Safinamide is a new add-on drug to levodopa for the treatment of Parkinson's disease (PD) with motor fluctuations. Due to the recent incorporation of safinamide into routine clinical practice, no post-authorisation phase IV studies on the safety of safinamide have been conducted to date. This study provides clinical management guidelines for safinamide based on the opinion of a group of experts in movement disorders. This project was developed in 2 phases: 16 local meetings in phase 1 and a national meeting in phase 2. The meetings followed a pre-established agenda. The present clinical practice guidelines are based on the main conclusions reached during the national meeting. The group concluded that safinamide is effective in reducing motor and non-motor fluctuations. PD patients with mild-to-moderate fluctuations benefit most from treatment, although the drug may also improve the clinical status of patients with advanced PD. The dose of other dopaminergic drugs may be reduced after introducing safinamide, which would contribute to reducing such adverse reactions as impulse control disorder. At doses higher than those usually prescribed, safinamide may also improve dyskinesia. The experts agreed that safinamide is well tolerated and causes few adverse reactions when compared with placebo.
La safinamida es un nuevo fármaco para el tratamiento de pacientes con enfermedad de Parkinson (EP) con fluctuaciones como tratamiento complementario a levodopa. Dado que por el momento aún no existen estudios de fase IV postautorización debido a la reciente incorporación de la safinamida a la práctica clínica habitual, el interés de este proyecto radica en el desarrollo de una guía de manejo clínico de la safinamida basada en las opiniones de expertos de trastornos del movimiento. Este proyecto se desarrolló en 2 fases: una primera fase que constó de 16 reuniones locales y una segunda fase que consistió en una reunión nacional. Dichas reuniones siguieron un guion de trabajo preestablecido. Tras la reunión nacional se recopilaron las principales conclusiones de los expertos, que han supuesto la base para redactar la presente guía clínica. Se concluyó que la safinamida es eficaz en la reducción de las fluctuaciones motoras y no motoras. Los pacientes con EP con fluctuaciones leves-moderadas son los que más se benefician del tratamiento, si bien el fármaco puede contribuir a mejorar diversos problemas clínicos en pacientes con EP avanzada. Se ha destacado la posibilidad de reducir la dosis de otros fármacos dopaminérgicos tras la introducción de la safinamida, lo cual contribuiría a reducir efectos adversos como el trastorno de control de impulsos. Se hipotetizó sobre el posible efecto de la safinamida sobre la mejoría de las discinesias a dosis más altas de las habitualmente utilizadas. Se ha consensuado que la safinamida es bien tolerada y presenta un perfil de efectos adversos favourable frente a placebo.
Safinamide has been approved as a treatment for adult patients with idiopathic Parkinson's disease (PD). It is administered as a complementary treatment to a stable dose of levodopa, either alone or in combination with other antiparkinsonian drugs, in patients with moderate or advanced PD with fluctuations.
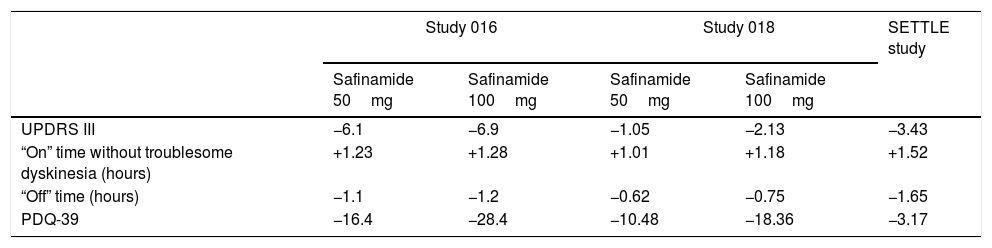
The drug has several mechanisms of action (dopaminergic and non-dopaminergic), including inhibition of Na+ and Ca++ channels; it also inhibits glutamate release in vitro and in vivo,1–4 and is a potent and reversible monoamine oxidase B (MAO-B) inhibitor,1 increasing extracellular dopamine levels in the striatum. The clinical use of safinamide is based on evidence from class I studies (Tables 1 and 2).5–7 The first study (Study 016) reported an improvement in motor fluctuations in patients with PD already receiving levodopa. At week 24, the group receiving safinamide (50 or 100mg) showed a 1.3 hour reduction in “off” time (vs 0.7 in the placebo group).5 The treatment group also scored better than patients receiving placebo on the Clinical Global Impression scale, part III of the Unified Parkinson's Disease Rating Scale (UPDRS III), and the 39-item Parkinson's Disease Questionnaire (activities of daily living). While the initial study did not demonstrate any significant improvement in dyskinesia, the extension of this study (Study 018) and various ad hoc and post hoc analyses found that patients with more basal dyskinesia did benefit from the drug,6,8,9 and showed improvements in “on” time, quality of life, and depressive symptoms.6,8 These results were corroborated in the SETTLE study,7 in a population similar to the Spanish population.
Main findings on the efficacy of safinamide.
| Study 016 | Study 018 | SETTLE study | |||
|---|---|---|---|---|---|
| Safinamide 50mg | Safinamide 100mg | Safinamide 50mg | Safinamide 100mg | ||
| UPDRS III | −6.1 | −6.9 | −1.05 | −2.13 | −3.43 |
| “On” time without troublesome dyskinesia (hours) | +1.23 | +1.28 | +1.01 | +1.18 | +1.52 |
| “Off” time (hours) | −1.1 | −1.2 | −0.62 | −0.75 | −1.65 |
| PDQ-39 | −16.4 | −28.4 | −10.48 | −18.36 | −3.17 |
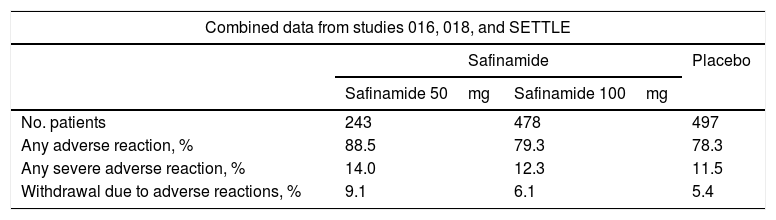
Main findings on the safety of safinamide.
| Combined data from studies 016, 018, and SETTLE | |||
|---|---|---|---|
| Safinamide | Placebo | ||
| Safinamide 50mg | Safinamide 100mg | ||
| No. patients | 243 | 478 | 497 |
| Any adverse reaction, % | 88.5 | 79.3 | 78.3 |
| Any severe adverse reaction, % | 14.0 | 12.3 | 11.5 |
| Withdrawal due to adverse reactions, % | 9.1 | 6.1 | 5.4 |
Source: European public assessment report for safinamide.16
This project aimed to develop a series of clinical guidelines to assist in the management of safinamide, based on the opinions of a group of movement disorder specialists with over a year's experience using the drug in clinical practice in Spain.
MethodsThe project was developed in 2 phases: first, 16 local meetings were held, with the attendance of more than 80 neurologists specialising in movement disorders, each of whom had over a year's experience with safinamide. In the second phase, a national meeting was held with 14 representatives of 14 local meetings (2 local representatives were unable to attend, although minutes from these local meetings were provided and interpreted at the national meeting).
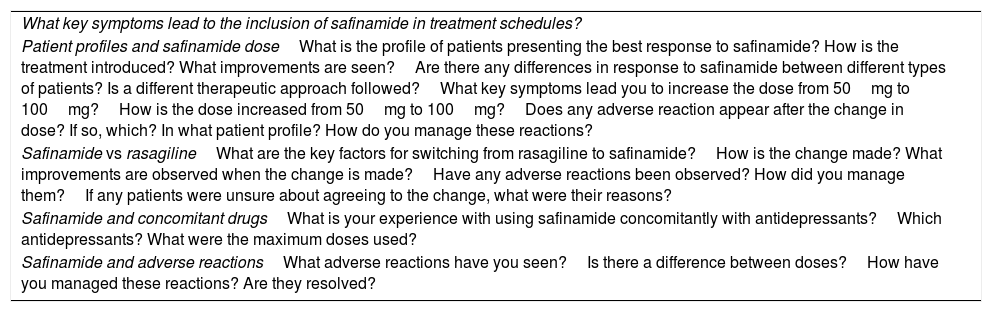
To ensure the homogeneity of the different local meetings, they followed a work agenda addressing the main aspects of safinamide treatment (Table 3).
Work agenda used in the local meetings.
| What key symptoms lead to the inclusion of safinamide in treatment schedules? |
| Patient profiles and safinamide doseWhat is the profile of patients presenting the best response to safinamide? How is the treatment introduced? What improvements are seen?Are there any differences in response to safinamide between different types of patients? Is a different therapeutic approach followed?What key symptoms lead you to increase the dose from 50mg to 100mg?How is the dose increased from 50mg to 100mg?Does any adverse reaction appear after the change in dose? If so, which? In what patient profile? How do you manage these reactions? |
| Safinamide vs rasagilineWhat are the key factors for switching from rasagiline to safinamide?How is the change made? What improvements are observed when the change is made?Have any adverse reactions been observed? How did you manage them?If any patients were unsure about agreeing to the change, what were their reasons? |
| Safinamide and concomitant drugsWhat is your experience with using safinamide concomitantly with antidepressants?Which antidepressants? What were the maximum doses used? |
| Safinamide and adverse reactionsWhat adverse reactions have you seen?Is there a difference between doses?How have you managed these reactions? Are they resolved? |
The national meeting was structured with 3 phases: a plenary session, group work, and a second plenary session to discuss the conclusions. With the assistance of a moderator, each group discussed the results of the local sessions and established conclusions for discussion in the final plenary session.
After the national meeting, the main conclusions were gathered to draft these consensus guidelines for the clinical use of safinamide in Spain.
ResultsThe experts agreed to group the conclusions according to different aspects of the disease, such as motor and non-motor fluctuations, inadequate symptom control, and advanced PD; and practical considerations, such as dosage, substitution of other MAO-B inhibitors for safinamide, concomitant administration of other drugs, and tolerability.
Motor complicationsAll participants agreed that simple motor fluctuations (e.g., wearing-off, “delayed on”) are the main indication for administration of safinamide in everyday practice. There was also consensus that the drug is more effective in patients with mild to moderate fluctuations than in those with more severe or complex complications. The majority of experts preferentially used safinamide in patients at the beginning of the intermediate stage of PD, when fluctuations are mild, or intermediate progression of advanced PD.
Regarding dyskinesia, the experts agreed that there is currently a shortage of clear evidence of the drug's efficacy. The only study addressing this subject is a post hoc analysis.9 According to the study participants’ experience, dyskinesia does not decrease after safinamide treatment in most patients, whether the drug is dosed at 50mg or 100mg. In patients with moderate or severe dyskinesia, the drug was sometimes associated with clinical worsening. Therefore, while this drug is considered to be a potent dopaminergic agent, its antidyskinetic effects have not been confirmed in clinical trials or observed in clinical practice when it is administered at normal doses.
Therefore, the ideal patient profile for treatment with safinamide would be those patients presenting mild or moderate fluctuations, with no dyskinesia or mild or non-troublesome dyskinesia. The experts also agreed that if dyskinesia were to worsen after onset of safinamide treatment, the recommended course of action would be to reduce the dose of other dopaminergic agents (e.g., levodopa), and especially dopamine agonists. In this way, the patient may benefit from the dopaminergic effects of safinamide, with fewer adverse reactions and an optimal clinical response.
Non-motor symptomsPost hoc analyses have addressed the potential usefulness of safinamide as a treatment for pain10,11 or depression12 associated with PD. Some participants reported improvements in sleep fragmentation, pain, mood, and other non-motor symptoms after treatment with safinamide. There would be great benefit in designing and conducting prospective studies that may truly verify the drug's efficacy in treating these common complications of PD.
In parallel, several participants proposed using safinamide in the management of patients with impulse control disorders. Therefore, despite the current lack of published studies, administering this drug may in some cases enable the withdrawal or reduction of dopamine agonists, which are known to be the main cause of this highly relevant clinical problem.
Poor symptom controlSome of the participating neurologists used the drug in patients either not presenting fluctuations, or in whom fluctuations were barely perceptible, but who reported a suboptimal clinical status throughout the day. This condition is frequently reported in very different forms: even in the absence of fluctuations, patients often comment that they previously felt more agile, functional, active, etc., and that they have perceived an overall decline in their physical state. In these circumstances, even in patients without clear fluctuations, there are cases when safinamide can bring about a sustained improvement in global clinical status. This may be a further indication for the drug in daily clinical practice, in addition to those mentioned in the summary of product characteristics. In fact, initial studies found direct antiparkinsonian effects (improved UPDRS scores); this supports our experience and reasoning.13
Advanced Parkinson's diseaseThe term “advanced PD” fundamentally refers to the stage at which optimised first-line treatment no longer achieves adequate motor control. It is also used in reference to patients who, over time, stop showing adequate improvements with treatment, either due to the appearance of disabling motor complications or due to symptoms that do not respond to dopaminergic treatment, such as balance or gait disorders or cognitive impairment. At other times, advanced PD has been applied as a requirement for the indication of deep brain stimulation, continuous subcutaneous apomorphine infusion, or intestinal levodopa/carbidopa infusion, due to fluctuations refractory to conventional treatment.
The experts discussed the possibility of using safinamide in this subgroup of patients with advanced PD. Generally, in their experience, the drug is well tolerated in these patients, although for obvious reasons it shows less clinical effectiveness. Some attendees noted the possibility of a degree of improvement of such symptoms as freezing and better cognitive performance after onset of safinamide treatment. The participants concluded that prospective studies into the drug's possible effectiveness in these areas would be beneficial. In no case does advanced PD represent a contraindication for the administration of safinamide, as the drug's tolerability profile is very good, even in this patient group.
Dosage of the drug at treatment onsetThere was disagreement on the most appropriate dose to administer at treatment onset. Most experts followed the indications of the summary of product characteristics, starting with a dose of 50mg and subsequently increasing it to 100mg if the desired effect was not achieved or if no adverse reactions were observed.2 Some participants reported using an initial dose of 100mg, citing the fact that the most relevant studies in the literature report no significant differences between doses in terms of adverse reactions. In line with the literature,6,9 the attendees considered the 100-mg dose to have a greater likelihood of reducing dyskinesia, as this higher dose would probably enable more complete development of the drug's potential antiglutamatergic effects. They also agreed on the need for prospective studies corroborating this possibility, with doses of 100mg or even up to 200mg (as in the studies into early PD13,14), and even with much higher doses than those used for PD: previous studies into the drug's efficacy for treating epilepsy demonstrated a good safety profile for doses of up to 300mg.15
Substituting other MAO-B inhibitors for safinamideThere were a range of opinions on how to proceed when substituting rasagiline with safinamide, as administering both drugs in combination may increase the risk of adverse reactions. Most participants reported that, in principle, they follow the recommendations of the summary or product characteristics, withdrawing rasagiline 2 weeks before onset of treatment with safinamide. However, approximately half of the participants reported switching the drugs overnight with no wash-out period, without observing any problems. A third group supported an intermediate approach: they had reduced the wash-out period from 2 weeks to one week or less without observing any adverse reactions.
The experts agreed that long periods without rasagiline administration may exacerbate parkinsonism before onset of treatment with safinamide.
Without doubt, clinical experience will shed light on the best way to substitute other MAO-B inhibitors with safinamide, although the general trend seems to be overnight changes or very short wash-out periods.
TolerabilityGenerally, safinamide was considered to be very well tolerated, and the experts reported no severe adverse reactions associated with its use. Isolated cases were mentioned of patients complaining of headache, drowsiness, general discomfort, confusion, nervousness, and epigastric pain. Participants generally recommended contacting the primary care physician in the event of any new symptoms that the patient believes to be related with safinamide, in order to clinically evaluate the possible causal and temporal relationship.
A point worth mentioning is the appearance of dyskinetic symptoms after administration of safinamide. Regarding this problem, the following recommendations were made:
- •
If dyskinesia increases when safinamide is administered at 50mg per day, the dose may be increased to 100mg to verify the possibility of an antidyskinetic effect related to the drug's antiglutamatergic action.
- •
Secondly, as mentioned earlier, physicians may attempt to reduce doses of other antiparkinsonian medications, especially drugs that may cause more adverse reactions, such as dopamine receptor agonists, or even reduce global levodopa dose.
According to the summary of product characteristics, in patients receiving selective serotonin reuptake inhibitors (SSRI), a wash-out period of 5 half-lives of the drug should be considered before administering safinamide. However, the experts participating in our study reported that in their experience, concomitant use of SSRIs and safinamide had not been associated with serotonin syndrome. This combination was considered safe for all SSRIs, serotonin-norepinephrine reuptake inhibitors, and classic tricyclic antidepressants. Some attendees recommended exercising particular caution with fluvoxamine and fluoxetine, as per the official recommendations. Although no adverse effects were reported in association with concomitant use of safinamide and antidepressants, the experts recommended instructing patients to seek immediate medical care should they present significant dysautonomic symptoms or fever. Similarly, in patients receiving particularly high doses of any type of antidepressant, they advised consulting with the patient's psychiatrist before prescribing safinamide.
Combining safinamide with sympathomimetic drugs (nasal decongestants) was not advised. The experts also recommended taking special precaution and warning patients of possible interactions with dextromethorphan, tramadol, or pethidine.
Similarly, we should be cautious when safinamide is administered in combination with metformin, aciclovir, or ganciclovir due to the possibility of increased exposure to these substances.
Therefore, we concluded that the drugs described can initially be coadministered, with the exceptions mentioned above, for all antiparkinsonian drugs except for MAO-B inhibitors.
Conclusions and discussionThis article reports the experience, opinions, and proposals of the group of experts who participated in this consensus statement. According to the most robust evidence published in the literature, safinamide is useful for reducing “off” time in patients with motor fluctuations; all participants were in agreement on this matter. The drug may also be beneficial for non-motor fluctuations, such as pain, anxiety, paraesthesias, and tiredness. The ideal candidates for this treatment (those presenting the best response) are those with PD with mild to moderate fluctuations. Safinamide may also be effective for patients with more complicated or advanced PD, offering the same potential benefits as well as the possibility of reducing the doses of other medications that may be causing adverse effects, particularly impulse control disorders or other psychiatric symptoms derived from the use of dopaminergic agonists. Positive responses were reported in some patients with advanced PD and receiving special therapies, such as levodopa/carbidopa intestinal gel infusion or deep brain stimulation, but with persistence of motor fluctuations. To summarise, the most pronounced improvement associated with safinamide is increased “on” time, in any of the clinical situations described. This drug is generally very well tolerated, with a favourable safety profile as compared to placebo.
Further research is needed to establish a more detailed profile of the drug's efficacy in diverse situations commonly associated with PD. These studies should follow a double-blind, randomised, multicentre design, although less ambitious phase IV (post-marketing) studies, prospectively analysing specific clinical issues, are also of great merit. Important lines of research would be the drug's role in improving patients’ overall quality of life, or more specific aspects such as sleep quality or pain; in fact, evidence has already been published that suggests safinamide may be of some benefit for treating pain associated with PD.10
Greater information is needed about the potential improvement in dyskinesia associated with higher doses of safinamide.
Given the drug's antiglutamatergic effect, research should also be conducted into its possible benefits for symptoms presenting little response to dopaminergic therapy, such as gait disorders, on-state freezing of gait, and restless legs syndrome.
Conflicts of interestThe authors have no direct conflicts of interest to declare; this article represents our personal opinions.
The authors received honoraria from Zambon, S.A.U. for attending the meetings.
Please cite this article as: Valldeoriola F, Grandas F, Arbelo JM, Blázquez Estrada M, Calopa Garriga M, Campos-Arillo VM, et al. Consenso de expertos españoles sobre el uso de la safinamida en la enfermedad de Parkinson. Neurología. 2021;36:666–672.