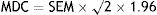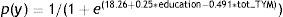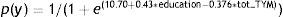To validate a Spanish version of the TYM, a self-administered cognitive screening test designed for the detection of Alzheimer's disease and mild cognitive defect.
MethodsA cross-sectional study was conducted in a neurology outpatient clinic. The TYM was administered to individuals of 50 years or more who came to the clinic because of any symptom. Their cognitive state was evaluated regardless of the outcome of TYM. They were categorised into 3 groups: (1) Cognitively normal (739), (2) with mild cognitive impairment (183), (3) with dementia (127). An analysis of items was made and the psychometric properties of the TYM were defined. There was a cross-validation, and the predictive validity of the TYM score, adjusted to the demographic variables, was determined by evaluating their performance in ROC curves.
ResultsThe internal consistency, interobserver reliability, short term and long-term test-retest reliability were adequate. The TYM correlated with the MMSE (r=0.779, P<.0001). The cross validation showed consistent results. With the TYM Score adjusted according to the educational level, a sensitivity of 0.86 with a specificity of 0.88 in the cut-off point of ≤40/50 was obtained to identify subjects with cognitive impairment, and a sensitivity of 0.94 with a specificity of 0.89 in the cut-off point of ≤36/50 to identify subjects with dementia.
ConclusionsThe TYM is a self-administered global cognitive test, possessing excellent psychometric properties and good predictive validity. It can be used as a cognitive screening test in subjects with 4 years or more of formal education.
Validar una versión en español del TYM, una prueba cognitiva de cribado autoadministrada, diseñada para la detección de la enfermedad de Alzheimer y defecto cognitivo ligero.
MétodosRealizamos un estudio transversal en una clínica ambulatoria neurológica. El TYM fue administrado a todas las personas de 50 años o más que acudieron a la consulta, sin tener en cuenta el síntoma por el que consultaban. Independientemente del resultado del TYM se evaluó su estado cognoscitivo. Se clasificaron en 3 grupos: 1) cognitivamente normales (739), 2) con deterioro cognitivo leve (183) y 3) con demencia (127). Se analizaron los ítems y se definieron las propiedades psicométricas del TYM. También se procedió a una validación cruzada y se determinó la validez predictiva del TYM, corregido por las variables demográficas, mediante la evaluación de su desempeño en curvas ROC.
ResultadosLa consistencia interna, fiabilidad interobservador y fiabilidad test-retest a corto y largo plazo fueron adecuadas. El TYM está correlacionado con el MMSE (r=0,779; p<0,0001). La validación cruzada mostró resultados consistentes. Con la puntuación TYM ajustada por nivel educativo, se obtuvo una sensibilidad de 0,86 con una especificidad de 0,88 en el punto de corte de ≤40/50 para identificar a sujetos con defecto cognitivo y una sensibilidad de 0,94 con una especificidad de 0,89 en el punto de corte de ≤36/50 para identificar a sujetos con demencia.
ConclusionesEl TYM es una prueba cognitiva global autoadministrada, que posee excelentes propiedades psicométricas y buena validez predictiva. Puede usarse como un test cognitivo de cribado en sujetos con 4 o más años de instrucción formal.
Every day, doctors must determine whether patients have cognitive deficiencies. This task involves specific difficulties made more onerous by the fact that consultation times have been shortened in almost all care settings.1
Time constraints have made it necessary to apply brief screening tests to patients with cognitive concerns. Unfortunately, the vast majority of currently available brief tests do not possess sufficient diagnostic efficacy to detect cognitive impairment.2
We feel that the best approach, when time is limited, is to combine 2 strategies: using effective clinical screening tests and extending consultation times by letting the subject begin the tests while he or she is still in the waiting room. The assessment can later be completed with any tests that would have to be administered during the consultation itself. To achieve this end, doctors can first provide a self-administered cognitive test requiring only moderate supervision by support staff at the clinic.
In light of the above, we were particularly intrigued by the Brown et al. study3 presenting a self-administered cognitive test (Test Your Memory [TYM]) that seems to display excellent psychometric properties, including acceptable predictive validity. The TYM has been used in several countries and in many translated versions with good results.4–7 Furthermore, it compares favourably with the Mini-Mental State Examination (MMSE) as a screening test for cognitive impairment.8 This article presents a Spanish-language version of the TYM and its validation process in a private neurology clinic.
Subjects, material, and methodsSubject selectionOur aim was to administer the TYM to every new patient aged 50 or older examined by one of the authors (JFA) regardless of their initial symptoms. Subjects were asked to sign their informed consent once the objective of the study, validation of a Spanish-language version of the test, had been explained.
We excluded individuals with sensory or motor impairments that would prevent them from completing the TYM, as well as illiterate subjects or those with less than 4 years of formal education. We also excluded patients with chronic alcoholism, major psychiatric disorders, and those taking significant amounts of psychotropic substances. Subjects filled out the TYM before being admitted to the consultation room; they were supervised, and assisted if necessary, by the same person (MTR). JFA reviewed and scored the TYM at a different time, following the consultation and after having determined whether or not the subject was cognitively impaired.
As sociodemographic data, we recorded age, sex, education level (as one of 4 levels: 4-8 years, 9-12 years, 13-15 years, and more than 15 years of schooling). Scores on each item of the TYM and the total were also recorded, along with results from other neurocognitive tests; the subject's cognitive and functional state expressed as a Clinical Dementia Rating (CDR) score9; and the definitive diagnosis.
Cognitively normal subjectsThe group of cognitively normal subjects (CNS) included patients’ family members and carers, as well as other neurological patients examined at the clinic. All had a CDR score of 0. These subjects had their histories taken and a neurological examination performed in addition to at least one cognitive screening test (MMSE,10,11 five by five test,12 or Fototest13) administered no more than 4 weeks before or after the TYM. Inclusion criteria were absence of subjective cognitive complaints, no history of major head trauma, and no functional limitations due to cognitive impairment. The CNS group contained 739 individuals divided into 3 subgroups:
- 1.
Controls sensu stricto (n=62, CSS). This subgroup consisted of patients’ family members and carers, or patients who visited the neurology clinic by mistake or were determined to have non-neurological conditions. They had provided their histories and undergone a complete neurological examination. These subjects had no history of nervous system disease.
- 2.
Cognitively normal patients with no brain impairment (n=319, CNP). These patients had neurological disorders with no cerebral involvement (muscular or peripheral nerve disorders, peripheral vertigo, etc.). These controls had no history of brain disease.
- 3.
Cognitively normal patients with brain involvement (n=358, CNB). These patients had historical or current cerebral impairment without extensive focal lesions (small meningioma, minor head trauma, etc.). In addition to the cognitive screening test, all controls took other formal neurocognitive tests within 4 weeks of the TYM (forward and backward digit span,14 semantic and phonological fluency,15 Free and Cued Selective Reminding Test16 or the Rey Auditory Verbal Learning test,17 and a clock-drawing test).18 Subjects were eliminated from this group when they had an abnormal result on one test (≥1 SD below the mean corresponding to their population).
Since values on the TYM do not follow a normal distribution according to the Kolmogorov–Smirnov test, we compared values from the TYM among the 3 CNS subgroups defined above using a non-parametric test (Kruskal–Wallis). To this end, each CSS subject was paired with 2 CNP subjects and 2 CNB subjects and matched by sex, education level (with/without university studies), and 5-year age group (range, 50 to older than 85). A total of 55 CSS, 110 CNP, and 110 CNB subjects were contrasted. No intergroup differences were observed for TYM scores (χ2 1.28; GL 2; P>.5). In light of this result, we decided to merge all CNS into a single control group.
Subjects with acquired cognitive deficitThis category includes subjects who were examined at JFA's neurology clinic and whose evaluation showed that they had a non-focal acquired cognitive deficit. In addition to the cognitive impairment screening test, patients without severe impairment (CDR>1) underwent a neurocognitive examination whose content depended on the patient's initial complaint. These subjects are divided into 2 subgroups:
- 1.
Subjects with acquired mild cognitive impairment (MCI, n=183), without dementia, meeting the Petersen criteria19 for MCI in CDR stage 0.5. All of these subjects scored lower than 3 on the AD8 questionnaire.20 The criterion for objective abnormality was met when at least one test yielded a result≥1.5 SD, or 2 tests had results≥1 SD, below the mean corresponding to the population group.
- 2.
Subjects with dementia (n=127), meeting DSM-IV21 criteria for that condition with a CDR stage of 1 (74%) or 2 (26%). Presence of a functional deficit was confirmed by the interview and a survey given to close acquaintances or carers (AD8 or the brief version of the IQCODE).20,22 Subjects whose cognitive impairment had a focal cause were excluded from the study.
TYM is a comprehensive cognitive screening test originally designed to detect Alzheimer disease in the clinical setting. It can be administered in a short amount of time and requires very little training. The test includes a total of 10 tasks and possible total scores range from 1 to 50 points. High scores indicate better performances. The Spanish language version was created based on a translation of the original English-language version (see Appendix provided as additional online material). The 2 questions in the semantic knowledge section were substituted such that subjects were asked to name the Prime Minister of Spain rather than the UK, and to provide the year in which the Spanish Civil War broke out instead of World War I. Likewise, subjects were asked to name 4 creatures beginning with ‘P’ rather than ‘S’. The example animal was omitted since some subjects reused it as the first on their list. See Appendix for the TYM scoring method. A bilingual individual also produced a back-translation that coincided with the original English-language version. The resulting text is nearly identical to another recently published Spanish-language version.6
Psychometric properties of Test Your MemoryWe ran an analysis of items from a subsample of subjects (175 with cognitive impairment and 525 controls) with similar demographic variables. In this process, 3 controls were matched by sex, education, and age (5-year range) to each subject with cognitive impairment (CDR 0.5 or 1). The scale's internal consistency was tested using Cronbach's alpha. Interobserver reliability, short- and long-term test/retest reliability, and concurrent validity were tested with coefficients of correlation and determination. The kappa statistic was used to test both interrater reliability and agreement between TYM scores from 2 tests administered a short time apart. Concurrent validity was tested by calculating the correlation coefficient with the score obtained on a validated Spanish-language version of the MMSE,11 which was administered to 106 subjects on the same day.
The minimum detectable change (MDC) was calculated according to the following formula:
where SEM is the standard error of the mean.Predictive validity, meaning the test's ability to distinguish between subjects with and without cognitive impairment, was measured using ROC curves to analyse the performance of the TYM. We also ran a cross validation with half of the subjects (explained in detail below) to determine the consistency of the rule and its potential for generalisation.
TYM explores different cognitive domains that are affected by disorders that include cognitive decline, and its content is thus reasonably appropriate for our purpose.
Method used to create the rulesCross-validation was used to verify consistency of the rule. The subjects were listed in alphabetical order by their last names and divided into 2 approximately equal groups. Each half set then underwent logistic regression analysis with total TYM score as the predictor variable. The logistic function obtained from 50% of the subjects was then applied to the other half as a bijective function. The Hanley and McNeil statistic23 was used to compare ROC curves plotted for each half to the probabilities calculated from the logistic function obtained for the other half.
After confirming the stability of the rule using cross validation, we analysed the ability to predict cognitive abnormality based on demographic factors (age, sex, education) and on the total TYM score. Stepwise logistical regression analysis was performed with the set of all subjects to determine the relative effect of those variables. The Hosmer–Lemeshow test was applied to check goodness of fit for the logistic regression models.
To simplify use of the TYM and adapt it to clinical practice, we corrected raw TYM scores by the relevant demographic variables such that scores would be the same when subjects had equal probabilities of belonging to the control group.
Once this correction had been applied, we evaluated performance of the adjusted TYM score using a ROC curve. Lastly, we calculated values of sensitivity, specificity, likelihood ratios, and predictive values for the cut-off point with the maximum Youden index, plus the 2 higher and lower points.
This analysis assumed an alpha risk of 0.05 in a bilateral contrast. Estimates are provided with their 95% confidence intervals (95% CI). Statistical analyses were carried out using SPSS 10.0.7™ and MEDCAL 12.5.00™.
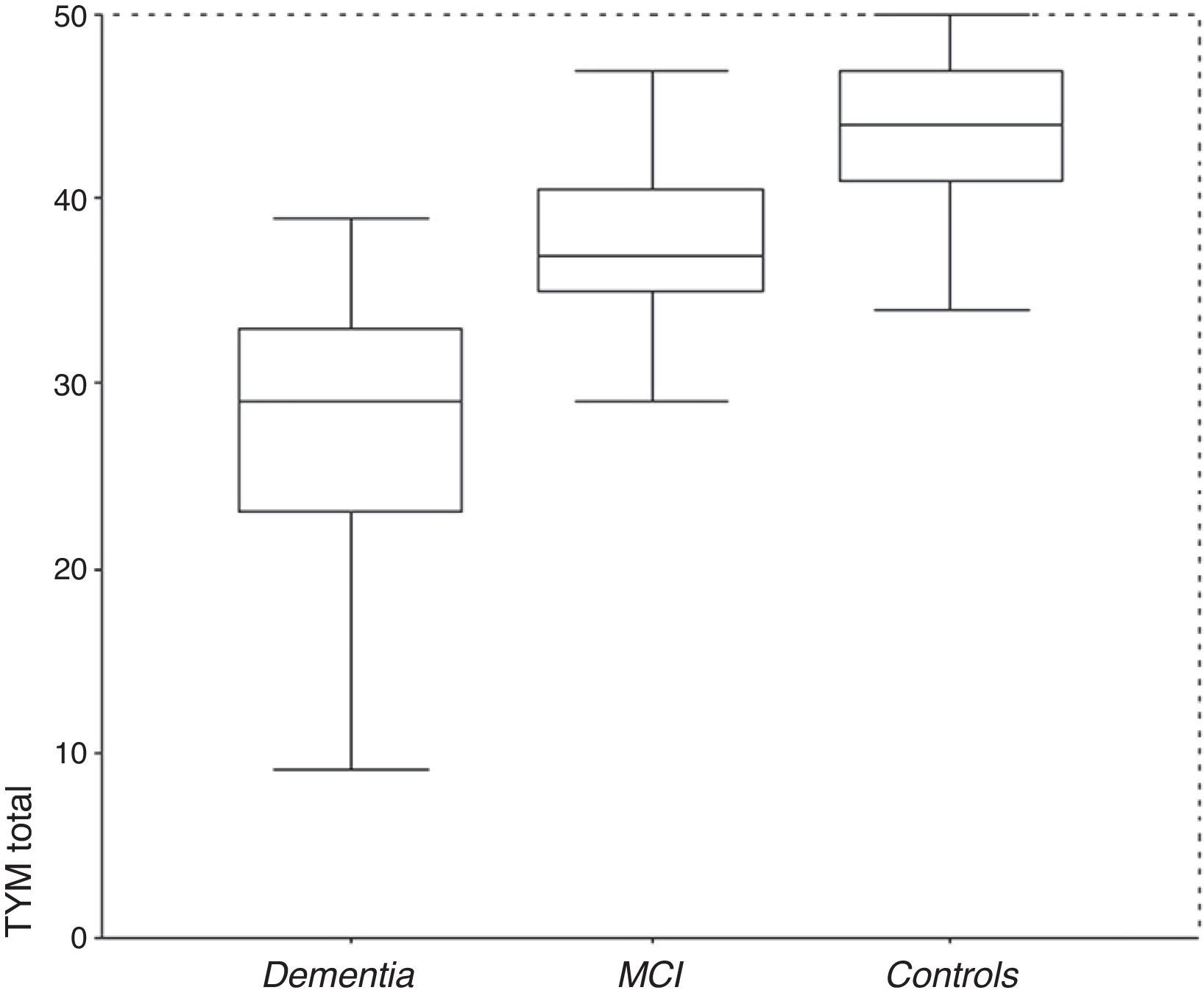
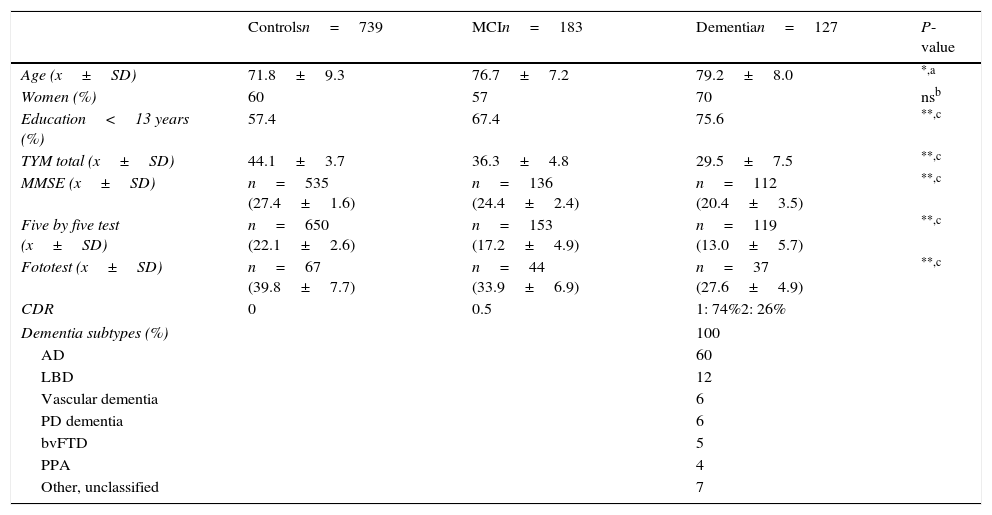
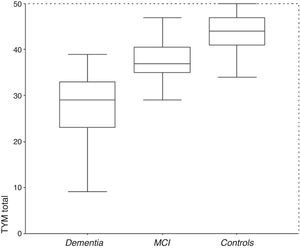
ResultsDescription of the sampleRecruitment took place between 1 July 2009 and 31 December 2012. Table 1 displays the distribution of the demographic data and scores on the cognitive screening tests, including TYM. The total sample consisted of 1049 subjects (183 with MCI, 127 with dementia, and 739 controls). The table also shows results from bivariate analyses with their statistical significance levels. As was expected, older age was associated with decreasing cognitive performance and function. There were no sex differences between groups, although women were predominant among patients with dementia. Education level was lower among patients with MCI or dementia than in control subjects. TYM scores showed a gap of 7 to 8 points between controls and patients with MCI, and between patients with MCI and dementia patients (Table 1 and Fig. 1).
Demographic data and cognitive screening tests by group (all subjects).
| Controlsn=739 | MCIn=183 | Dementian=127 | P-value | |
|---|---|---|---|---|
| Age (x±SD) | 71.8±9.3 | 76.7±7.2 | 79.2±8.0 | *,a |
| Women (%) | 60 | 57 | 70 | nsb |
| Education<13 years (%) | 57.4 | 67.4 | 75.6 | **,c |
| TYM total (x±SD) | 44.1±3.7 | 36.3±4.8 | 29.5±7.5 | **,c |
| MMSE (x±SD) | n=535 (27.4±1.6) | n=136 (24.4±2.4) | n=112 (20.4±3.5) | **,c |
| Five by five test (x±SD) | n=650 (22.1±2.6) | n=153 (17.2±4.9) | n=119 (13.0±5.7) | **,c |
| Fototest (x±SD) | n=67 (39.8±7.7) | n=44 (33.9±6.9) | n=37 (27.6±4.9) | **,c |
| CDR | 0 | 0.5 | 1: 74%2: 26% | |
| Dementia subtypes (%) | 100 | |||
| AD | 60 | |||
| LBD | 12 | |||
| Vascular dementia | 6 | |||
| PD dementia | 6 | |||
| bvFTD | 5 | |||
| PPA | 4 | |||
| Other, unclassified | 7 | |||
PPA: progressive primary aphasia; CDR: Clinical Dementia Rating; AD: Alzheimer disease; LBD: Lewy body dementia; bvFTD: behavioural variant frontotemporal dementia; ns: not significant; TYM: Test Your Memory.
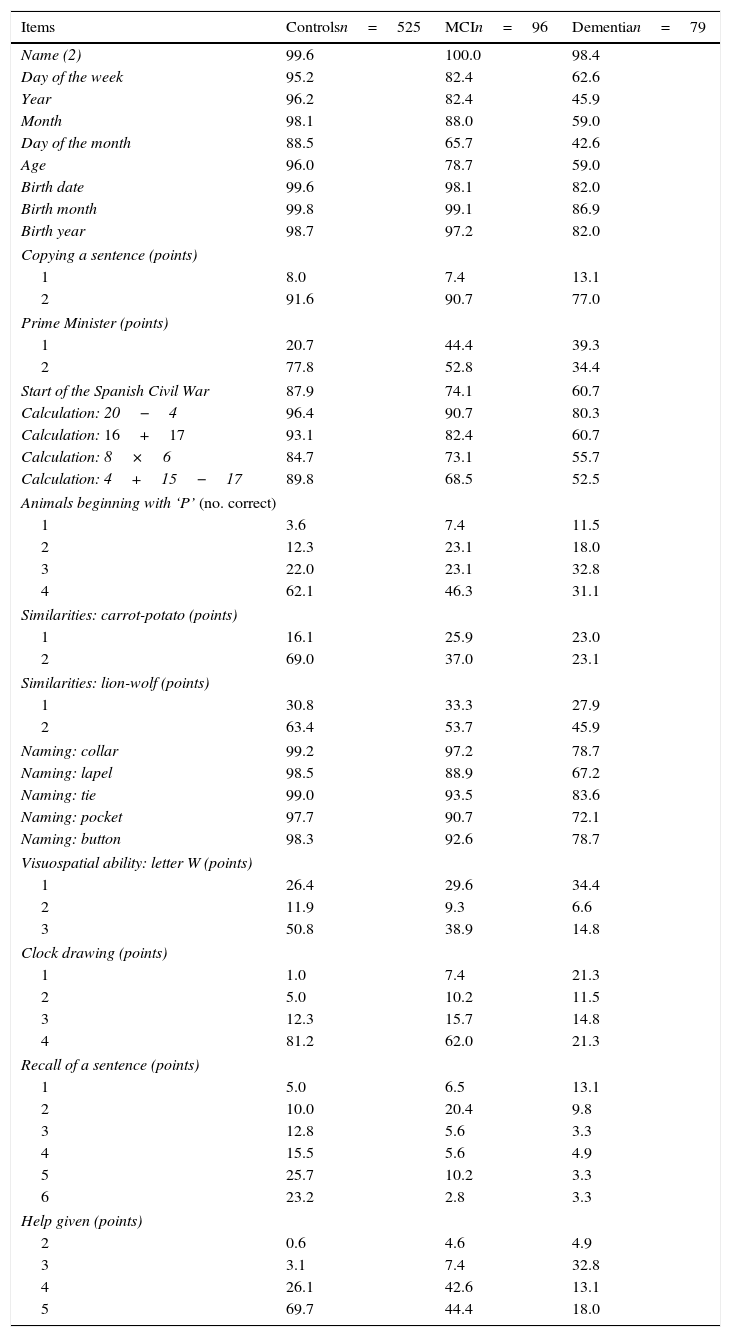
Table 2 lists the percentages of correct answers per item, broken down by each of the 3 subject groups (controls, MCI, dementia). This table was elaborated using the subsample of subjects matched by demographic variables. The correlation matrix in this subsample indicates that there are no strong correlations between the items (none of the coefficients reaches 0.7). Items measuring time orientation show a slight correlation with one another (0.2-0.3); there is also a moderate correlation between date of birth items (0.4-0.5) and the naming section (0.5-0.6). The test includes 28 items; easy items (those answered correctly by more than 90% of the subjects with MCI) include the 3 for date of birth, copying the sentence, one of the calculation tasks, and 4 of the 5 naming tasks. Difficult items (answered correctly by less than 50% of all subjects with MCI) are listing animals beginning with ‘P’, describing similarities, visualising a hidden letter, and recalling the sentence. Finding the letter also poses difficulties for many subjects with no cognitive impairment. More than 50% of subjects with cognitive impairment needed some degree of help with the test. Items best able to distinguish between cognitively normal subjects and those with MCI were recall of a previously copied sentence, evoking similarities, the clock-drawing test, and needing help with the test.
Item analysis: subjects matched by demographic variables.a
| Items | Controlsn=525 | MCIn=96 | Dementian=79 |
|---|---|---|---|
| Name (2) | 99.6 | 100.0 | 98.4 |
| Day of the week | 95.2 | 82.4 | 62.6 |
| Year | 96.2 | 82.4 | 45.9 |
| Month | 98.1 | 88.0 | 59.0 |
| Day of the month | 88.5 | 65.7 | 42.6 |
| Age | 96.0 | 78.7 | 59.0 |
| Birth date | 99.6 | 98.1 | 82.0 |
| Birth month | 99.8 | 99.1 | 86.9 |
| Birth year | 98.7 | 97.2 | 82.0 |
| Copying a sentence (points) | |||
| 1 | 8.0 | 7.4 | 13.1 |
| 2 | 91.6 | 90.7 | 77.0 |
| Prime Minister (points) | |||
| 1 | 20.7 | 44.4 | 39.3 |
| 2 | 77.8 | 52.8 | 34.4 |
| Start of the Spanish Civil War | 87.9 | 74.1 | 60.7 |
| Calculation: 20−4 | 96.4 | 90.7 | 80.3 |
| Calculation: 16+17 | 93.1 | 82.4 | 60.7 |
| Calculation: 8×6 | 84.7 | 73.1 | 55.7 |
| Calculation: 4+15−17 | 89.8 | 68.5 | 52.5 |
| Animals beginning with ‘P’ (no. correct) | |||
| 1 | 3.6 | 7.4 | 11.5 |
| 2 | 12.3 | 23.1 | 18.0 |
| 3 | 22.0 | 23.1 | 32.8 |
| 4 | 62.1 | 46.3 | 31.1 |
| Similarities: carrot-potato (points) | |||
| 1 | 16.1 | 25.9 | 23.0 |
| 2 | 69.0 | 37.0 | 23.1 |
| Similarities: lion-wolf (points) | |||
| 1 | 30.8 | 33.3 | 27.9 |
| 2 | 63.4 | 53.7 | 45.9 |
| Naming: collar | 99.2 | 97.2 | 78.7 |
| Naming: lapel | 98.5 | 88.9 | 67.2 |
| Naming: tie | 99.0 | 93.5 | 83.6 |
| Naming: pocket | 97.7 | 90.7 | 72.1 |
| Naming: button | 98.3 | 92.6 | 78.7 |
| Visuospatial ability: letter W (points) | |||
| 1 | 26.4 | 29.6 | 34.4 |
| 2 | 11.9 | 9.3 | 6.6 |
| 3 | 50.8 | 38.9 | 14.8 |
| Clock drawing (points) | |||
| 1 | 1.0 | 7.4 | 21.3 |
| 2 | 5.0 | 10.2 | 11.5 |
| 3 | 12.3 | 15.7 | 14.8 |
| 4 | 81.2 | 62.0 | 21.3 |
| Recall of a sentence (points) | |||
| 1 | 5.0 | 6.5 | 13.1 |
| 2 | 10.0 | 20.4 | 9.8 |
| 3 | 12.8 | 5.6 | 3.3 |
| 4 | 15.5 | 5.6 | 4.9 |
| 5 | 25.7 | 10.2 | 3.3 |
| 6 | 23.2 | 2.8 | 3.3 |
| Help given (points) | |||
| 2 | 0.6 | 4.6 | 4.9 |
| 3 | 3.1 | 7.4 | 32.8 |
| 4 | 26.1 | 42.6 | 13.1 |
| 5 | 69.7 | 44.4 | 18.0 |
MCI: mild cognitive impairment.
- -
Internal consistency. Cronbach's alpha for all subjects was 0.86 (lower limit of 95% CI=0.85).
- -
Interrater reliability. Tests completed by 36 subjects were scored by each author working independently. The correlation coefficient was 0.996 (R2=0.992). The weighted kappa coefficient was 0.926 (95% CI=0.896-0.956).
- -
Short-term test-retest reliability. A total of 42 clinically stable subjects (24 controls and 18 with cognitive impairment) took the TYM on 2 occasions separated by a mean of 24 days (range, 8–63). The test-retest stability coefficient was 0.865 (R2=0.748) (P<.0001). There were no significant differences between controls and subjects with cognitive impairment, either in the mean difference in TYM score (t-test, t=−0.05; DF=40; P>.9) or in the number of days elapsed between the tests (t-test, t=−0.157; DF=40; P>.8) The weighted kappa coefficient was 0.597 (95% CI=0.473-0.721). Short-term MDC was +3.43 (upper limit of 95% CI).
- -
Long-term test-retest reliability. A total of 45 cognitively intact subjects took the TYM on 2 occasions separated by a mean of 1.59 (±0.71) years. The test-retest stability coefficient was 0.549 (R2=0.301) (P<.0001). Long-term MDC was 5.8 (upper limit of 95% CI).
- -
Concurrent validity. The Spearman rank correlation coefficient (rho) between the TYM and the MMSE in the 106 subjects who took both tests on the same day was 0.779 (P<.0001).
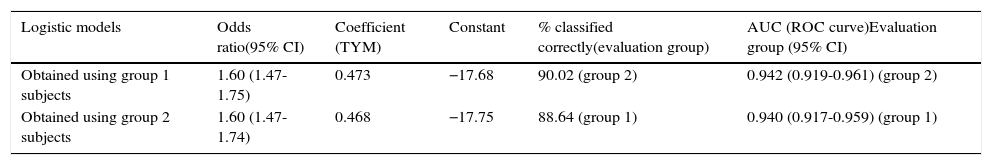
Regarding consistency of the predictive rule, diagnostic performance is excellent. Results are comparable between the two ROC curves: the one showing probabilities calculated according to the function obtained from the first half of the subjects and applied to the other half, and the curve plotted with probabilities calculated from the logistic function obtained from the second half of the subjects and applied to the first half. A comparison of both ROC curves (Hanley and McNeil test) revealed no significant differences; the coefficients for calculating probabilities, derived from both logistic regression analyses, are superimposable (Table 3).
Comparison of logistic models obtained from half of the subjects vs performance by the other half (cognitive deficit vs normal).
| Logistic models | Odds ratio(95% CI) | Coefficient (TYM) | Constant | % classified correctly(evaluation group) | AUC (ROC curve)Evaluation group (95% CI) |
|---|---|---|---|---|---|
| Obtained using group 1 subjects | 1.60 (1.47-1.75) | 0.473 | −17.68 | 90.02 (group 2) | 0.942 (0.919-0.961) (group 2) |
| Obtained using group 2 subjects | 1.60 (1.47-1.74) | 0.468 | −17.75 | 88.64 (group 1) | 0.940 (0.917-0.959) (group 1) |
AUC: area under the ROC curve; 95% CI: 95% confidence interval; TYM: Test Your Memory.
Group 1: subjects 1-512. Group 2: subjects 513-1049.
The logistic regression analysis, using presence/absence of cognitive deficit (MCI+dementia) as its criterion variable, with stepwise entry of the variables sex, age, education level, and total TYM score, yielded only total TYM score and education level as predictor variables and obtained the following function:
To achieve equal predictive probabilities for the TYM between the lower levels and the highest level of education, we apply this simple rule: add 2 points to the observed score for subjects with less than 9 years of schooling and 1 point to the total for subjects with 9 to 12 years of schooling. Scores obtained by patients with more than 12 years of education are not modified.
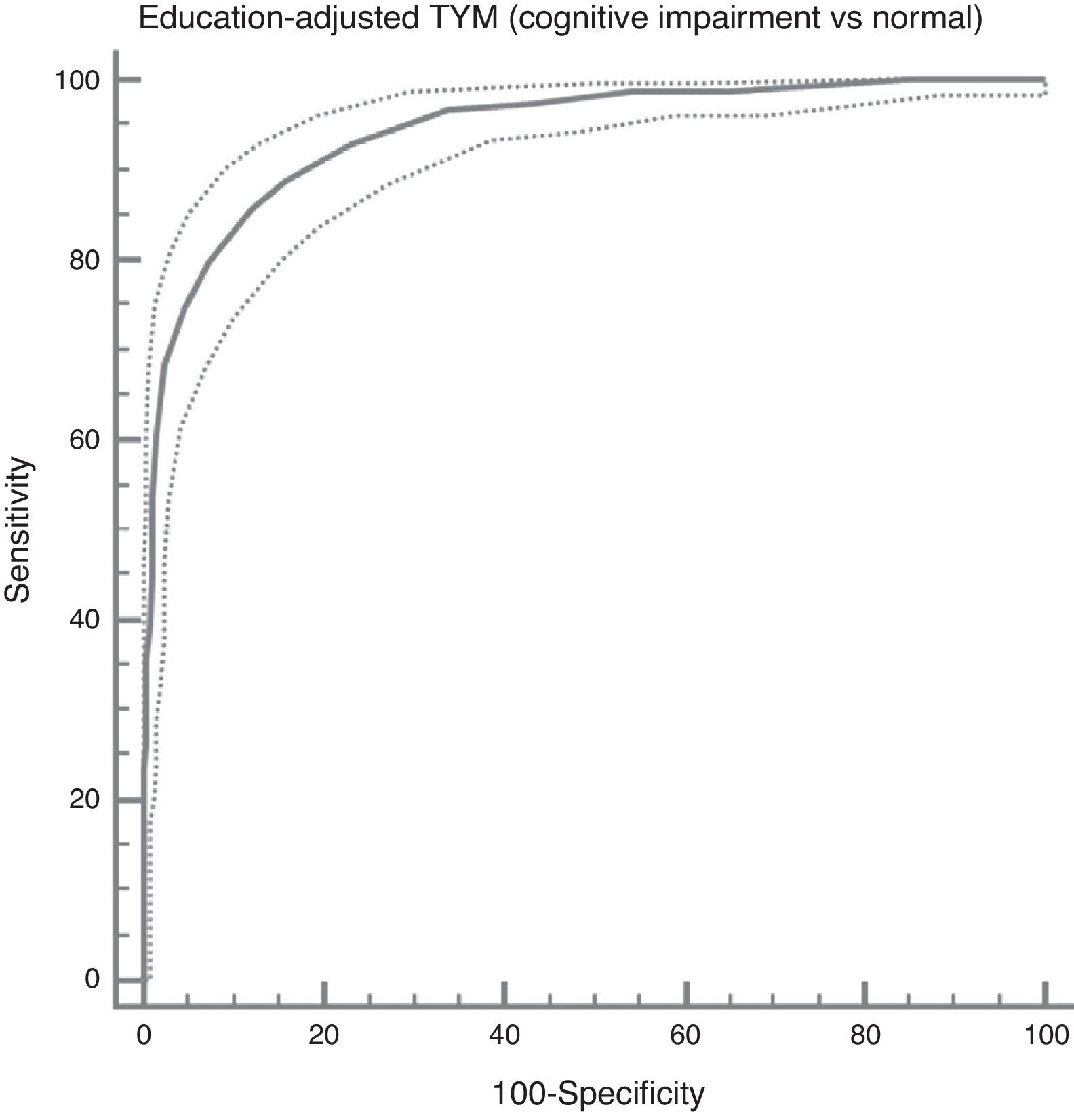
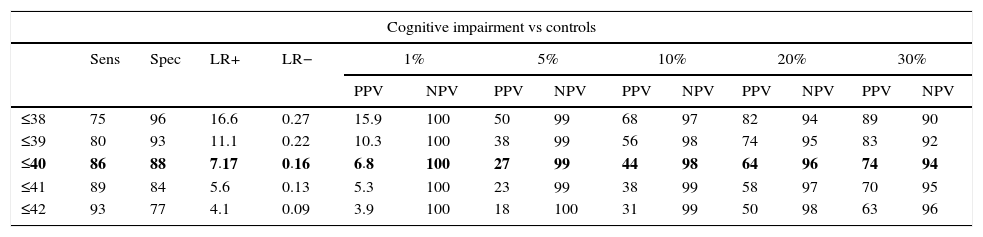
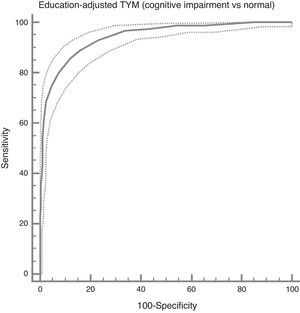
In the total subject set, TYM scores corrected for education allow us to distinguish between controls and subjects with cognitive impairment (ROC curve (Fig. 2)). Table 4 summarises the sensitivities, specificities, likelihood ratios, and positive and negative predictive values for different prevalence rates with different selected cut-off points. The cut-off point providing the best balance between sensitivity and specificity is at 40/41 total TYM points (corrected by education level). This cut-off point delivers a sensitivity of 0.86 and a specificity of 0.88.
Cognitive impairment vs controls; dementia vs no dementia.
| Cognitive impairment vs controls | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | LR+ | LR− | 1% | 5% | 10% | 20% | 30% | ||||||
| PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | |||||
| ≤38 | 75 | 96 | 16.6 | 0.27 | 15.9 | 100 | 50 | 99 | 68 | 97 | 82 | 94 | 89 | 90 |
| ≤39 | 80 | 93 | 11.1 | 0.22 | 10.3 | 100 | 38 | 99 | 56 | 98 | 74 | 95 | 83 | 92 |
| ≤40 | 86 | 88 | 7.17 | 0.16 | 6.8 | 100 | 27 | 99 | 44 | 98 | 64 | 96 | 74 | 94 |
| ≤41 | 89 | 84 | 5.6 | 0.13 | 5.3 | 100 | 23 | 99 | 38 | 99 | 58 | 97 | 70 | 95 |
| ≤42 | 93 | 77 | 4.1 | 0.09 | 3.9 | 100 | 18 | 100 | 31 | 99 | 50 | 98 | 63 | 96 |
| Logistic model | |||||||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | AUC (95% CI) | Cut-off point | YI | Sens (95% CI) | Spec (95% CI) | ||
| Corrected TYM | 0.608 (0.570-0.649) | 89.34 | 0.943 (0.928-0.957) | 40/41 | 0.737 | 86 (81-89) | 88 (85-90) |
| Dementia vs no dementia | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | LR+ | LR− | 1% | 5% | 10% | 20% | 30% | ||||||
| PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | |||||
| ≤34 | 82 | 93 | 12.5 | 0.19 | 10.6 | 100 | 38 | 99 | 57 | 98 | 76 | 95 | 83 | 92 |
| ≤35 | 89 | 91 | 10.2 | 0.12 | 9.1 | 100 | 34 | 99 | 52 | 99 | 71 | 97 | 81 | 95 |
| ≤36 | 94 | 89 | 8.4 | 0.07 | 7.9 | 100 | 31 | 100 | 49 | 99 | 68 | 98 | 79 | 97 |
| ≤37 | 97 | 86 | 6.4 | 0.04 | 6.5 | 100 | 27 | 100 | 44 | 100 | 63 | 99 | 75 | 99 |
| ≤38 | 98 | 82 | 5.4 | 0.02 | 5.2 | 100 | 22 | 100 | 38 | 100 | 58 | 99 | 70 | 99 |
| Logistic model | |||||||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | % CC | AUC (95% CI) | Cut-off point | YI | Sens (95% CI) | Spec (95% CI) | |
| Corrected TYM | 0.690 (0.651-0.731) | 93.38 | 0.965 (0.953-0.975) | 36/37 | 0.826 | 94 (88-97) | 89 (87-91) |
AUC: area under the ROC curve; CC: classified correctly; Spec: specificity; 95% CI: 95% confidence interval; YI: Youden index; LR: likelihood ratio; Sens: sensitivity; TYM: Test Your Memory; NPV: negative predictive value; PPV: positive predictive value.
Rows including cut-off points with the maximum Youden index are shown in bold.
In turn, the logistic function derived from using presence/absence of dementia as the criterion variable, and demographic data and total TYM score as the predictor variables, once more consisted only of the education level and the TYM score:
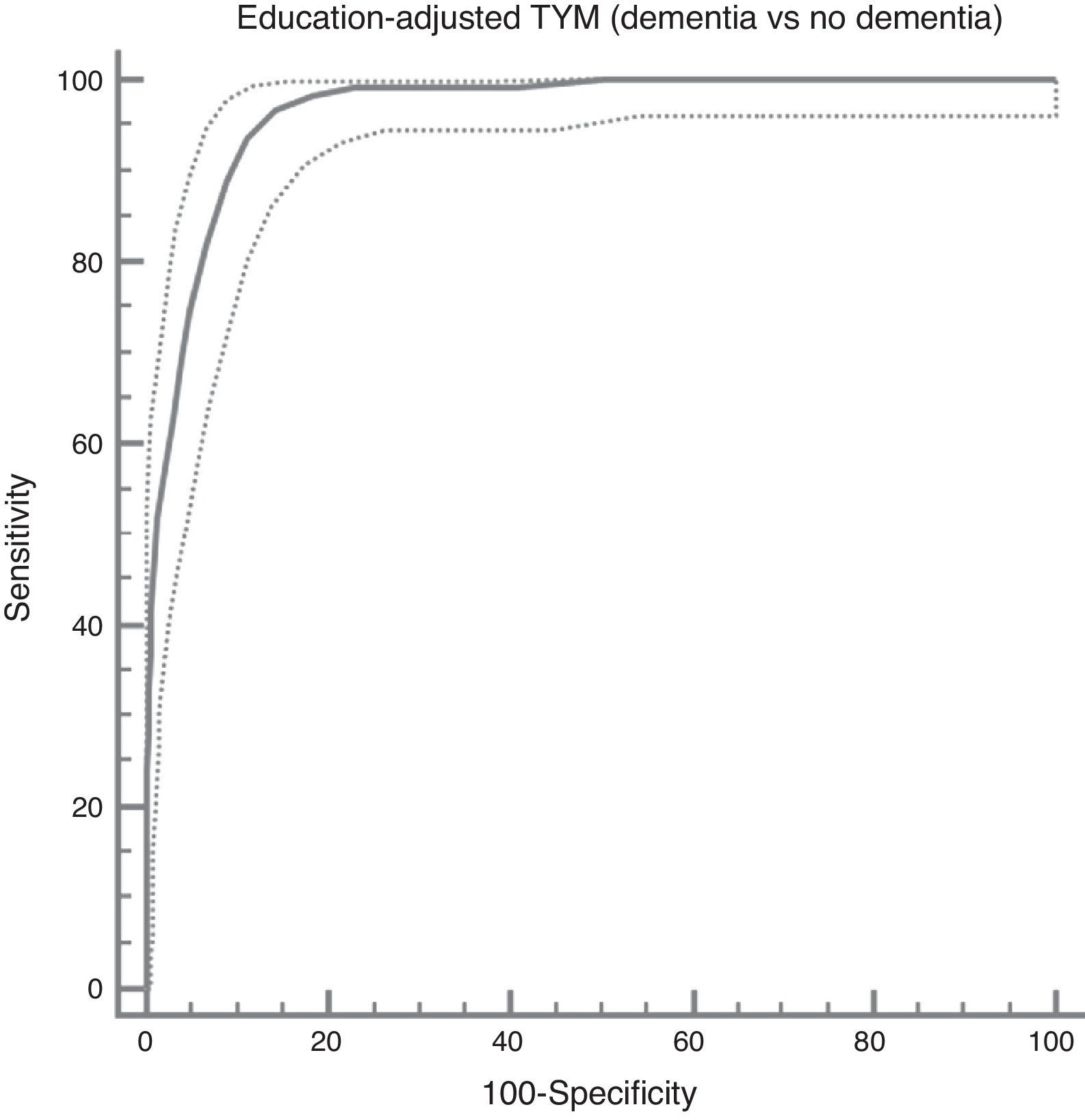
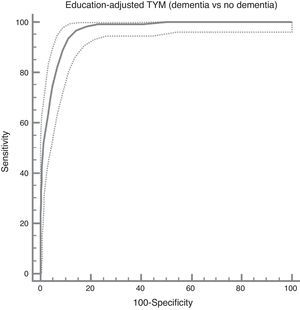
The ROC curve created with the corrected TYM score mentioned above, using presence/absence of dementia as the criterion variable, places the best cut-off point at 36/37 points, which provides a sensitivity of 0.94 and a specificity of 0.89 (Fig. 3). Table 4 describes the logistic regression model and the values of parameters for different prevalence rates with different selected cut-off points.
DiscussionMany neurodegenerative disorders begin developing years or even decades before there are signs of frank disease that can be clinically diagnosed.24 It is therefore likely that little can be done at time of diagnosis to prevent disease progression and its functional consequences. For this reason, diseases of this type must now be targeted in their prolonged preclinical phase. In theory, if we are able to act when the process is just beginning, we will have better possibilities of halting it. Researchers have proposed multiple neurocognitive, biochemical, and neuroimaging markers that may improve our ability to detect Alzheimer disease and other types of dementia.25–27 The most effective method will probably entail use of a carefully selected array of these markers.28 Nevertheless, most potentially helpful biochemical or imaging markers are not yet investigated in normal clinical practice because of their high costs or because they would require specialists to apply or interpret the tests. This being the case, it is important to continue searching for simple early detection methods that can be used in a conventional clinic in a short amount of time and which are sufficiently reliable.
The US-based chapter of the Alzheimer's Association recently issued recommendations for detecting cognitive impairment in a primary care setting.29
The algorithm they propose includes a brief screening test for cognitive impairment and a survey for close acquaintances or carers, which seems reasonable. Nevertheless, the cognitive tests they indicate for practical reasons are not very sensitive. Specifically, a number of different neurocognitive tests, such as the Fototest,13 the clock-drawing test,18 SPMSQ,30 MIS,31 the Montreal Cognitive Assessment,32 and others33 are effective for discriminating between patients with and without dementia, but much less so for identifying patients with MCI. The MMSE also displays low sensitivity for identifying MCI.34,35
Our study was carried out in a skewed sample mostly consisting of a middle- to upper-middle class population with regard to socioeconomic and educational status. These people had visited a single general neurology clinic. This being the case, these study results should not be extrapolated to the general population, although we believe that they could be applied, with caution, to a population segment resembling the one in our study. In this context, the predictive strength of the rule seems to have been reliable given the result of the cross validation. TYM should not be used as a cognitive screening test in uneducated individuals (illiterate subjects or those with little formal schooling), and this is its main drawback. In contrast, research has shown other tests, such as the Fototest, to be feasible for illiterate and uneducated subjects, although they may not be as useful in populations with more formal education. In conclusion, there is no universally valid cognitive screening test, and every clinic should therefore use the one best matching the characteristics of its target population.
TYM appears to be a strong candidate as a cognitive screening test for the type of population represented by our study sample. It possesses excellent psychometric properties, requires very little time from doctors and other healthcare professionals, and allows us to identify patients with cognitive symptoms that require closer monitoring or more extensive testing. It also shows acceptable concurrent validity with the MMSE, a test widely used by healthcare professionals for detecting cognitive impairment. Its results are stable and reproducible in the short term, and sufficiently stable over the long term. In addition, the TYM is being used in a variety of languages and countries, and results seem to confirm that it is efficient for clinical use. It is true that some areas must be studied further, such as its prospective validity and sensitivity to change, as well as its usefulness for different populations and clinical settings.
The TYM's multifaceted structure provides a qualitative sketch of the subject's cognitive function that helps us decide which specific areas require more in-depth examination. For example, most subjects with MCI will score below 3 on the recall of a sentence item, but make few mistakes on the rest of the items. For patients with recurrent memory complaints, the best approach is systematic administration of a more specialised learning and episodic verbal memory test during the visit.36
The adjustments to the TYM score proposed here consist of adding 2 points to the total score of subjects with primary studies or less (between 4 and 8 years of schooling) and 1 point to those who did not attend university (with 9-12 years of schooling). This correction is sufficient to make TYM scores comparable between all subjects, regardless of their age.
The score of 42 established by the original study as the cut-off point for Alzheimer disease3 is higher than the cut-off point of 40 for cognitive impairment which we obtained here. The cut-off point for dementia in general is 36 points. This discrepancy, also observed in another study,7 may be due to differences in the interpretation of dementia criteria (that is, what constitutes functional loss), or to certain linguistic or cultural peculiarities. In any case, the discrepancy highlights the need for population-specific calibration of cognitive tests. In addition, it shows that researchers need a more homogeneous approach to applying criteria for dementia and MCI.
This study did not attempt to analyse patients with Alzheimer disease independently from others. We wish to highlight that the TYM is useful for the exact purpose we examined here: red-flagging patients so that we know which ones will require a more in-depth examination.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank Dr J. Brown for being so kind as to review an early version of this study.
The copyright holders of the original version of the TYM have authorised the authors to use the original test concept and format to create the Spanish-language version.
The Spanish-language version of the TYM presented here has not been registered.
Please cite this article as: Ferrero-Arias J, Turrión-Rojo M. Validación de una versión española del Test Your Memory. Neurología. 2016;31:33–42.