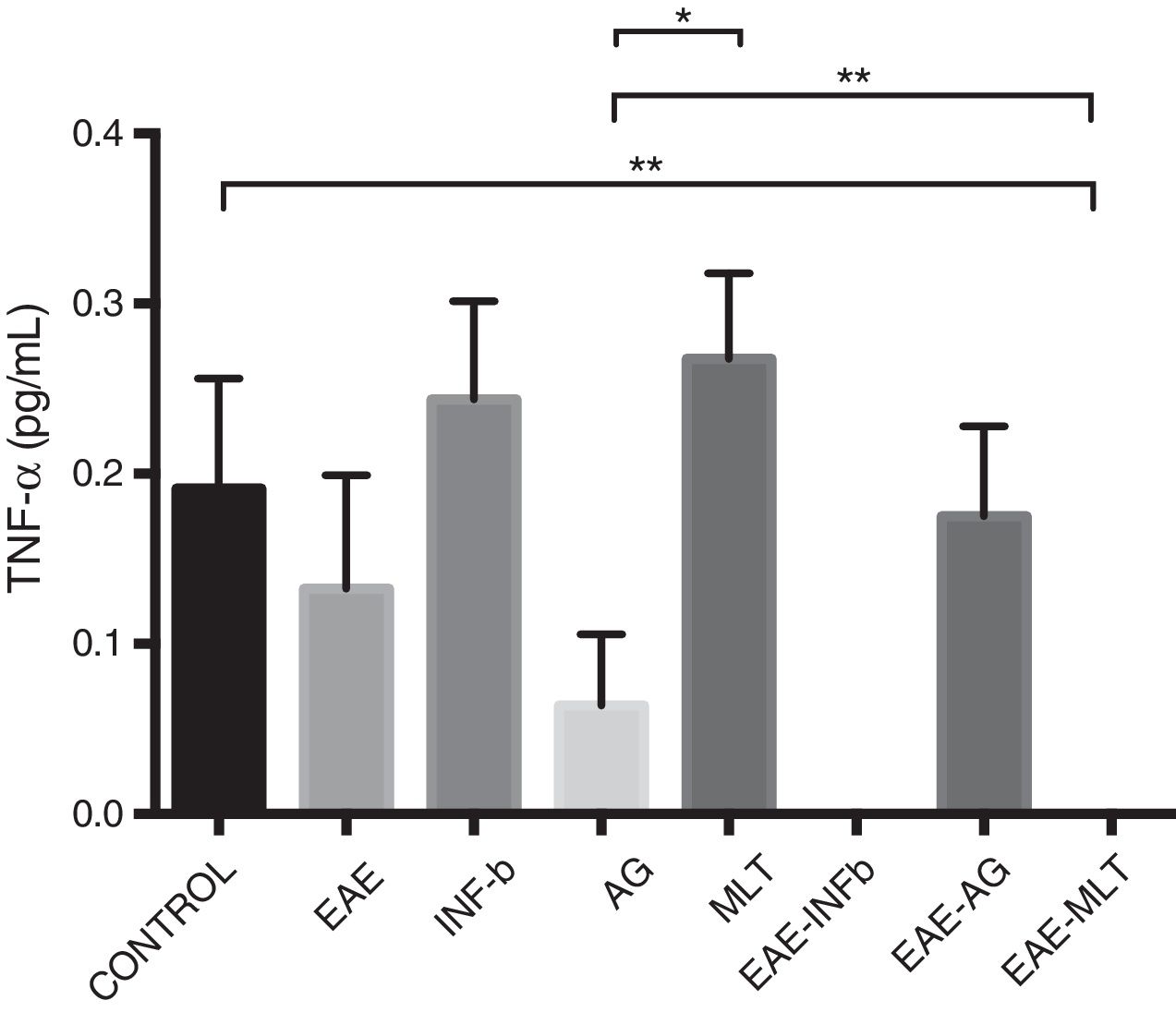
TNF-〈 concentration showed statistically significant differences between the control and EAE–IFN-® groups, control and EAE-MLT groups, IFN-® and GA groups, IFN-® and EAE–IFN-® groups, IFN-® and EAE-MLT groups, GA and MLT groups, MLT and EAE–IFN-® groups, and MLT and EAE-MLT groups (P < .030, 95% CI) (Fig. 4).
CSF TNF-〈 concentration of each experimental group: control, EAE, IFN-®, GA, MLT, EAE–IFN-®, EAE-GA, and EAE-MLT.
CSF: cerebrospinal fluid; EAE: rats with experimental autoimmune encephalitis with no treatment; EAE-GA: rats with EAE treated with glatiramer acetate; EAE–IFN-β: rats with EAE treated with interferon beta; EAE-MLT: rats with EAE treated with melatonin; GA: rats without EAE treated with glatiramer acetate; IFN-β: rats without EAE treated with interferon beta; IL: interleukin; MLT: rats without EAE treated with melatonin; SEM: standard error of the mean; TNF-α: rats without EAE treated with tumour necrosis factor alpha.
*P ≤ .030; **P < .010, 95% CI. Kruskal-Wallis test and Mann-Whitney U test.
The present study compared the effect of the first-line MS drugs used in clinical practice (IFN-® and GA) with MLT in EAE. EAE is the most widely used model for assessing potential drugs for treating MS,21 and therefore the appropriate model for our aim of comparing IFN-®, GA, and MLT. Our model achieved a maximum of grade 3 on the established clinical scale; we can therefore consider it a non-aggressive model of EAE, at least in the first cycle of the disease. Were the disease to progress, we would be likely to observe a significant increase in severity with each cycle, approaching the maximum clinical grade; this pattern would correspond to RRMS, in which the natural course of exacerbations ends with a period of recovery, leading to clinical remission. However, residual deficit after a relapse may persist and contribute to the stepwise progression of disability.22
IFN-® showed a greater effect than GA and MLT in the clinical assessment of EAE, demonstrating why this drug is considered a gold standard in MS treatment, since it suppressed EAE faster and more effectively (preventing severity from surpassing grade 2) than the other treatments assessed. The action mechanism of IFN-® is complex, causing several effects in different areas of cell function.23 The most relevant action mechanisms include proinflammatory cytokine inhibition and regulation of Th2 cytokines by T cells in IFN-®–treated patients.24 However, no previous study has evaluated the effect of IFN-® on the CSF. In our study, we observed no statistically significant intergroup differences in IL-1® and IL-12p70 cytokine concentration; their concentration therefore remains stable at the time of CSF collection. This was not the case with TNF-〈, which was depleted or below the lower limit of detection of the equipment used. This is consistent with the results of other studies reporting that IFN-® decreases TNF-〈 expression in T cells and macrophages25,26; this effect would promote inhibition of TNF-〈 production and therefore its absence in CSF quantification.
Furthermore, in our model, GA was less effective than the other treatments, as it did not decrease disease severity or accelerate recovery; rats receiving this treatment showed a plateau of greater severity, lasting 4 days. Regarding CSF cytokine concentrations, we observed downward trends in IL-1®, IL-12p70, and TNF-α in the group receiving GA only, which points to regulation of the immune system despite absence of any inflammatory process.
MLT suppressed EAE, reducing the relapse phase duration and promoting the remitting phase sooner than in the EAE group. However, animals presented a second relapse during the experimental period. MLT seems to promote inflammation, as it plays a dual role: it has both an anti-inflammatory and a proinflammatory effect, depending on the cellular microenvironment. Franco and Markus27 demonstrated this effect by assessing cerebellar cells stimulated with lipopolysaccharide and treated with MLT, which presented a higher degree of protection against lipopolysaccharide cytotoxicity than cells that had not been stimulated but which were treated with MLT; cell death was promoted in the latter cell group. Other study groups have described the anti-inflammatory effect of MLT. In a trial with an EAE model, Chen et al.28 administered high doses of MLT, which promotes inhibition of Th17 cells in the CNS, reducing cell migration and inhibiting cell proliferation in the CNS.29,30 Other research groups have described several cell models and lines in which the anti-inflammatory effect of MLT presents by inhibiting the expression and activation of NF-κB,31 a transcription factor involved in the development of the immune response and inflammatory processes.32 MLT has also been reported to have a significant proinflammatory effect, upregulating MHC class II molecules,33 and to promote the Th1-type immune response.34,35 The negative impact of exogenous MLT on the recovery of acute EAE is reported to be caused by increased serum IFN-®, astrocytic activation, and infiltration of T cells into the CNS.36 This dual behaviour of MLT has been described as an “immune system buffer,” acting as a stimulant under baseline or immunosuppressive conditions or as an anti-inflammatory suppressor in the presence of an exacerbated immune response, for example in acute inflammation.20 This EAE model has demonstrated that in a proinflammatory microenvironment, MLT has a beneficial effect, reducing severity of EAE, whereas in a controlled environment it presents a negative effect, as it promotes an inflammatory process that exacerbates EAE symptoms.
MLT inhibits proinflammatory cytokines (TNF-α) through suppression of inflammatory mediators mediated by various signalling pathways, such as ERK/p38 MAPK, c/EBPb, NFκB, and p300.37 We observed no significant differences in IL-1® and IL-12p70 concentration with any of the treatments used in this model due to the matrix solution used; studies with other models of EAE report detecting IL-1® in perivascular infiltrates of MHC class II cells at the edges of demyelinating lesions or in resident microglia or differentiated macrophages, which suggests that IL-1® expression is induced within the CNS.38 IL-12 expression has been identified in sections of brain tissue affected by EAE.39 Therefore, the concentration of cytokines remains constant in the CSF, without a need for a remote stimulus to increase concentration.
In conclusion, this model enables us to study and compare alternative treatments for MS. IFN-® shows greater immunomodulation of the inflammatory process of EAE. GA does not control EAE as effectively, but does show an anti-inflammatory effect mediated by other mechanisms not assessed in this study. MLT has been demonstrated to present both a proinflammatory and an anti-inflammatory effect, depending on the cellular microenvironment. Further studies are needed to assess the molecular mechanisms involved in the effects of MLT on EAE, and to quantify these and other cytokines in other biological matrices, in order to confirm the role of MLT as an effective anti-inflammatory agent in the treatment of MS.
FundingThis study was financed by the Health Research Fund of the Mexican Institute of Social Security (registry number FIS/IMSS/PROT/G14/123).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ramos González EJ, Ramirez Jirano LJ, García Martínez DZ, Ortiz GG, Jave Suárez LF, Leal Cortes CA, etal. Estudio comparativo de melatonina contra los tratamientos inmunomoduladores (interferón beta y acetato de glatirámero) en un modelo murino de esclerosis múltiple. Neurología. 2021;36:262–270.