This study evaluated the protective effects of 2 commercial formats of Ginkgo biloba on motor alterations induced by cassava (Manihot esculenta Crantz) juice consumption in male Wistar rats.
MethodsThe effects were evaluated with the open field and swim tests at 0, 7, 14, 21, and 28 days of treatment, one hour after administering the product.
ResultsCompared to controls, open field crossings increased after day 21 of cassava juice consumption, and lateral swimming in the swim test was reported after day 7.
ConclusionGinkgo biloba extracts prevented motor alterations associated with cassava juice consumption, probably due to the flavonoid content in both formats of Ginkgo biloba.
Se evaluó el efecto protector de 2 presentaciones comerciales de Ginkgo biloba sobre las alteraciones motoras inducidas por el consumo de jugo de yuca (Manihot esculenta Crantz) en ratas macho Wistar.
MétodoLos efectos se evaluaron en las pruebas de campo abierto y nado a los 0, 7, 14, 21 y 28 días de tratamiento, una hora después de la administración correspondiente.
ResultadosA partir del día 21 del consumo de jugo de yuca incrementó el número de cuadros cruzados en campo abierto y, en la prueba de nado, produjo el nado lateral a partir del día 7, con respecto al grupo control.
ConclusiónLos extractos de Ginkgo biloba previnieron las alteraciones motoras asociadas al consumo de jugo de yuca, probablemente por el contenido de flavonoides presentes en ambas presentaciones de Ginkgo biloba.
Cassava (Manihot esculenta Crantz), also known as yuca and manioc, is a perennial woody shrub of the Euphorbiaceae family.1 Due to the high starch content of its roots and its ability to adapt to nutrient-poor soils and dry climates, it is widely consumed in tropical regions of Asia, Africa, and the Americas.2 However, cassava contains 2 cyanogenic glucosides: linamarin and lotaustralin.3 When linamarin is exposed to the enzyme linamarase, which is also present in cassava, the glucoside is hydrolysed and hydrocyanic acid is released.4 Inadequate processing and excessive consumption of cassava have been associated with such disorders as hypothyroidism and 2 cyanide-related neurological diseases: tropical ataxic neuropathy (peripheral polyneuropathy associated with sensorineural deafness and optic atrophy) and konzo (spastic paraparesis).5 According to experimental studies on male Wistar rats, cassava juice causes motor hyperactivity in the open-field test and motor alterations in the forced swim test (lateral swimming). An association has been suggested between these alterations and the presence of linamarin in cassava juice, although the action of other compounds in cassava juice cannot be ruled out.6
Ginkgo biloba is a tree of the Ginkgoaceae family which contains several active compounds with biological and pharmacological properties.7 According to several phytochemical studies, Ginkgo biloba leaf extract contains 24% flavonoids, 6% terpenoids, 5% to 10% organic acids, and less than 5% flavonoid-based polymers.8–10Ginkgo biloba leaf extract has been used to manage cerebrovascular accidents due to its antioxidant and membrane stabilising properties.8 It has also been used in patients with Alzheimer disease due to its beneficial effects on attention, memory, and psychomotor function.11Ginkgo biloba leaf extract also has significant antioxidant properties. In a study by Mohanta et al.,12Ginkgo biloba leaf extract was found to protect against oxidation when added to a water-in-oil emulsion. Peroxidation of the lipids present in the said emulsion was delayed by between 24 and 43 hours, depending on the concentration of the antioxidant. Furthermore, treatment with Ginkgo biloba protects neurons from excitotoxicity induced by N-methyl-d-aspartate receptor (NMDAR) overactivation and focal cerebral ischaemia. The neuroprotective effect of Ginkgo biloba may also be linked to its ability to inhibit reactive oxygen species (ROS) and prevent seizures.13 This suggests that Ginkgo biloba leaf extract could prevent motor and cognitive impairment associated with cassava juice consumption; this hypothesis, however, should be further explored.
Several preparations of Ginkgo biloba leaf extract are commercially available12,14; chemical composition varies across standardised and non-standardised preparations. However, there is no scientific evidence of the alleged therapeutic properties of most of these preparations; this constitutes a potential risk to consumers. Exploring the pharmacological effects of non-standardised preparations, and comparing their benefits with those of the preparations marketed by pharmaceutical companies, is therefore essential. The purpose of our study was to evaluate whether treatment with 2 commercial preparations of Ginkgo biloba leaf extract has a protective effect against motor alterations induced by long-term consumption of cassava (Manihot esculenta Crantz) juice in Wistar rats.
MethodsSubjectsWe used 48 adult male Wistar rats weighing around 250g at the beginning of the study. Rats were housed at room temperature in transparent acrylic cages in the vivarium at the biological and pharmaceutical chemistry school at Universidad Veracruzana, with a 12:12 light-dark cycle (lights on at 7:00am). Rats had ad libitum access to food and water. All experiments were carried out in accordance with the official Mexican guidelines for the care and use of laboratory animals (NOM-062-ZOO-1999)15 and the “Guide for the care and use of laboratory animals” (National Research Council, 1996).16
Cassava juice extractionCassava roots were collected in La Defensa, a village in Yecuatla, in the state of Veracruz; the village is located at an altitude of 260m above sea level. Cassava was identified as Manihot esculenta Crantz by Dr Sergio Avendaño Reyes, coordinator of the Herbarium at the Institute of Ecology (INECOL).
Cassava juice was extracted following the procedures described in previous studies. To prevent degradation, juice was extracted daily before administration. Immediately after collection, cassava roots were thoroughly washed, peeled, and cut into sections measuring approximately 5×2×2cm to simplify standard dose calculation. Cassava juice was obtained using a liquidiser (Moulinex Centri III, Celaya, Guanajuato, Mexico) and administered to rats immediately afterwards.6
Ginkgo biloba leaf extractThe first commercial preparation of Ginkgo biloba leaf extract was allopathic drug VASODIL® 25mL (Altana Pharma, Mexico); each mL contains 40mg of dry Ginkgo biloba extract standardised to 9.6mg flavonoid glycosides (quercetin, kaempherol). The second commercial preparation was Ginkgo biloba nutritional supplement, a herbal preparation containing Ginkgo biloba leaf extract (AVANFARMA COHONASA, Mexico).
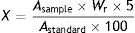
Quantification of flavonoids by spectrophotometryWe refluxed a sample of 1mL of each of the commercial preparations of Ginkgo biloba leaf extract for 2 hours with 20mL sulphuric acid 10% and 20mL ethanol 50%; samples were then cooled and filtered under vacuum. Residues were washed with 30mL ethanol 50%. The filtrate was evaporated to half of the initial volume, cooled down, and filtered again. The precipitate was washed with 40mL distilled water and dissolved in 70mL ethanol 96% to reach a final volume of 100mL. Absorbance was determined at 258nm. The standard solution contained 0.04g quercetin dissolved in ethanol 96% to reach a volume of 50mL; 1mL of this solution was dissolved in 100mL ethanol 50%. The blank solution used was ethanol 50%.17 Calculations were conducted using the following formula:
whereX: total flavonoid content expressed as quercetin (%);Asample: absorbance of the sample solution (nm);Wr: weight of the reference substance (g);Astandard: absorbance of the standard solution (nm).Identification and quantification of quercetin, catechin, and gallic acid using high-performance liquid chromatographyWe used 20mL of the extracts obtained for the previous measurements; these were filtered through a 0.45mm microfilter before being injected. We used high-performance liquid chromatography (HPLC; Varian ProStar 210) module equipped with a UV detector and 2 pumps, and a C18 reversed-phase column (4.6×250mm, 5μm; Agilent, Wilmington, USA). Mobile phase A was water/acetonitrile (80/20) and mobile phase B was HPLC-grade acetonitrile. We used a flow rate of 0.8mL, a wavelength suitable for the compound we were studying and an injection volume of 20μL.18 Flavonoids were quantified with the peak enrichment technique using quercetin, gallic acid, and catechin as standards (Sigma-Aldrich).
Behavioural trialsLocomotor activity testDuring the locomotor activity test, a rat was placed for a period of 5 minutes in an opaque acrylic cage (44×33×20cm) whose base was divided into 12 squares measuring 11×11cm. The test evaluated the number of instances and the duration of vertical behaviour, that is, when the rat stood on its hind legs, and the number of crossed squares (the rat was considered to have crossed a square when at least three quarters of its body passed from one square to another). The latter variable was used to identify or rule out treatment-induced motor changes (hypoactivity, hyperactivity, or no motor changes). Vertical behaviour was assessed to detect any potential alterations in motor coordination.
Forced swim testRats were placed for 5 minutes in a glass tank (26×29×50cm) filled with water at a temperature of 25±1°C. The water level was such that rats could touch the bottom of the tank with their back feet and/or tails. At the beginning of the test, rats were placed in a corner of the tank. Rats began to swim vigorously as soon as they were placed in the water. None of the rats drowned. After this initial swimming behaviour, rats displayed lateral swimming. The dependent variable was the number of times rats displayed lateral swimming during the test. Lateral swimming is defined as a behaviour in which a rat swims slowly on one side, holding its head horizontally, rather than displaying coordinated swimming. The back legs are extended, rigid, and parallel to the water surface for short periods of time and do not move in coordination,6 whereas one or both front legs remain flexed. After lateral swimming, rats eventually display normal swimming behaviour for short periods of time.
All sessions in the open-field and forced swim tests were recorded with a video camera. Two independent observers recorded the studied variables until they reached 95% inter-rater agreement.
Experimental groupsRats were divided into 6 groups. One group (VEH; n=8) received the vehicle (purified water), another group received cassava juice (CAS; n=8) equivalent to 28.56mg cassava per kg of rat weight. A third group received allopathic Ginkgo biloba leaf extract (GINK-AL; n=8), and a fourth group received Ginkgo biloba nutritional supplement (GINK-NUT; n=8). Two further groups received a combination of allopathic Ginkgo biloba leaf extract plus cassava juice (GINK-AL+CAS; n=8) and Ginkgo biloba nutritional supplement plus cassava juice (GINK-NUT+CAS; n=8). We used the minimum effective dose of cassava juice necessary to trigger motor alterations in the open-field and forced swim tests, according to the findings of a previous study.6 All treatments were administered by oral route at a volume of 4mL/kg. Ginkgo biloba leaf extract was dosed at 160mg per kg of rat weight, equivalent to the recommended dose for humans indicated by the supplier, and administered orally. Treatments were administered every day between 9:00 and 9:30am for 28 consecutive days; Ginkgo biloba was administered 30 minutes before cassava juice. Treatment effects on the open-field and forced swim tests were evaluated on days 0, 7, 14, 21, and 28.
Statistical analysisData were analysed with the 2-way ANOVA; factor A was treatment and factor B was days of treatment. Given P-values≤.05 in ANOVA, we applied the post hoc Student-Newman-Keuls test. Results were expressed as the mean±SD.
ResultsFlavonoid contentTable 1 shows the flavonoid content of the 2 commercial formats of Ginkgo biloba leaf extract. No significant differences were found in gallic acid content. Catechin and quercetin content, however, were significantly higher in the allopathic Ginkgo biloba leaf extract. Total flavonoid content was nearly twice as high in the nutritional supplement than in allopathic Ginkgo biloba leaf extract, which may be explained by presence of other types of flavonoids (for example, kaempherol, as reported in other studies).
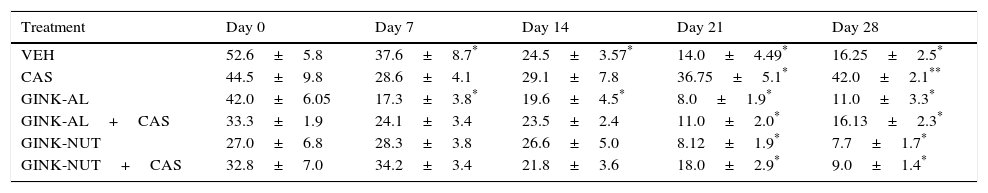
Locomotor activity in the open-field testCrossed squaresWe found significant differences in the number of crossed squares for treatment type (F[5,210]=13.816; P<.001), days of treatment (F[4,210]=17.145; P<.001), and the interaction between factors (F[20,210]=2.44; P<.001). According to the post hoc test, the number of crossed squares did not change significantly compared to the baseline session in the CAS group. On days 21 and 28, however, these rats were observed to cross a greater number of squares than those in the VEH, GINK-AL, and GINK-NUT groups. Furthermore, we observed that the effect of cassava juice on this variable was prevented by both treatment with GINK-AL+CAS and GINK-NUT+CAS (Table 2).
Number of crossed squares in the open-field test.
| Treatment | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| VEH | 52.6±5.8 | 37.6±8.7* | 24.5±3.57* | 14.0±4.49* | 16.25±2.5* |
| CAS | 44.5±9.8 | 28.6±4.1 | 29.1±7.8 | 36.75±5.1* | 42.0±2.1** |
| GINK-AL | 42.0±6.05 | 17.3±3.8* | 19.6±4.5* | 8.0±1.9* | 11.0±3.3* |
| GINK-AL+CAS | 33.3±1.9 | 24.1±3.4 | 23.5±2.4 | 11.0±2.0* | 16.13±2.3* |
| GINK-NUT | 27.0±6.8 | 28.3±3.8 | 26.6±5.0 | 8.12±1.9* | 7.7±1.7* |
| GINK-NUT+CAS | 32.8±7.0 | 34.2±3.4 | 21.8±3.6 | 18.0±2.9* | 9.0±1.4* |
Data are expressed as means±SD for each group (n=8).
GINK-AL: allopathic Ginkgo biloba leaf extract; GINK-AL+CAS: allopathic Ginkgo biloba leaf extract plus cassava juice; GINK-NUT: Ginkgo biloba nutritional supplement; GINK-NUT+CAS: Ginkgo biloba nutritional supplement plus cassava juice; VEH: vehicle; CAS: cassava juice.
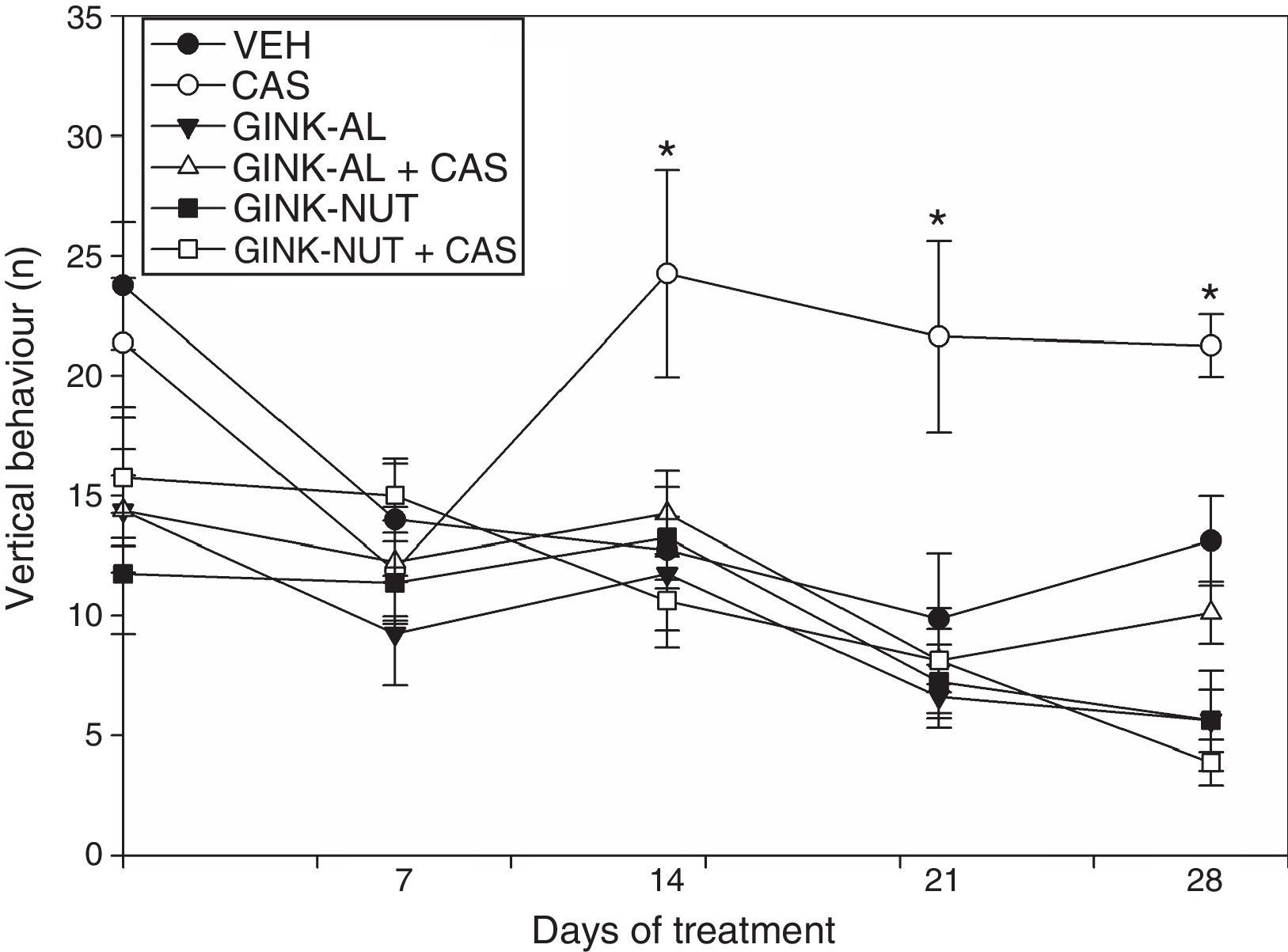
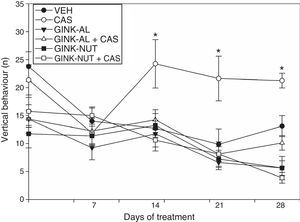
The analysis of vertical behaviour revealed significant differences for treatment type (F[5,210]=12.95; P<.001), days of treatment (F[4,210]=8.21; P<.001), and the interaction between factors (F[20,210]=1.719; P<.001). The post hoc test revealed no significant change in the number of instances of vertical behaviour throughout the study in the CAS group. On day 14, however, rats receiving cassava juice were observed to cross a greater number of squares than those in the VEH, GINK-AL, and GINK-NUT groups. Again, we observed that the effect of cassava juice on this variable was prevented by both treatment with GINK-AL+CAS and GINK-NUT+CAS (Fig. 1).
Vertical behaviour in the open-field test. The group receiving cassava juice displayed a similar number of instances of vertical behaviour throughout the study. This effect was prevented with Ginkgo biloba leaf extract.
*P<.05 when comparing the CAS group to the remaining groups for the same day of treatment.
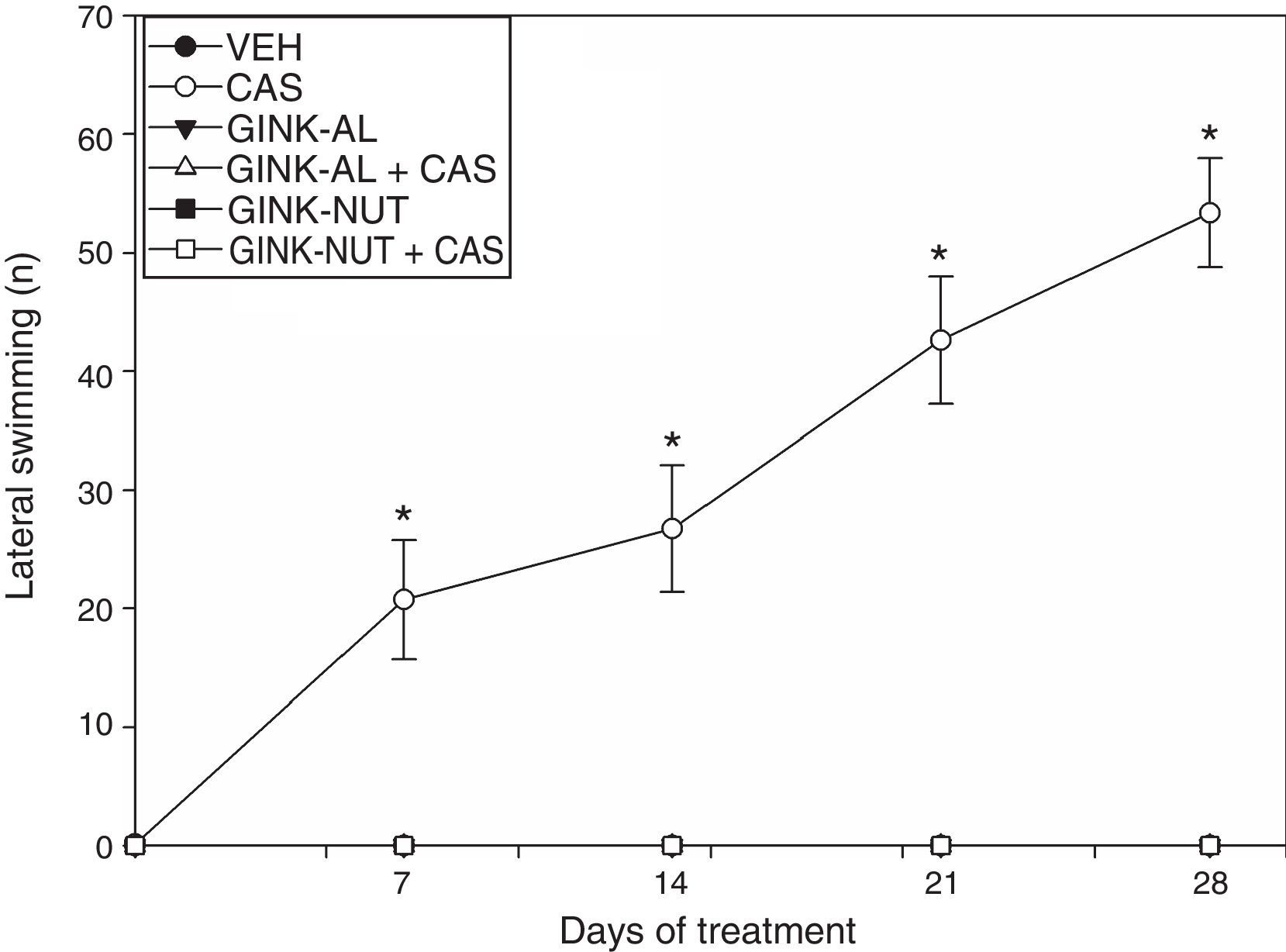
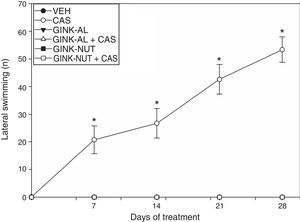
Only the rats receiving cassava juice displayed lateral swimming. Statistical analysis of this variable revealed significant differences by treatment type (F[5,220]=237.76; P<.001), days of treatment (F[4,210]=24.256; P<.001), and the interaction between factors (F[20,210]=24.325; P<.001) (Fig. 2). According to the post hoc test, the CAS group displayed a significantly greater number of instances of lateral swimming behaviour on day 7 and onwards, compared to the baseline results and the results of the VEH group for the same sessions. The effect of cassava juice on this variable was not observed when cassava juice was administered in combination with either of the commercial presentations of Ginkgo biloba leaf extract (Fig. 2).
DiscussionThe open-field test is used to assess motor activity, an innate and specific behaviour dependent on central nervous system maturation and the preservation of the motor pathways that control movement.19,20 In these tests, animals move in response to environmental stimuli or pharmacological treatments that inhibit or promote motor activity.19,20 Rats repeatedly evaluated with the open-field test have been found to explore less due to habituation to their setting.21 Habituation is determined by a decrease in the number of squares crossed and other behaviours associated with exploration, such as vertical behaviour.22 Animals experiencing stress or receiving such neurotoxic substances as methylazoxymethanol (an active ingredient of the cycad Dioon spinulosum) or linamarin (present in cassava juice) do not become acclimated to the test conditions6,22; this appears to be associated with alterations in neurotransmission pathways and systems involved in movement and locomotor control. This seems to be associated with increased release of free radicals at the neuronal level.19,23
We found that rats consuming cassava juice displayed motor hyperactivity in the open-field test; this is consistent with results from previous studies.6 These alterations in motor activity would appear to be associated with the toxicity of the linamarin present in the cassava.6 The vertical behaviour displayed during the open-field test constitutes a response to novel stimuli and involve short- and long-term memory consolidation.24 In our sample, the CAS group was the only one to display a consistent level of vertical behaviour throughout the study. This suggests that the CAS group rats had memory deficits: they failed to recognise the setting and therefore explored the cage as though it were the first time they had been exposed to these conditions. This hypothesis is based on the fact that humans consuming cassava or cassava derivatives display memory impairment in addition to motor alterations.6,25 Rats receiving cassava juice plus either of the commercial formats of Ginkgo biloba leaf extract displayed no behavioural alterations in the open-field test, suggesting that Ginkgo biloba may have a protective effect; this will be addressed later on.
The forced swim test has mainly been used to evaluate the effects of antidepressant substances.26 However, it has also been used to study motor alterations associated with the consumption of substances which are either damaging to the vestibular system27,28 or neurotoxic.6,22,29 Long-term consumption of Dioon spinulosum seeds or microinjections of methylazoxymethanol (one of its active ingredients) result in abnormal swimming (spinning).22,29 A previous study showed that cassava juice promotes lateral swimming, which has been suggested as an indicator of impaired motor coordination.6 In the present study, rats receiving cassava juice were the only ones to display lateral swimming, confirming the association between consumption of cassava metabolites (linamarin) and motor impairment. Linamarin metabolism produces cyanide. Cyanide triggers a series of biochemical changes in the brain and oxidative stress, leading to neuronal death; this has been associated with motor function alterations.30 Cyanide exposure results in neurodegeneration of the spinal cord and CNS ganglia and inhibits the electron transport chain, generating ROS which are responsible for oxidative stress, a mechanism involved in neurodegeneration and motor and cognitive impairment, among other processes.31–33 As in the open-field test, motor alterations in the forced swim test were prevented by administering Ginkgo biloba extract, which has been suggested to have a neuroprotective effect.34
Although we did not explore the mechanism behind the protective effect of Ginkgo biloba extracts against motor alterations associated with the consumption of cassava juice, we suspect that it may be due to their high concentration of flavonoids and sesquiterpene lactones. These compounds act as neuroprotective agents as they prevent the neuronal damage induced by excitotoxicity resulting from NMDAR overactivation. Furthermore, flavonoids act as antioxidant agents, inhibiting the formation of the radicals hidroxile and adriamicile by 65% and 50%, respectively, and consequently leading to reduced lipid peroxidation.35,36Ginkgo biloba extract has also been found to reduce neuroinflammation and promote memory, learning, and cognitive function by decreasing the rate of muscarinic and α-adrenergic receptor disappearance, in addition to increasing choline acetyltransferase and somatostatin levels in the cortex and hippocampus.13,37,38 Considering that both presentations of Ginkgo biloba leaf extract had a high concentration of flavonoids, we hypothesise that Ginkgo biloba may have a protective effect against toxicity associated with the neurotoxic metabolites present in cassava, preventing the motor alterations described in this study.
In conclusion, standardised Ginkgo biloba leaf extract prevents the motor alterations associated with consumption of cassava juice (Manihot esculenta Crantz) in Wistar rats. Our findings may pave the way for prevention and treatment strategies for motor alterations associated with consumption of cassava or cassava derivatives in vulnerable populations.
FundingThis study was partially supported by the study group for the biology, chemistry, and molecular functionality of vegetable metabolites (UV-CA-368) at Universidad Veracruzana.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rivadeneyra-Domínguez E, Vázquez-Luna A, Rodríguez-Landa JF, Mérida-Portilla CV, Díaz-Sobac R. Efecto protector de 2 presentaciones comerciales de Ginkgo biloba sobre las alteraciones motoras inducidas por el jugo de yuca (Manihot esculenta Crantz) en la rata Wistar. Neurología. 2017;32:516–522.