Status epilepticus (SE) is an epileptic condition that can cause cerebellar atrophy and loss of Purkinje cells in both humans and research animals. Cerebellum is a region rich in γ-amino butyric acid (GABA) and glutamate, and some studies have shown that their concentrations may be altered after convulsions. However, there are no studies showing the effect of seizures on different cerebellar regions in developing rats. Time course of the effect of status epilepticus induced in the developing rat on γ-amino butyric acid and glutamate cerebellar concentration.
MethodsSE was induced using the lithium-pilocarpine model; control rats were injected with saline solution. At 6hours, 24hours, and 1month after SE o saline injection, rats were anaesthetised with pentobarbital and decapitated, and cerebella were extracted. The vermis and hemispheres were dissected and homogenised in 0.1M perchloric acid containing 4mM sodium bisulfite. Homogenates were centrifuged and supernatant was used to quantify GABA, and glutamate tissue concentrations by HPLC coupled with fluorometric detection.
ResultsSE did not alter GABA and glutamate tissue concentration in the cerebellar vermis and hemispheres.
ConclusionThe developing rat cerebellum is resistant to both short- and long-term neurochemical changes induced by SE.
El status epilepticus (SE) es un tipo de actividad epiléptica que causa atrofia cerebelar y pérdida de células de Purkinje en humanos y en animales de experimentación. El cerebelo es una región con alto contenido de ácido γ-aminobutírico (GABA) y glutamato, y algunos estudios refieren cambios en su concentración después de las convulsiones. Sin embargo, hasta la fecha no existen estudios que hayan analizado su efecto en diferentes regiones cerebelares en ratas en desarrollo. El objetivo del presente estudio fue realizar un curso temporal del efecto del SE inducido en ratas Wistar de 14 días de edad (P14) sobre el contenido tisular de GABA y glutamato en el vermis y los hemisferios cerebelares.
MétodosEl SE se indujo con el modelo de litio-pilocarpina; las ratas control se inyectaron con salina. Seis h, 24h o 30 días después del inicio del SE o de la aplicación de solución salina, las ratas se anestesiaron y decapitaron, se extrajo su cerebelo y se separaron el vermis y los hemisferios. Las ratas de ambos grupos se anestesiaron y decapitaron, se extrajo su cerebelo y se separaron el vermis y los hemisferios. Ambas regiones se homogeneizaron (ácido perclórico 0,1M conteniendo metabisulfito de sodio 4mM) y centrifugaron, y el sobrenadante se empleó para cuantificar la concentración tisular de GABA y glutamato por cromatografía de líquidos de alta resolución acoplada a un detector fluorométrico.
ResultadosEl SE no modificó la concentración de GABA y glutamato a los diferentes tiempos de análisis ni en el vermis ni en los hemisferios cerebelares.
ConclusionesEl cerebelo en desarrollo es resistente a los cambios neuroquímicos a corto y largo plazo producidos por el SE.
The International League Against Epilepsy recently defined status epilepticus (SE) as a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms that lead to abnormally prolonged seizures. The long-term consequences of SE depend on seizure type and duration and include neuronal death, neuronal damage, and alteration of neural networks.1 SE is considered the most extreme form of epilepsy.2 Its incidence varies according to a number of factors, including age, ethnicity, genetics, and socioeconomic status.3 Five epidemiological studies conducted in specific populations in Europe and the United States report a higher incidence of convulsive SE in children younger than one or 5 years.3 This suggests that the paediatric population is particularly vulnerable to this type of epilepsy and its consequences.
Cerebellar development begins during the embryonic phase and finishes after birth.4,5 This structure is involved in multiple functions, including balance, movement and postural control, and motor learning5,6; it has also been associated with sexual and addictive behaviour.7–10 Unlike such other central nervous system diseases as ataxia and autism,11,12 epilepsy has not traditionally been associated with cerebellar function. However, some studies have shown that the modulation of cerebellar function may have antiepileptic effects.13–16 Furthermore, patients with epilepsy or a history of SE display cerebellar atrophy17–20 and Purkinje cell loss21–23; this has also been observed in experimental models of epilepsy.24–26 The cerebellum is rich in amino acids, mainly γ-amino butyric acid (GABA) and glutamate: Purkinje and granule cells (the most abundant types of cells in the brain) use these amino acids as inhibitory and excitatory neurotransmitters, respectively, to integrate information transmitted to and from the brain.27
Little is known about the consequences of epileptic activity on the neurochemistry of the cerebellum, especially in the developing brain. Induction of febrile seizures in 10-day-old rats results in decreased GABA, taurine, and alanine concentrations and increased levels of aspartic acid in the whole cerebellum 24hours after seizures.28 Induction of SE in 14-day-old rats has been found not to change tissue GABA or glutamate concentration in the cerebellum 24hours after seizures.29 These findings underscore the importance of studying whether the changes in the concentration of these neurotransmitters are observable immediately after SE, or whether they occur over a longer period. This study aimed to describe the time course of the effects of lithium-pilocarpine-induced SE on tissue concentrations of GABA and glutamate in the vermis and hemispheres of the developing rat cerebellum.
Material and methodsExperimental subjectsWe studied 36 male and female Wistar rats of 14 days postnatal age (P14) at the time that seizures were induced (body weight 25-30g); the rats were raised in the vivarium of the Brain Research Centre at Universidad Veracruzana. The day of birth was considered postnatal day 0 (P0). Newborn rats were kept with their mothers in transparent acrylic cages measuring 15×24×37cm until the end of the experiment or until weaning on postnatal day 21. Adult rats were all male and were housed in collective acrylic boxes measuring 20×30×50cm. All rats were housed at ambient temperature and humidity levels with 12:12 light/darkness cycles starting at 8:00 am, and had free access to food and water. All experiments observed domestic and international standards and complied with the official Mexican guidelines on the use and care of experimental animals (NOM-062-ZOO-1999) and the National Research Council guide for the care and use of laboratory animals (2011 version).
Experimental groupsRats were divided into 6 groups (n=6 per group). They were euthanised 6hours, 24hours, or 30days after induction of SE; control rats were manipulated and euthanised at the same time points as the experimental rats.
Induction of status epilepticus with lithium-pilocarpineRats were injected with lithium chloride (3mEq/kg, intraperitoneal administration) on day P13; 20hours later, on day P14, SE was induced with pilocarpine chlorhydrate (100mg/kg, subcutaneous administration). We used the scale proposed by Haas et al.30 to monitor behavioural manifestations of SE; only animals displaying this behaviour were included in the study. Control rats were injected with lithium chloride and saline solution. All rats were rehydrated with glucose solution (5%) 7hours after the experiment started (1mL, subcutaneous administration) and were immediately returned to their mothers. We chose this time point because seizures had stopped after 7hours in the rats with SE. Experimental and control rats were separated from their mothers for similar periods of time.
Tissue processingRats were anaesthetised with pentobarbital sodium (60mg/kg, intraperitoneal administration). Following decapitation, the cerebellum was dissected on ice (approximately 4°C), and the vermis was separated from the hemispheres (both hemispheres were processed together). Brain tissue was then mechanically homogenised by adding 30μL perchloric acid 0.2N (HClO4, Baker) and sodium metabisulfite (S1516, Baker) for every 10mg of tissue. Tissue homogenates were centrifuged at 10000rpm for 20minutes at 4°C; the supernatant was collected and filtered through a 0.45-μm pore-size HV filter (Millipore). The filtrate and sediment were stored at −70°C until amino acid and protein quantifications were performed.
Determination of tissue amino acid concentration using high-performance liquid chromatographyGABA and glutamate concentrations in the vermis and hemispheres of the cerebellum were quantified according to the procedure described by Luna-Munguía et al.31 High-performance liquid chromatography was performed using a Waters 474 scanning fluorescence detector, a Nova-Pak C18 pre-column (4μm-60A [3.9×20]), and a reversed-phase Nova-Pak C18 column (4μm-60A [3.9-150]). The fluorescence detector operated at an excitation wavelength of 360nm and an emission wavelength of 450nm. For amino acid quantification, we prepared a supernatant dilution (1:250-1:450), mixed 20μL of the dilution with 6μL of the derivatisation reagent, and injected the mixture manually (25μL) into the chromatograph 2minutes after the reaction started. The derivatisation reagent was prepared by mixing 4.7mg o-phthalaldehyde (OPA), 94μL methanol, 0.874mL potassium tetraborate 0.4M, and 8.8μL 2-mercaptoethanol 0.116M. Chromatography was performed with a binary gradient system. Mobile phase A was a buffer solution of sodium acetate 40mM in 10% methanol (pH 6.7). Mobile phase B was a buffer solution of sodium acetate 8mM in 80% methanol (pH 5.7). The elution profile was as follows: 77% A and 23% B at 0min; 55% A and 45% B at 1min; 30% A and 70% B at 6.5min; 3% A and 97% B at 11-13min; 77% A and 23% B at 16min (flow rate: 0.5mL/min). GABA and glutamate concentrations were determined with linear regression analysis using the Millennium package (Waters®) based on an external standard calibration curve (100, 300, and 500ng/mL) of GABA and glutamate. Amino acid concentration was expressed in pg/mg protein.
Protein quantification using the Bradford protein assayProteins were quantified with the Bradford protein assay (Quick Start Bradford protein assay kit 1x dye reagent, Bio-Rad) using the sediment of the homogenised cerebellum. To this end, we prepared a standard curve with bovine serum albumin (Sigma). Absorbance was determined using a SpectraMax 190 microplate reader (Molecular Devices) at a wavelength of 595nm.
Statistical analysisThe results were analysed with two-way ANOVA for independent samples; the independent variables were treatment (SE vs control) and assessment time point (6hours, 24hours, and 30days), and the dependent variables were GABA and glutamate concentrations. The Tukey test for multiple comparisons was used to identify differences between factors. Statistical analysis and graphing were performed with GraphPad Prism 5.0. Results are presented as means±standard error of the mean (SEM); statistical significance was set at P<.05.
ResultsStatus epilepticus lasted 5 to 6hours. Maximum seizure severity in our sample was stage 5; rats displayed generalised seizures characterised by bilateral forelimb clonus and rearing, with or without loss of postural tone.
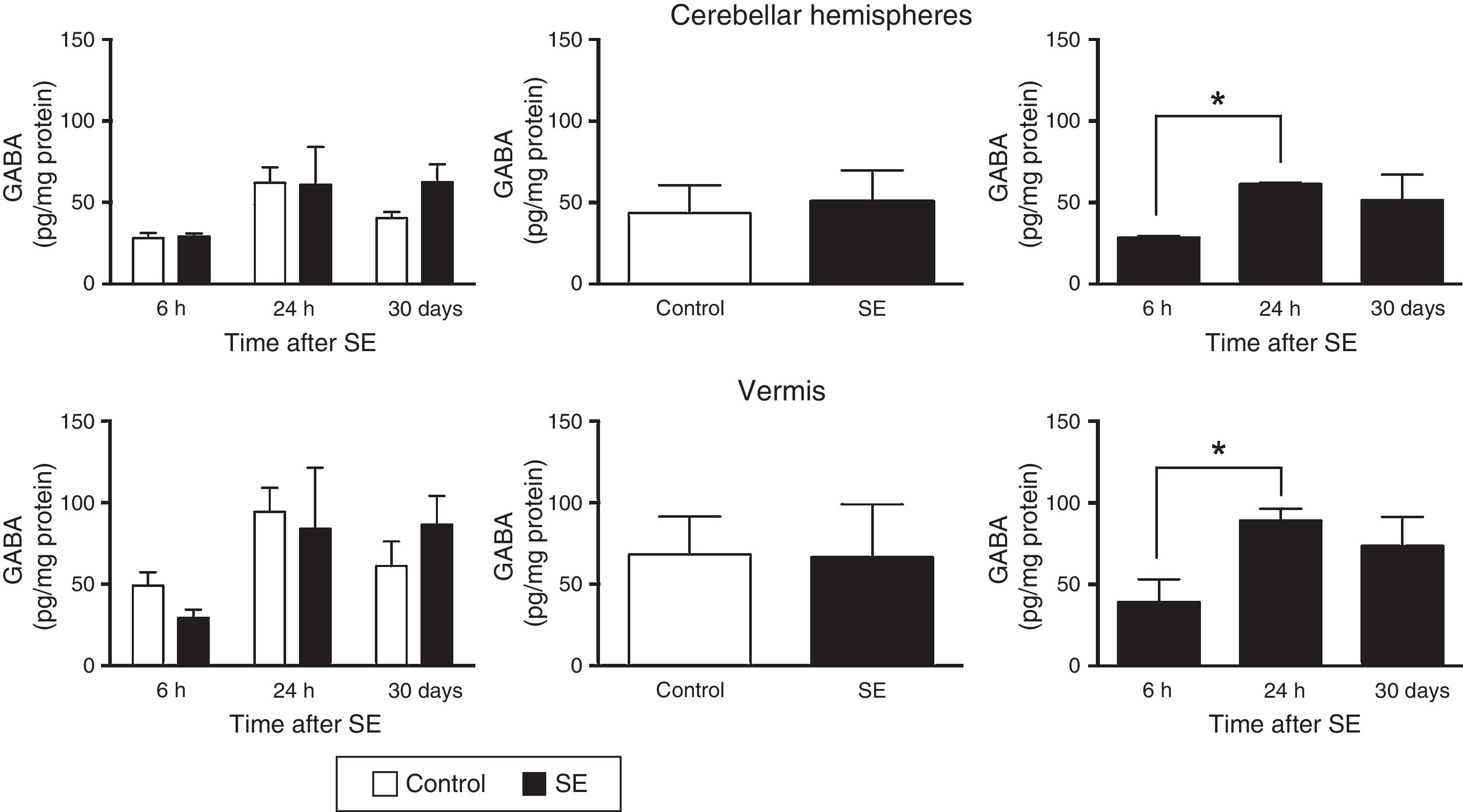
The two-way ANOVA revealed differences in tissue GABA concentrations in the cerebellar hemispheres; these differences were dependent on the assessment time point (F(2,30)=4.47; P=.0199) but not on treatment (F(1,30)=0.645; P=.4282) or the interaction between treatment and assessment time point (F(2,30)=0.651; P=.5299). A similar effect was observed in the cerebellar vermis: statistical analysis revealed differences in tissue GABA concentration, which were dependent on the time of assessment (F(2,30)=3.5; P=.0430) but not on treatment (F(1,30)=0.010; P=.9190) or the interaction between treatment and time of assessment (F(2,30)=0.749; P=.4813). The post hoc test revealed higher GABA concentrations in the cerebellar hemispheres and vermis (P<.05) at 24hours than at 6hours (Fig. 1).
GABA (γ-amino butyric acid) concentration in the cerebellar hemispheres and vermis after status epilepticus (SE). The graphs on the left show the time course of GABA concentration after SE. The graphs in the centre show the effect of treatment (induced SE or sham manipulation) on GABA concentration regardless of time. The graphs on the right show GABA concentration at different time points, regardless of treatment. Bars indicate mean (±SEM) GABA concentration (n=6 rats per group). *P<.05.
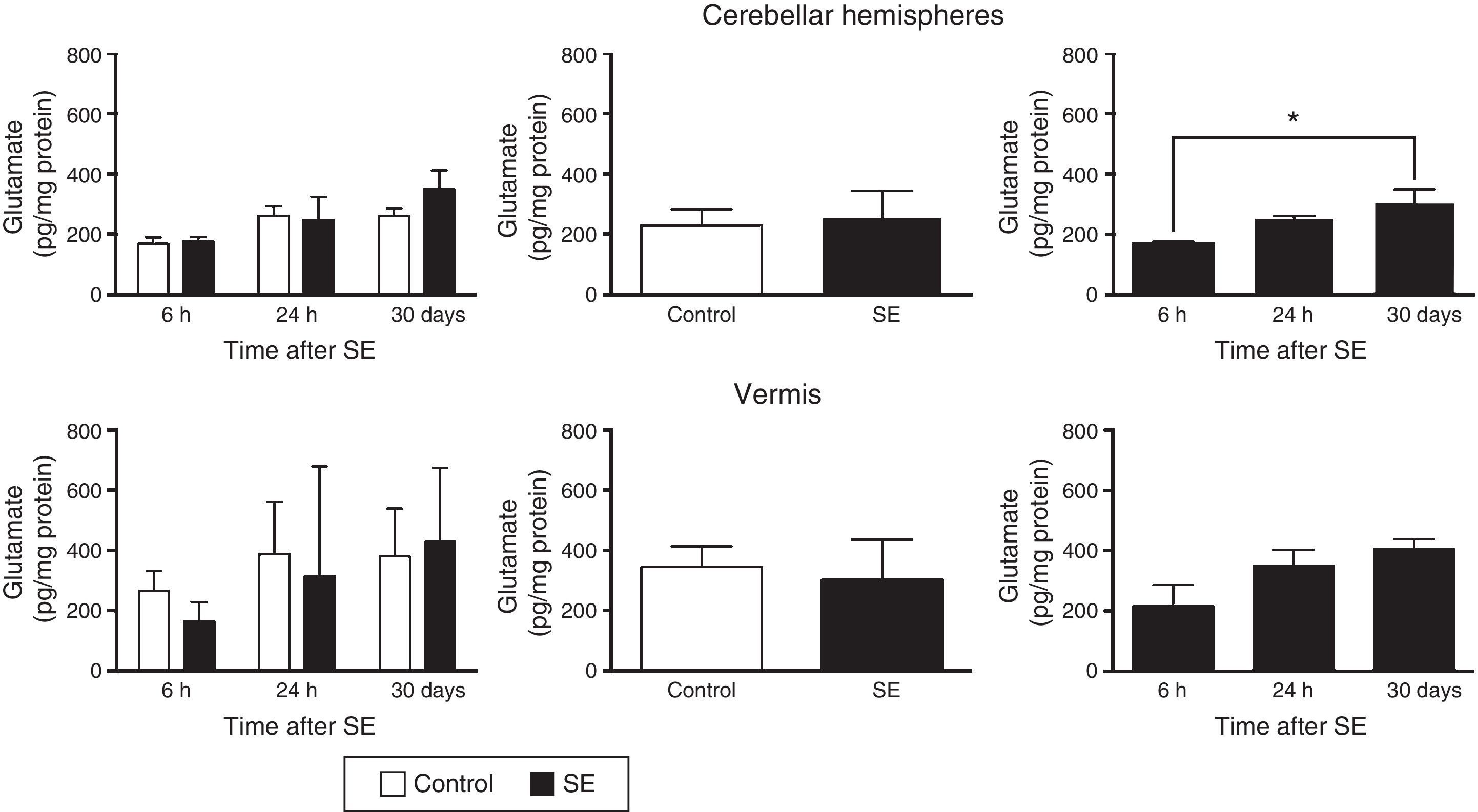
Similarly, the differences in tissue glutamate concentration in the cerebellar hemispheres were found to depend on the time of assessment (F(2,30)=4.37; P=.0216) but not on treatment (F(1,30)=0.556; P=.4615) or the interaction between treatment and time of assessment (F(2,30)=0.710; P=.4995). According to post hoc test results, glutamate concentration in the hemispheres is lower at 6hours than at 30days (P<.05). Glutamate concentration in the cerebellar vermis did not change in relation to time (F(2,30)=2.67; P=.0855), treatment (F(1,30)=371; P=.5473), or the interaction between these 2 factors (F(2,30)=0.44; P=.6481) (Fig. 2).
Glutamate concentration in the cerebellar hemispheres and vermis after status epilepticus (SE). The graphs on the left show the time course of glutamate concentration after SE. The graphs in the centre show the effect of treatment (induced SE or sham manipulation) on glutamate concentration regardless of time. The graphs on the right show glutamate concentration at different time points, regardless of treatment. Bars indicate mean (±SEM) glutamate concentration (n=6 rats per group). *P<.05.
Our study shows that SE induced in 14-day-old rats does not result in either acute or long-term changes in GABA or glutamate concentrations in either the vermis or the hemispheres of the cerebellum.
The causes and consequences of SE have been studied with experimental models mimicking this type of epileptic activity. We used the lithium-pilocarpine model to study SE during rat development as this technique causes motor symptoms as well as neuronal damage in different brain regions.32–35 Our results show that the neurochemistry of GABA and glutamate in the cerebellum of developing rats is not significantly affected by SE either immediately after seizure, 24hours after the episode, or in the long term. Few studies have evaluated the effect of SE on the extracellular concentration of cerebellar amino acids. In a previous study, SE induced in 14-day-old rats did not change GABA or glutamate concentrations in the cerebellar vermis or hemispheres, but did increase alanine, taurine, and glutamine levels in the cerebellar hemispheres 24hours after the episode. This effect was accompanied by a lack of change in radioligand binding to the GABAA receptor in the cerebellar vermis.29 The induction of febrile seizures in 10-day-old rats is known to increase aspartate levels in the cerebellum, whereas the concentration of such other amino acids as GABA and glutamate decreases 1 day after the seizure.28 The fact that SE does not affect GABA or glutamate concentrations suggests that the developing cerebellum resists changes induced by seizures, even seizures lasting several hours. This may be accompanied by changes typically occurring during the development of the rat brain or associated with brain plasticity.36 In fact, the anatomical patterns and the intensity of SE-induced neural damage in rats are known to depend on the age at which SE is induced. Spontaneous epileptic seizures due to SE are less frequent in rats in which SE is induced at the age of 2 weeks than at ages of 3 or 4 weeks.32
According to our results, GABA concentration in the vermis and cerebellar hemispheres was higher when evaluated 24hours after seizure (or after manipulation with saline solution, in the case of control rats), when rats were 14 days old, than 6hours after sample collection, when they were 13 days old. Glutamate concentration was higher 30days after SE or sham manipulation than at 6hours. This suggests that changes may be due to rat cerebellar maturation itself; marked cell proliferation and neurogenesis have been observed to extend until approximately postnatal day 25.37 Furthermore, GABAergic synapses are known to form during the second and third weeks of life.38
In conclusion, this study shows that induced SE causes neither short- nor long-term changes to GABA or glutamate concentration in the vermis and hemispheres of the cerebellum of 14-day-old rats; this may be due to neuroplasticity in this region of the brain.
Conflicts of interestThe authors have no conflicts of interest to declare.
To the Mexican Secretariat of Public Education's Improving Teaching Programme (PROMEP) for supporting MLLM in becoming a full-time professor (PROMEP/103.5/10/5006). To the Mexican Secretariat of Public Education's Institutional Strengthening Integral Program (PIFI) for supporting the neurophysiology study group (UV-CA-333).
Please cite this article as: Hernández-Martínez D, Rocha L, Martínez-Quiroz J, Morgado-Valle C, Manzo J, López-Meraz ML. Evaluación temporal del efecto del status epilepticus inducido en la rata en desarrollo en la concentración cerebelar de ácido γ-aminobutírico y glutamato. Neurología. 2018;33:577–582.