To update the recommendations of the Spanish Society of Neurology on primary and secondary stroke prevention in patients with arterial hypertension.
DevelopmentWe proposed several questions to identify practical issues for the management of blood pressure (BP) in stroke prevention, analysing the objectives of blood pressure control, which drugs are most appropriate in primary prevention, when antihypertensive treatment should be started after a stroke, what levels we should aim to achieve, and which drugs are most appropriate in secondary stroke prevention. We conducted a systematic review of the PubMed database and analysed the main clinical trials to address these questions and establish a series of recommendations.
ConclusionsIn primary stroke prevention, antihypertensive treatment should be started in patients with BP levels > 140/90 mmHg, with a target BP of < 130/80 mmHg. In secondary stroke prevention, we recommend starting antihypertensive treatment after the acute phase (first 24 hours), with a target BP of < 130/80 mmHg. The use of angiotensin-II receptor antagonists or diuretics alone or in combination with angiotensin-converting enzyme inhibitors is preferable.
Actualizar las recomendaciones de la Sociedad Española de Neurología para la prevención de ictus, tanto primaria como secundaria, en pacientes con hipertensión arterial.
DesarrolloSe han planteado diferentes preguntas para identificar cuestiones prácticas para el manejo de la presión arterial (PA) en prevención de ictus, analizando cuál debe ser el objetivo de control de la presión arterial y cuáles son los fármacos más adecuados en prevención primaria, cuándo iniciar el tratamiento antihipertensivo después de un ictus, cuáles son las cifras que debemos alcanzar y qué fármacos son los más adecuados en prevención secundaria de ictus. Se ha realizado una revisión sistemática en Pubmed analizando los principales ensayos clínicos para dar respuesta a estas preguntas y se han elaborado unas recomendaciones.
ConclusionesEn prevención primaria se recomienda iniciar tratamiento antihipertensivo con cifras de PA > 140/90 mmHg, con un objetivo de control de PA < 130/80 mmHg. En prevención secundaria de ictus se recomienda iniciar tratamiento antihipertensivo pasada la fase aguda (primeras 24 h) con un objetivo de control de PA < 130/80 mmHg, siendo preferible el empleo de ARA-II o diuréticos solos o en combinación con IECA.
Arterial hypertension is the most prevalent modifiable risk factor for stroke, and one of the most significant factors contributing to the development of the disease. Furthermore, it is associated with greater stroke severity and poorer outcomes. Controlling blood pressure reduces the risk of stroke in primary prevention, but can also reduce the risk of recurrence following a first stroke. In this consensus statement, we review the effect of arterial hypertension on stroke risk and the objectives of blood pressure control both in primary and in secondary stroke prevention.
MethodsWe addressed a series of questions identifying practical issues regarding blood pressure management in stroke prevention. We analysed: 1) target blood pressure values in primary prevention; 2) the most appropriate drugs in primary prevention; 3) when to begin antihypertensive treatment after a stroke; 4) target blood pressure values in secondary prevention; and 5) the most appropriate drugs in secondary prevention. In order to respond to these questions, we conducted a systematic review of the PubMed database and analysed the main clinical trials and meta-analyses, and drafted a series of recommendations. For our recommendation on when to begin antihypertensive treatment, we excluded studies addressing blood pressure management in patients receiving reperfusion therapy, which we considered to be part of acute treatment.
Evidence was classified as level A (high-quality evidence from more than one randomised trial, meta-analyses of high-quality clinical trials, or randomised clinical trial data corroborated by high-quality registry studies), level B (moderate-quality evidence, based on one or more randomised clinical trials; one or more non-randomised, observational, or high-quality registry studies; meta-analyses of moderate-quality clinical trials; or meta-analyses of non-randomised studies), or level C (limited evidence, with data from observational studies or registry studies presenting methodological limitations in design or execution). Grades of recommendation were as follows: class I (strong recommendation: benefits far greater than risk), class IIa (moderate recommendation: benefit outweighs risk), class IIb (weak recommendation), and class III (no benefit: benefit is equal to risk).1
Blood pressure management in primary preventionThe definition of arterial hypertension has changed over the years in the light of growing evidence on the effect of blood pressure on vascular risk. While in the 1950s arterial hypertension was defined as systolic blood pressure (SBP) > 180 mm Hg and diastolic blood pressure (DBP) > 100 mm Hg, lower values are considered in current definitions: blood pressure is now considered normal when SBP is below 120 mm Hg and DBP is below 80 mm Hg. We refer to arterial hypertension in patients with values greater than 130/80 mm Hg, defining stage 1 hypertension as SBP between 130 and 139 mm Hg or DBP between 80 and 89 mm Hg and stage 2 hypertension as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg. SBP values between 120 and 129 mm Hg are considered high.2
Arterial blood pressure values are directly associated with vascular risk. One observational study including a million individuals showed that above 115/75 mm Hg, every increment of 20 mm Hg in SBP or 10 mm Hg in DBP doubled the risk of death due to stroke or ischaemic heart disease.3 It is also well demonstrated that controlling blood pressure decreases the risk of vascular disease. A meta-analysis conducted in 2016, including over 600 000 patients, demonstrated that every decrease of 10 mm Hg in SBP was associated with a 20% decrease (relative risk [RR]: 0.80; 95% confidence interval [CI], 0.77-0.83) in the risk of severe vascular events (acute myocardial infarction, vascular death, coronary revascularisation, stroke, or heart failure), a 17% decrease in the risk of ischaemic heart disease (RR: 0.83; 95% CI, 0.78-0.88), a 27% decrease in the risk of ischaemic stroke (RR: 0.73; 95% CI, 0.68-0.77), and a 13% decrease in all-cause mortality (RR: 0.87; 95% CI, 0.84-0.91).4
While good control of arterial pressure is known to decrease the risk of stroke and other vascular diseases, it is important to establish target values for primary prevention, and to identify which drugs are most effective in reducing blood pressure.
What are the target blood pressure values in primary prevention?Various studies have demonstrated that controlling blood pressure reduces the risk of stroke and other vascular diseases in patients with arterial hypertension. In 2014, a meta-analysis analysed individual data from patients included in clinical trials evaluating different antihypertensive drugs against placebo, or comparing intensive blood pressure control strategies against more conservative strategies. The main objective of the study was to analyse the risk of vascular events, defined as stroke, acute myocardial infarction, heart failure, or vascular death.5 The meta-analysis included 11 clinical trials with a total of 67 475 participants, who were stratified into 4 groups according to the estimated risk of presenting a vascular event in 5 years: group 1 (< 11%), group 2 (11%-15%), group 3 (15%-21%), and group 4 (> 21%). Treatment to control blood pressure reduced the relative risk of vascular events in all groups (18% in group 1, 15% in group 2, 13% in group 3, and 15% in group 4).
While controlling blood pressure is known to be beneficial for reducing vascular risk, target blood pressure values in primary prevention are subject to controversy. The Systolic Hypertension in the Elderly Program (SHEP) study aimed to establish the effect of antihypertensive treatment on stroke risk in primary prevention in patients with SBP > 160 mm Hg.6 A diuretic (chlortalidone) was compared against placebo, with a follow-up period of 4.5 years. In individuals receiving the diuretic, SBP values decreased to 143 mm Hg, whereas the placebo group achieved values of 155 mm Hg. This effect translated to a 36% reduction in the relative risk of stroke in the treatment group. The authors also observed a 27% reduction in the relative risk of acute myocardial infarction and a 13% reduction in the relative risk of all-cause mortality. Another study found that in patients older than 80 years with SBP higher than 160 mm Hg, the use of indapamide (alone or in combination with perindopril) to reduce blood pressure below 150/80 mm Hg achieved a 30% reduction in the RR of stroke, a 39% reduction in the RR of fatal stroke, and a 21% reduction in all-cause mortality, compared to placebo.7
A meta-analysis conducted in 2011 aimed to establish the blood pressure values at which antihypertensive treatment should be started.8 The study analysed the effect of blood pressure control in a total of 201 566 participants included in 22 clinical trials; participants were grouped according to baseline SBP: < 140, 140-159, 160-179, and ≥ 180 mm Hg. All groups achieved a reduction in the relative risk of vascular disease: a 15% reduction in the SBP < 140 mm Hg group, 18% in the 140-159 mm Hg group, 20% in the 160-179 mm Hg group, and 35% in the ≥ 180 mm Hg group. The differences between groups in the magnitude of the reduction were not statistically significant (P = .17); use of different cut-off SBP/DBP values also did not identify significant differences. Furthermore, no significant differences were found between groups in the magnitude of the reduction in risk for each mm Hg of reduction in SBP or DBP values, indicating that the benefit was similar in all groups.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial compared the effect of intensive and standard blood pressure control (target SBP values < 120 mm Hg and < 140 mm Hg, respectively) in patients with diabetes.9 The study found no significant difference between groups in the primary outcome, a composite of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death (hazard ratio [HR]: 0.88; 95% CI, 0.73–1.06; P = .20) or for all-cause mortality (HR: 1.07; 95% CI, 0.85–1.35; P =.55). However, the authors did report a 41% reduction in the risk of stroke in patients receiving the intensive treatment (HR: 0.59; 95% CI, 0.39–0.89; P = .01).
The Systolic Blood Pressure Intervention Trial (SPRINT) also analysed whether intensive blood pressure control reduces the risk of vascular disease; this study excluded patients with diabetes.10 The trial included 9361 individuals with high vascular risk, without history of stroke, and with SBP values greater than 130 mm Hg. Participants were randomly allocated to receive intensive treatment (target SBP < 120 mm Hg) or standard treatment (target SBP < 140 mm Hg). The primary outcome variable was a composite of acute myocardial infarction, coronary disease, stroke, heart failure, and death from cardiovascular causes. This outcome occurred in 1.65% of the intensive treatment group and 2.19% of the standard treatment group; therefore, the intensive treatment reduced risk by 25% (HR: 0.75; 95% CI, 0.64–0.89; P < .001). The intensive treatment group also presented a 27% reduction in mortality (HR: 0.73; 95% CI, 0.60–0.90; P = .003). In the subgroup of participants older than 75 years, the primary outcome variable decreased by 35% and mortality decreased by 33% in the intensive treatment group.11
In 2016, a meta-analysis of 16 clinical trials with a total of 52 235 participants analysed the effect of intensive blood pressure control on vascular risk.12 Globally, the intensive treatment reduced the relative risk of stroke by 29%, ischaemic heart disease by 20%, and vascular death by 21%. However, different studies used different target blood pressure values; therefore, the authors stratified the effect of treatment according to the SBP values achieved: < 130 vs ≥ 130 mm Hg; 130-139 vs ≥ 140 mm Hg; and 140-149 vs ≥ 150 mm Hg. In all groups, intensive treatment achieved a reduction in the risk of stroke, ischaemic heart disease, and vascular death. While no significant differences were observed between groups in the magnitude of the reduction in relative risk, the reduction in absolute risk was smaller in the < 130 mm Hg group than in the ≥ 130 mm Hg group, suggesting that patients with lower initial SBP values present lower vascular risk.
Recommendations:
- 1
We recommend starting antihypertensive treatment for primary stroke prevention in patients with blood pressure values greater than 140/90 mm Hg (class I recommendation, level of evidence A).
- 2
In patients with arterial hypertension, target blood pressure values should be < 130/80 mm Hg (class I recommendation, level of evidence A).
Different antihypertensive drugs have been studied in the prevention of vascular events, including diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), and calcium channel blockers. The results of the main clinical trials are evaluated in a 2018 meta-analysis comparing different antihypertensive drugs in primary prevention.13 Overall, no statistically significant differences were found between drugs with respect to the prevention of acute myocardial infarction or death due to vascular causes. The study only observed a trend towards greater vascular mortality in patients receiving beta-blockers, compared to those using thiazide diuretics (odds ratio [OR]: 1.20; 95% CI, 0.98-1.40) or calcium channel blockers (OR: 1.2; 95% CI, 0.98-1.40). The specific analysis of stroke risk found that beta-blockers were associated with a 30% increase in the risk of stroke when compared to thiazide diuretics (OR: 1.30; 95% CI, 1.1-1.6), and a 40% increase when compared to calcium channel blockers (OR: 1.4; 95% CI. 1.1-1.7). No differences were observed between ACE inhibitors and ARBs and the remaining classes of antihypertensive drugs.
A recent study aimed to establish when antihypertensive treatment should be administered.14 The study included 19 084 patients with arterial hypertension, who were randomly allocated to take an antihypertensive drug either when going to bed or upon waking. The primary outcome variable was vascular risk, defined as vascular death, acute myocardial infarction, coronary revascularisation, heart failure, or stroke. Patients taking the drug at night presented 45% less vascular risk (OR: 0.55; 95% CI, 0.50-0.61). In the specific analysis of cerebrovascular disease, night-time administration was associated with a 49% reduction in the relative risk of stroke (OR: 0.51; 95% CI, 0.41-0.63).
Recommendations:
- 1
It is unclear which drugs are the most appropriate for lowering blood pressure in the primary prevention of stroke. As an initial treatment, it is reasonable to use thiazide diuretics and calcium channel blockers rather than beta-blockers (class I recommendation, level of evidence A).
- 2
Taking antihypertensive medications at night may be helpful in reducing the risk of stroke and other vascular diseases (class IIa recommendation, level of evidence B).
Most patients present increased arterial blood pressure during the acute phase of stroke,15 with a subsequent progressive decrease to baseline levels in the following days.16 Observational studies have shown that blood pressure values during acute stroke present a U-shaped relationship with stroke outcomes: both the high and low extreme values are associated with poorer functional prognosis, with a higher percentage of early neurological deterioration, stroke recurrence, and mortality.17,18 This effect may be explained by the fact that high blood pressure increases the risk of oedema and haemorrhagic transformation,19 whereas low pressure may reduce perfusion of the ischaemic area.20 It may also be the result of an increase in the inflammatory response (associated with poor prognosis) that is observed during acute stroke in patients with high blood pressure values but no previous diagnosis of arterial hypertension.21
These findings raise the question of when antihypertensive treatment should be started after stroke, what target blood pressure values we should establish in secondary prevention of stroke, and whether any specific drug is most appropriate for lowering blood pressure after stroke.
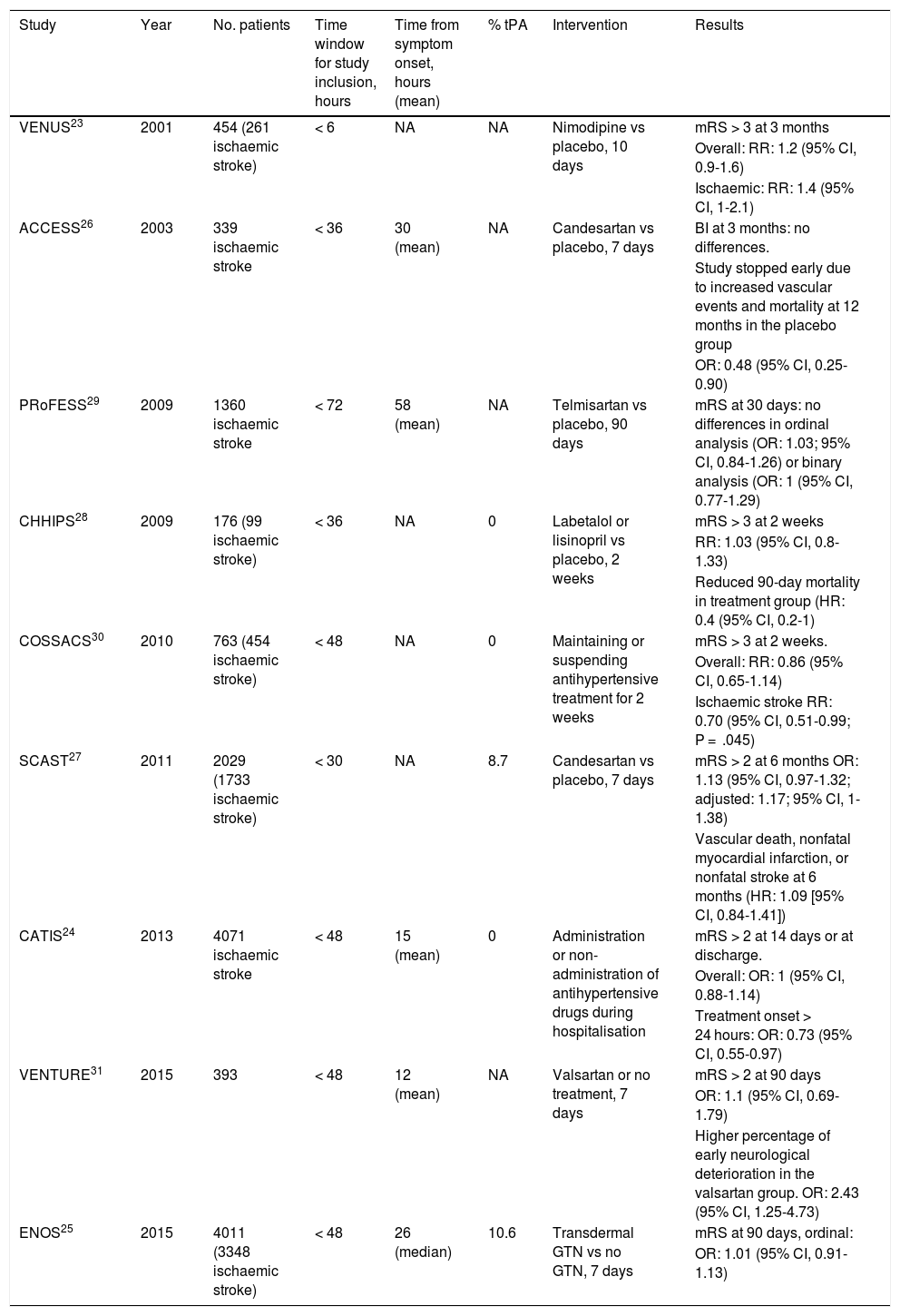
When should antihypertensive treatment be started after stroke?With the exception of blood pressure management in the context of reperfusion therapies, most of the data available to answer this question are from studies into the effect of antihypertensive treatment during the first 72 hours after stroke (Table 1); however, some of these studies also include patients with haemorrhagic stroke and a very small percentage of patients who underwent thrombolysis.
Summary of the main studies assessing the early impact of antihypertensive treatment.
| Study | Year | No. patients | Time window for study inclusion, hours | Time from symptom onset, hours (mean) | % tPA | Intervention | Results |
|---|---|---|---|---|---|---|---|
| VENUS23 | 2001 | 454 (261 ischaemic stroke) | < 6 | NA | NA | Nimodipine vs placebo, 10 days | mRS > 3 at 3 months |
| Overall: RR: 1.2 (95% CI, 0.9-1.6) | |||||||
| Ischaemic: RR: 1.4 (95% CI, 1-2.1) | |||||||
| ACCESS26 | 2003 | 339 ischaemic stroke | < 36 | 30 (mean) | NA | Candesartan vs placebo, 7 days | BI at 3 months: no differences. |
| Study stopped early due to increased vascular events and mortality at 12 months in the placebo group | |||||||
| OR: 0.48 (95% CI, 0.25-0.90) | |||||||
| PRoFESS29 | 2009 | 1360 ischaemic stroke | < 72 | 58 (mean) | NA | Telmisartan vs placebo, 90 days | mRS at 30 days: no differences in ordinal analysis (OR: 1.03; 95% CI, 0.84-1.26) or binary analysis (OR: 1 (95% CI, 0.77-1.29) |
| CHHIPS28 | 2009 | 176 (99 ischaemic stroke) | < 36 | NA | 0 | Labetalol or lisinopril vs placebo, 2 weeks | mRS > 3 at 2 weeks |
| RR: 1.03 (95% CI, 0.8-1.33) | |||||||
| Reduced 90-day mortality in treatment group (HR: 0.4 (95% CI, 0.2-1) | |||||||
| COSSACS30 | 2010 | 763 (454 ischaemic stroke) | < 48 | NA | 0 | Maintaining or suspending antihypertensive treatment for 2 weeks | mRS > 3 at 2 weeks. |
| Overall: RR: 0.86 (95% CI, 0.65-1.14) | |||||||
| Ischaemic stroke RR: 0.70 (95% CI, 0.51-0.99; P = .045) | |||||||
| SCAST27 | 2011 | 2029 (1733 ischaemic stroke) | < 30 | NA | 8.7 | Candesartan vs placebo, 7 days | mRS > 2 at 6 months OR: 1.13 (95% CI, 0.97-1.32; adjusted: 1.17; 95% CI, 1-1.38) |
| Vascular death, nonfatal myocardial infarction, or nonfatal stroke at 6 months (HR: 1.09 [95% CI, 0.84-1.41]) | |||||||
| CATIS24 | 2013 | 4071 ischaemic stroke | < 48 | 15 (mean) | 0 | Administration or non-administration of antihypertensive drugs during hospitalisation | mRS > 2 at 14 days or at discharge. |
| Overall: OR: 1 (95% CI, 0.88-1.14) | |||||||
| Treatment onset > 24 hours: OR: 0.73 (95% CI, 0.55-0.97) | |||||||
| VENTURE31 | 2015 | 393 | < 48 | 12 (mean) | NA | Valsartan or no treatment, 7 days | mRS > 2 at 90 days |
| OR: 1.1 (95% CI, 0.69-1.79) | |||||||
| Higher percentage of early neurological deterioration in the valsartan group. OR: 2.43 (95% CI, 1.25-4.73) | |||||||
| ENOS25 | 2015 | 4011 (3348 ischaemic stroke) | < 48 | 26 (median) | 10.6 | Transdermal GTN vs no GTN, 7 days | mRS at 90 days, ordinal: |
| OR: 1.01 (95% CI, 0.91-1.13) |
BI: Barthel Index; CI: confidence interval; GTN: glyceryl trinitrate; HR: hazard ratio; mRS: modified Rankin Scale; NA: not available; OR: odds ratio; RR: relative risk.
A 2015 meta-analysis of 13 studies assessing the effect of early blood pressure control, including a total of 12 703 patients with acute stroke, found that starting antihypertensive treatment in the first 3 days after the event was not associated with poorer functional outcomes at 3 months (OR: 1.04; 95% CI, 0.96-1.13).22
The earliest treatment was reported in the Very Early Nimodipine Use in Stroke (VENUS) trial; in a sample of 454 patients, the study compared a 10-day course of nimopidine against placebo, with treatment onset within 6 hours of symptom onset.23 The authors found no significant differences in functional prognosis at 3 months (RR: 1.2; 95% CI, 0.9-1.6).
The largest studies include the China Antihypertensive Trial in Ischemic Stroke (CATIS) and the Efficacy of Nitric Oxide in Stroke (ENOS) trial. The CATIS trial studied the effect of starting antihypertensive treatment within 48 hours of symptom onset in a sample of over 4000 patients with acute ischaemic stroke.24 Globally, starting antihypertensive treatment did not modify functional prognosis at 3 months (OR: 0.99; 95% CI, 0.86-1.15). However, starting antihypertensive treatment between 24 and 48 hours was associated with an increased likelihood of good functional prognosis at 3 months (OR: 0.73; 95% CI, 0.55-0.97).
The ENOS study included 4011 patients (3348 with ischaemic stroke), and also studied the effect of treatment onset within 48 hours of stroke.25 The study compared transdermal glyceryl trinitrate against conventional treatment, finding no differences between treatments in the ordinal analysis of modified Rankin Scale (mRS) scores at 90 days.
The Acute Candesartan Ciletexil Therapy in Stroke Survivors (ACCESS) study compared a 7-day course of candesartan against placebo in patients with ischaemic stroke.26 Patients were recruited within 36 hours of treatment onset, and while the trial found no differences in the primary endpoint, dependence at 3 months, it was terminated prematurely when 339 patients had been recruited due to increased prevalence of vascular events (9.8% vs 18.7%; P = .026) and 12-month mortality (2.9% vs 7.2%; P = .07) in the placebo group. However, these results were not replicated in the Scandinavian Candesartan Acute Stroke Trial (SCAST), a subsequent study including 2029 patients (1733 with ischaemic stroke) recruited within 30 hours of symptom onset, which also evaluated the effect of a 7-day course of candesartan against placebo.27 The study was stopped prematurely due to slow recruitment and a lack of funding. This trial used 2 co-primary effect variables: mRS at 6 months and a composite endpoint of vascular death, nonfatal myocardial infarction, and nonfatal stroke at 6 months. Neither variable showed significant differences between groups. Although an ordinal analysis of mRS scores adjusted for age, type of stroke, clinical severity, and SBP showed a trend in favour of placebo, the difference was not statistically significant when 2 co-primary effect variables were used. Among the secondary effect variables, stroke progression was observed in a higher percentage of patients receiving candesartan (6% vs 4%; P = .04).
The Controlling Hypertension and Hypotension Immediately Post-Stroke (CHHIPS) study randomly allocated 176 patients (99 with ischaemic stroke) to receive a 2-week course of either antihypertensive treatment with labetalol and/or lisinopril, or placebo, starting within 36 hours of stroke onset.28 The primary endpoint, mRS score > 3 at 2 weeks, showed no differences between groups; however, the group receiving antihypertensive treatment showed a lower 90-day mortality rate.
The Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) study included a subanalysis comparing telmisartan administered in the first 72 hours after stroke onset against placebo, finding no difference between groups in functional prognosis at 3 months (OR: 1.03; 95% CI, 0.84-1.26).29
The multicentre Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS) included 763 patients with acute stroke (454 with ischaemic stroke) who were receiving antihypertensive treatment prior to the cerebrovascular event.30 The study evaluated the effect of maintaining or stopping treatment in the first 2 weeks after stroke in patients recruited within 48 hours of symptom onset. The trial had to be suspended early due to slow recruitment and a lack of funding; however, continuing antihypertensive treatment did not modify the risk of functional dependence at 3 months (RR: 0.86; 95% CI, 0.65–1.14; P = .30). The study also found no differences between groups in vascular risk, mortality at 6 months, or number of adverse events.
The Valsartan Efficacy on Modest Blood Pressure Reduction in Acute Ischemic Stroke (VENTURE) study randomly allocated 393 patients with ischaemic stroke to receive a 7-day course of valsartan or placebo.31 The primary outcome variable was mRS score at 90 days; no significant differences were found. However the researchers did observe a higher rate of early neurological deterioration in the valsartan group (OR: 2.43; 95% CI, 1.25-4.73).
Recommendations:
- 1
In patients with ischaemic stroke, antihypertensive treatment should be started after the first 24 hours following stroke onset (class I recommendation, level of evidence A).
- 2
Onset of antihypertensive treatment between 24 and 72 hours after onset is safe and may be appropriate (class I recommendation, level of evidence B).
- 3
In patients who were receiving antihypertensive treatment prior to stroke, treatment may be resumed between 24 and 48 hours after stroke onset (class I recommendation, level of evidence B).
While various studies have shown that lowering blood pressure reduces the risk of stroke recurrence, it is important to know what blood pressure values should move us to start antihypertensive treatment, and what values we should aim to achieve.
The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) included 6015 patients with or without arterial hypertension who had presented stroke in the previous 5 years and were randomly allocated to receive perindopril with or without indapamide, or placebo.32 Mean follow-up time was 3.9 years, and the study’s primary objective was to analyse stroke recurrence. Globally, antihypertensive treatment reduced the risk of stroke recurrence by 28%. The analysis of patient subgroups defined according to baseline SBP values found that the treatment reduced stroke recurrence in patients with values greater than 140 mm Hg. For baseline SBP between 120 and 139 mm Hg, a non-significant trend towards a beneficial effect was observed, and no effect in stroke prevention was observed for patients with values below 120 mm Hg.
Later studies were included in a meta-analysis published in 2009, which demonstrated that antihypertensive treatment was able to reduce stroke recurrence in patients with SBP greater than 130 mm Hg (OR: 0.75; 95% CI, 0.62-0.89) and DBP greater than 70 mm Hg at baseline (OR: 0.64; 95% CI, 0.50-0.80).33 No change in stroke recurrence was observed in patients with baseline SBP below 130 mm Hg (OR: 0.56; 95% CI, 0.26-1.17).
However, other studies have shown that the risk of recurrence decreases in line with the blood pressure values. A subanalysis from the PROGRESS trial found that the annual risk of stroke recurrence was 2.23% for achieved follow-up SBP values < 120 mm Hg, 2.81% for SBP of 120-139 mm Hg, 3.36% for SBP of 140-159 mm Hg, and 5.65% for SBP > 160 mm Hg.34
A meta-analysis published in 2017 also analysed the risk of stroke recurrence as a function of achieved follow-up SBP values.35 The subgroup of patients achieving SBP < 130 mm Hg presented a lower rate of recurrence (8.3%) than those achieving SBP of 130-140 mm Hg (9.2%) or > 140 mm Hg (11.7%); this difference was statistically significant (P = .048).
Other studies have analysed the effect of intensive blood pressure control on the risk of stroke recurrence. The Secondary Prevention of Small Subcortical Strokes (SPS3) study compared intensive blood pressure control (target SBP < 130 mm Hg) against standard treatment (target SBP 130-149 mm Hg) in patients with lacunar stroke.36 While no differences were observed in global stroke risk (HR: 0.81; 95% CI, 0.64-1.03), the intensive control group showed a 63% reduction in the risk of brain haemorrhage (OR: 0.37; 95% CI, 0.15-0.95). The Recurrent Stroke Prevention Clinical Outcome (RESPECT) study was published recently; the study included 1280 patients who had presented a stroke in the previous 3 years, who were randomly allocated to receive either intensive blood pressure control (target < 120/80 mm Hg) or standard treatment (140/90 mm Hg).37 The intensive treatment was not associated with a decrease in the risk of stroke (OR: 0.73; 95% CI, 0.49-1.11), ischaemic heart disease (OR: 1.23; 95% CI, 0.33-4.59), or mortality (OR: 0.80; 95% CI, 0.49-1.29), but was associated with a considerable reduction in the risk of brain haemorrhage (OR: 0.09; 95% CI, 0.01-0.70). The data from this study were included in a subsequent meta-analysis that found that the intensive treatment reduced the relative risk of stroke recurrence by 22% (OR: 0.78; 95% CI, 0.64-0.96), with target blood pressure values <130/80 mm Hg being associated with the greatest benefit.37
Recommendations:
- 1
In secondary prevention of stroke, antihypertensive treatment should aim to achieve blood pressure values < 130/80 mm Hg (class I recommendation, level of evidence B).
- 2
We recommend starting antihypertensive treatment in patients with ischaemic stroke who persistently present blood pressure values greater than 140/90 mm Hg (class I recommendation, level of evidence A).
- 3
In patients with lacunar stroke, treatment should be started if blood pressure values are higher than 130/80 mm Hg (class I recommendation, level of evidence B).
- 4
Patients with stroke who present blood pressure values above 140/90 mm Hg may benefit from antihypertensive treatment (class IIb recommendation, level of evidence B).
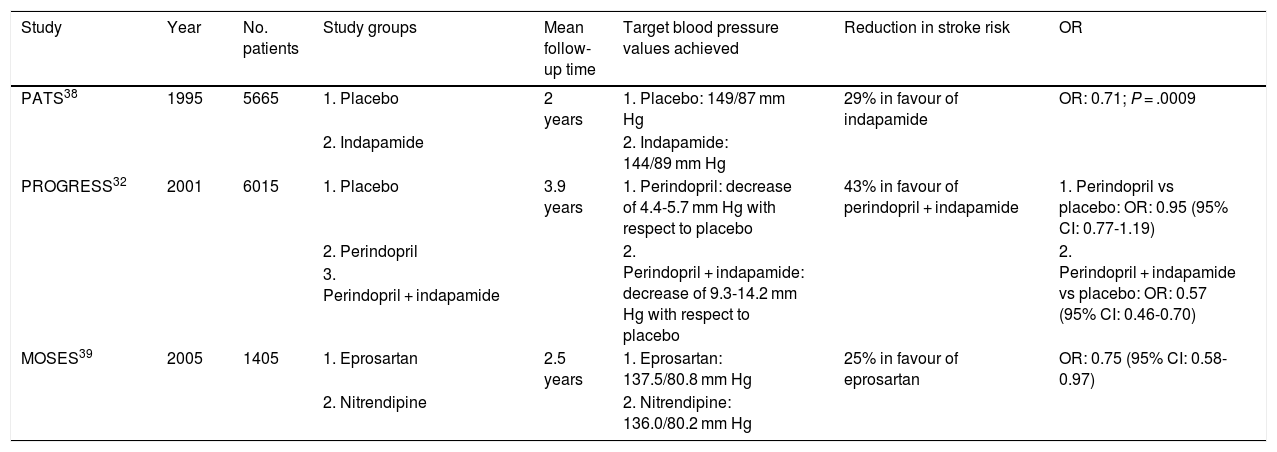
Several studies have employed different drugs in secondary prevention of stroke, including ACE inhibitors, ARBs, beta-blockers, and calcium channel blockers (Table 2). The Post-stroke Antihypertensive Treatment Study (PATS) was the first study to demonstrate that the diuretic indapamide reduces the relative risk of stroke by 29%, compared to placebo, in patients with stroke or transient ischaemic attack.38
Characteristics of the main clinical trials with antihypertensive drugs in secondary stroke prevention.
| Study | Year | No. patients | Study groups | Mean follow-up time | Target blood pressure values achieved | Reduction in stroke risk | OR |
|---|---|---|---|---|---|---|---|
| PATS38 | 1995 | 5665 | 1. Placebo | 2 years | 1. Placebo: 149/87 mm Hg | 29% in favour of indapamide | OR: 0.71; P = .0009 |
| 2. Indapamide | 2. Indapamide: 144/89 mm Hg | ||||||
| PROGRESS32 | 2001 | 6015 | 1. Placebo | 3.9 years | 1. Perindopril: decrease of 4.4-5.7 mm Hg with respect to placebo | 43% in favour of perindopril + indapamide | 1. Perindopril vs placebo: OR: 0.95 (95% CI: 0.77-1.19) |
| 2. Perindopril | 2. Perindopril + indapamide: decrease of 9.3-14.2 mm Hg with respect to placebo | 2. Perindopril + indapamide vs placebo: OR: 0.57 (95% CI: 0.46-0.70) | |||||
| 3. Perindopril + indapamide | |||||||
| MOSES39 | 2005 | 1405 | 1. Eprosartan | 2.5 years | 1. Eprosartan: 137.5/80.8 mm Hg | 25% in favour of eprosartan | OR: 0.75 (95% CI: 0.58-0.97) |
| 2. Nitrendipine | 2. Nitrendipine: 136.0/80.2 mm Hg |
CI: confidence interval; OR: odds ratio.
Subsequent studies have also shown that diuretics are beneficial in reducing the risk of stroke recurrence. The PROGRESS study included 6015 patients who had presented stroke or transient ischaemic attack in the last 5 years, who were randomly allocated to receive perindopril, perindopril + indapamide, or placebo.32 Compared to placebo, the group receiving perindopril only did not show reduced risk of stroke recurrence (OR: 0.95; 95% CI, 0.77-1.19); however, combination therapy with indapamide achieved a 43% reduction in the relative risk of stroke recurrence (OR: 0.57; 95% CI, 0.46-0.70).
The Morbidity and Mortality after Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention (MOSES) study compared an ARB (eprosartan) and a calcium channel blocker (nitrendipine) in patients presenting stroke in the previous 2 years.39 While both groups achieved similar blood pressure values (137.5/80.8 mm Hg vs 136.0/80.2 mm Hg), patients receiving eprosartan presented 25% less relative risk of stroke recurrence (OR: 0.75; 95% CI, 0.58-0.97).
Recommendations:
- •
We recommend using either an ARB or a diuretic, either alone or in combination with an ACE inhibitor, as an initial treatment in the secondary prevention of stroke (class I recommendation, level of evidence B). However, insufficient data are available to compare different antihypertensive drugs in secondary stroke prevention.
Both of these authors are the lead authors.
Please cite this article as: Rodríguez-Yañez M, Gómez-Choco M, López-Cancio E, Amaro S, Alonso de Leciñana M, Arenillas JF, et al. Prevención de ictus en pacientes con hipertensión arterial: recomendaciones del Grupo de Estudio de Enfermedades Cerebrovasculares de la Sociedad Española de Neurología. Neurología. 2021;36:462–471.