The safety and effectiveness of natalizumab in patients with relapsing-remitting multiple sclerosis (RRMS) has been demonstrated in clinical trials. However, due to the limitations of these trials, it is important to know how the condition behaves under long-term clinical practice conditions.
ObjectiveTo determine the long-term effectiveness of natalizumab in patients with RRMS by means of annual evaluation of the “no evidence of disease activity” (NEDA) parameter, which includes number of relapses, disability (measured with the Expanded Disability Status Scale), and brain MRI parameters.
Patients and methodsWe performed a retrospective study of patients with RRMS from 3 centres who were treated with one or more doses of natalizumab. Each year, we evaluated NEDA status and safety based on the percentage of patients who discontinued treatment with natalizumab and experienced adverse reactions.
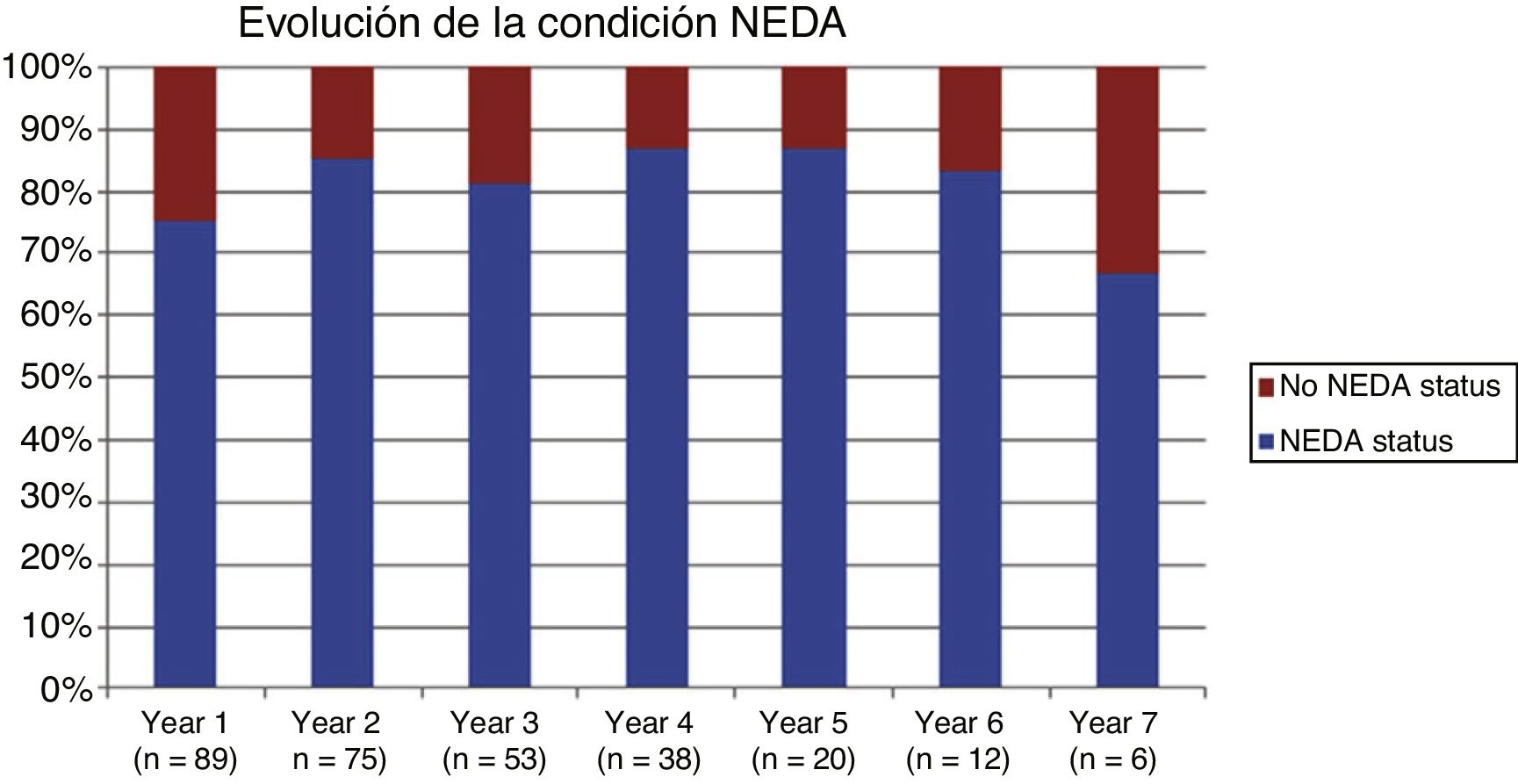
ResultsThe study included 89 patients, most of whom received treatment for 2 to 4 years, with a follow-up period of up to 7 years. Natalizumab significantly reduces the radiological and clinical progression of the disease, as well as the annual rate of relapses. The NEDA parameter demonstrates the effectiveness of the drug, with values of 75.28% for year one and 66.67% for year 7. Twenty-five patients (28.1%) dropped out after a median of 4 years. Fourteen of these patients (56%) dropped out due to the appearance of anti–JC virus antibodies, either in isolation or associated with another cause. Four dropouts (16%) were due to treatment ineffectiveness, with one patient dying due to progressive multifocal leukoencephalopathy.
ConclusionsNatalizumab is highly effective as measured by the NEDA long-term remission parameter.
La efectividad y seguridad de natalizumab en pacientes con esclerosis múltiple remitente recurrente (EMRR) se demostró en ensayos clínicos. Sin embargo, por las limitaciones de estos, es importante saber cómo se comporta en condiciones de práctica clínica a largo plazo.
ObjetivoConocer la eficacia a largo plazo de natalizumab en pacientes con EMRR mediante la evaluación anual del NEDA (no evidence of disease activity), que incluye número de brotes, discapacidad medida con EDSS y parámetros de RM cerebral.
Pacientes y métodosEstudio retrospectivo y multicéntrico (n = 3) de pacientes con EMRR tratados con una o más dosis de natalizumab. Se evaluó el estado NEDA cada año y la seguridad a partir del porcentaje de pacientes que discontinuaron y que presentaron efectos adversos.
ResultadosIncluimos 89 pacientes, la mayoría recibieron tratamiento durante 2 a 4 años, con una duración del seguimiento de hasta 7 años. Natalizumab reduce significativamente la progresión radiológica y clínica de la enfermedad, así como la tasa anual de brotes, demostrándose su eficacia con el parámetro NEDA, 75.28% al primer año y 66.67% al séptimo año. 25 pacientes (28.1%) han abandonado el estudio en una mediana de tiempo de 4 años. 14 pacientes (56%) fue por aparición de anticuerpos contra el virus JC, como causa única o asociada a otro motivo. 4 abandonos (16%) fueron por ineficacia, un paciente falleció a causa de LMP.
ConclusionesNatalizumab presenta una alta eficacia medida mediante el parámetro de remisión NEDA a largo plazo.
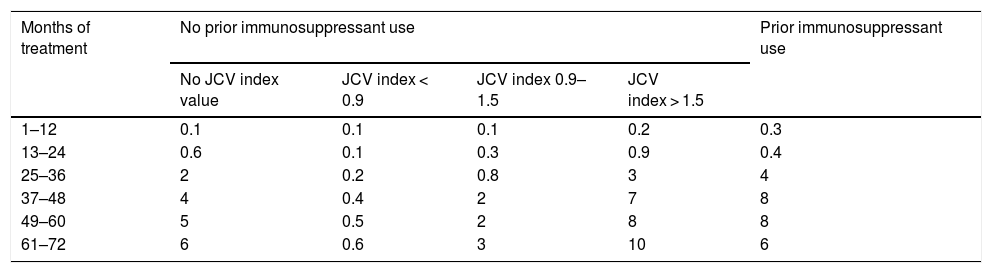
There is extensive evidence of the efficacy and safety of natalizumab for patients with relapsing-remitting multiple sclerosis (RRMS).1–3 However, clinical trials do not provide information on the long-term effects of treatment. Natalizumab should be administered only after careful patient selection due to the associated risk of progressive multifocal leukoencephalopathy (PML).4,5 The risk of developing PML is proportional to anti–JC virus antibody titres; prior use of immunosuppressants and long duration of natalizumab treatment are associated with increased risk.6 In patients testing negative for JC virus, the annual risk of developing PML is 0.1 cases per 1000 patients treated. The risk of PML in patients testing positive is shown in Table 1.7
Estimated risk of progressive multifocal leukoencephalopathy in patients testing positive for anti–JC virus antibodies (per 1000 treated patients).
| Months of treatment | No prior immunosuppressant use | Prior immunosuppressant use | |||
|---|---|---|---|---|---|
| No JCV index value | JCV index < 0.9 | JCV index 0.9–1.5 | JCV index > 1.5 | ||
| 1–12 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 |
| 13–24 | 0.6 | 0.1 | 0.3 | 0.9 | 0.4 |
| 25–36 | 2 | 0.2 | 0.8 | 3 | 4 |
| 37–48 | 4 | 0.4 | 2 | 7 | 8 |
| 49–60 | 5 | 0.5 | 2 | 8 | 8 |
| 61–72 | 6 | 0.6 | 3 | 10 | 6 |
The purpose of this study was to determine the long-term effectiveness of natalizumab based on annual and cumulative “no evidence of disease activity” (NEDA) status. NEDA status is defined as absence of clinical and radiological activity (no relapses or progression of disability as measured with the Expanded Disability Status Scale [EDSS], and no new lesions on T2-weighted sequences or gadolinium uptake).
Material and methodsThree hospitals in the Spanish region of Galicia participated in this retrospective study: Hospital Povisa in Vigo, Complejo Hospitalario Universitario de Santiago de Compostela, and Complejo Hospitalario Universitario de Pontevedra.
Participants were required to be at least 18 years old and to have legal capacity; all patients gave informed consent to the use of their clinical and radiological data.
The main inclusion criteria, based on the indications of the summary of product characteristics for natalizumab, were as follows:
- -
High disease activity despite treatment with a complete course (normally one year) of interferon beta or glatiramer acetate (high disease activity is defined as occurrence of a relapse during the previous year and presence of at least 9 T2-hyperintense lesions or at least one gadolinium-enhancing lesion). We also included patients showing a relapse rate equal to or greater than that of the year prior to treatment onset, and patients with severe relapses.
- -
Patients with severe, rapidly-progressing RRMS, defined as occurrence of 2 or more disabling relapses in one year and one or more gadolinium-enhancing lesions, or a significant increase in T2 lesion load as compared to an MR image from the previous year.
We excluded all patients older than 65 years or younger than 18, as well as pregnant women, as natalizumab is not approved for use in these population groups. We also excluded patients with hypersensitivity to natalizumab, immunosuppressed individuals, and patients with active neoplasia (except for basal cell carcinoma).
Participants were treated with intravenous perfusion of natalizumab dosed at 300 mg every 28 days. Infusion time was one hour; patients were kept under observation for an additional hour after infusion to monitor any hypersensitivity reactions. The drug was discontinued in patients with high risk of PML who refused to continue with treatment, and in cases of treatment failure, defined as occurrence of more relapses than in the year prior to treatment onset, severe relapses, or progression of disability.
Before treatment onset, all patients underwent clinical assessment, a brain MRI scan, and a complete blood count.
During treatment, monthly follow-up assessments were performed. At each assessment, we recorded the number of relapses the patient had presented since the previous consultation. A relapse was defined as the appearance of new symptoms or the recurrence of old symptoms, lasting at least 24 hours, without fever or infection. Retrospective quantification of the number of relapses may introduce bias, as patients may not remember mild symptoms not interfering with daily living activities. However, the fact that patients were assessed on a monthly basis minimises this risk.
Clinical progression was evaluated annually with the EDSS. An increase of 0.5 points at 3 months was regarded as progression of disability.
JC virus serology tests were performed every 6 months. Patients underwent follow-up neuroimaging scans annually, with the exception of those testing positive for JC virus, who underwent MRI scans every 3 or 6 months, according to the recommendations of the Spanish Agency for Medicines and Medical Devices and the summary of product characteristics for natalizumab. Brain MRI scans were performed with patient repositioning. Radiological progression was defined as the appearance of gadolinium-enhancing T1 lesions or new T2 lesions.
The objective of the study was to determine the number of patients meeting the NEDA parameter. NEDA status is defined as absence of clinical and radiological progression (no relapses or increase in disability; no new or active lesions on MRI); the variable is therefore dichotomous. Negative results for all 3 parameters indicates NEDA, or freedom from disease activity.
Statistical analysis of variables was performed using the SPSS software, version 21. The level of statistical significance was set at P < .05. We performed a descriptive analysis for all variables. To compare qualitative variables between 2 independent samples, we used the t test for normally distributed data or the non-parametric Mann-Whitney U test. To compare more than 2 independent samples, we used ANOVA for normally distributed data or the non-parametric Kruskal-Wallis test. The t test was used to compare means for unpaired data and the chi-square test to study the association between qualitative variables.
ResultsWe studied a total of 89 patients from treatment onset until treatment discontinuation or until September 2016 (whichever occurred first). The sample included 38 men (42.7%) and 51 women (57.3%), with a mean age (SD) of 41.09 (8.19) years (range, 22–63); age followed a normal distribution. Nearly half of the participants (43 patients) were attended at Complejo Hospitalario Universitario de Santiago de Compostela, with 25 attended at Hospital Povisa and 21 at Complejo Hospitalario Universitario de Pontevedra.
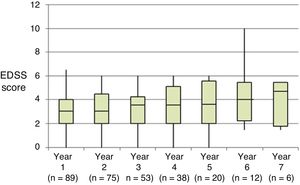
At the beginning of the study period, median EDSS score was 3 (Q1-Q3: 2.0–4.5); during the year prior to treatment onset, patients presented a mean (SD) of 1.67 (0.81) relapses. All patients showed radiological signs of MS at treatment onset.
Most participants had received treatment for 2 to 4 years. All patients completed one year of treatment, 74 completed 2 years, 53 completed 3 years, 38 completed 4, 20 completed 5, 12 completed 6, and 6 completed 7 years of treatment.
Reasons for treatment discontinuation were risk of PML considered unacceptable by the patient in 14% of cases (5 patients in year 2, 3 in year 3, 3 in year 4, one in year 5, and one in year 6), treatment ineffectiveness in 5% (3 patients in year 2, one in year 4, and one in year 6), personal reasons in 2% (2 patients in year 1), and adverse drug reactions in 6% (one patient in year 3, 2 in year 4, one in year 5, and one in year 6). Furthermore, treatment was temporarily suspended due to pregnancy in 2 patients (one during the first month and the other in treatment year 4).
Adverse drug reactions included one case of thrombocytopaenia (year 3), one case of breast cancer (year 4), and 3 cases of infection (tonsillar abscess requiring surgery in year 4, spondylodiscitis leading to a long hospital stay during year 5, and PML resulting in the patient’s death in year 6). The rate of adverse reactions in our sample was 0% during the first 2 years of treatment, 2% during year 3, 5% during years 4 and 5, 8% during year 6, and 0% during year 7.
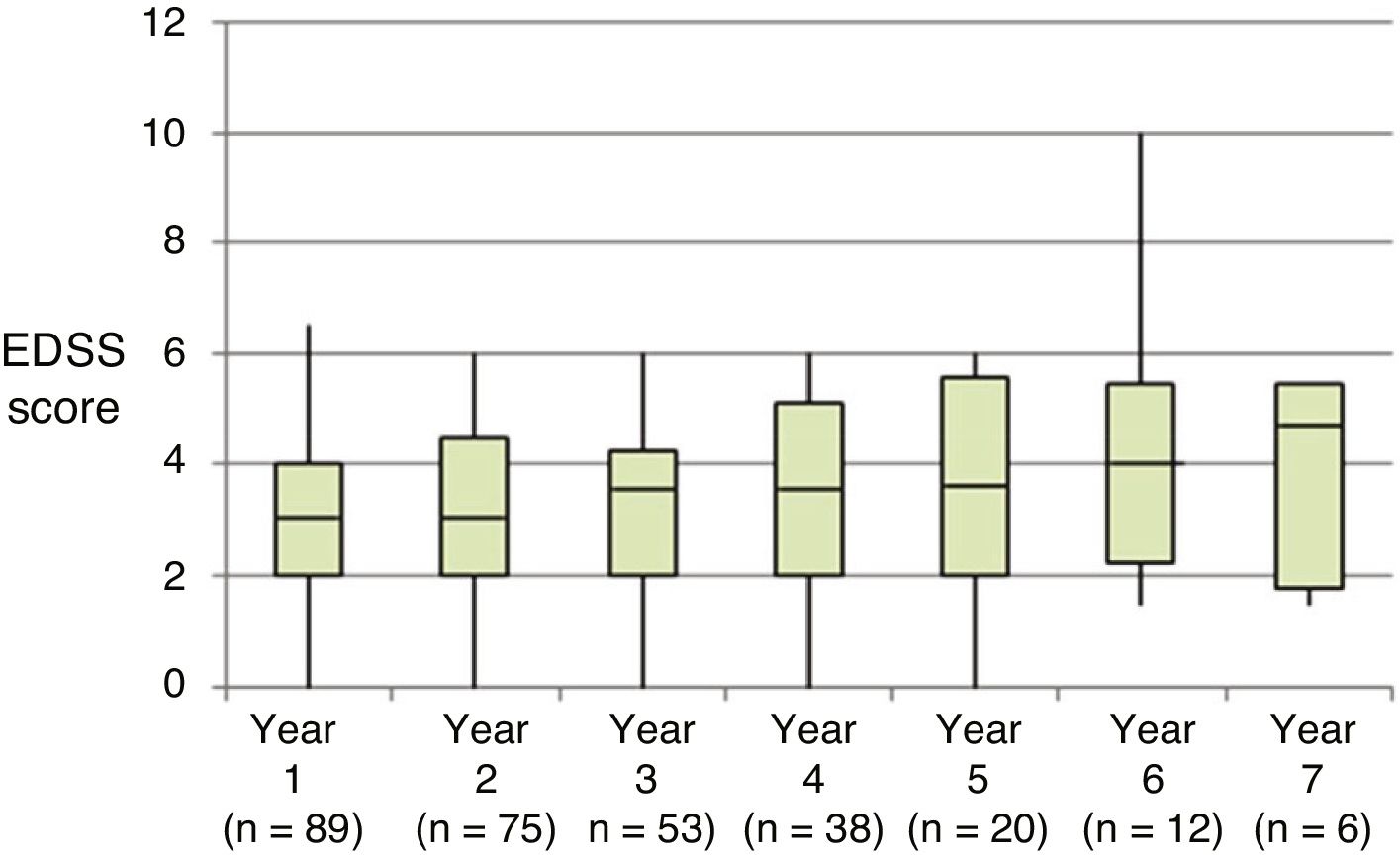
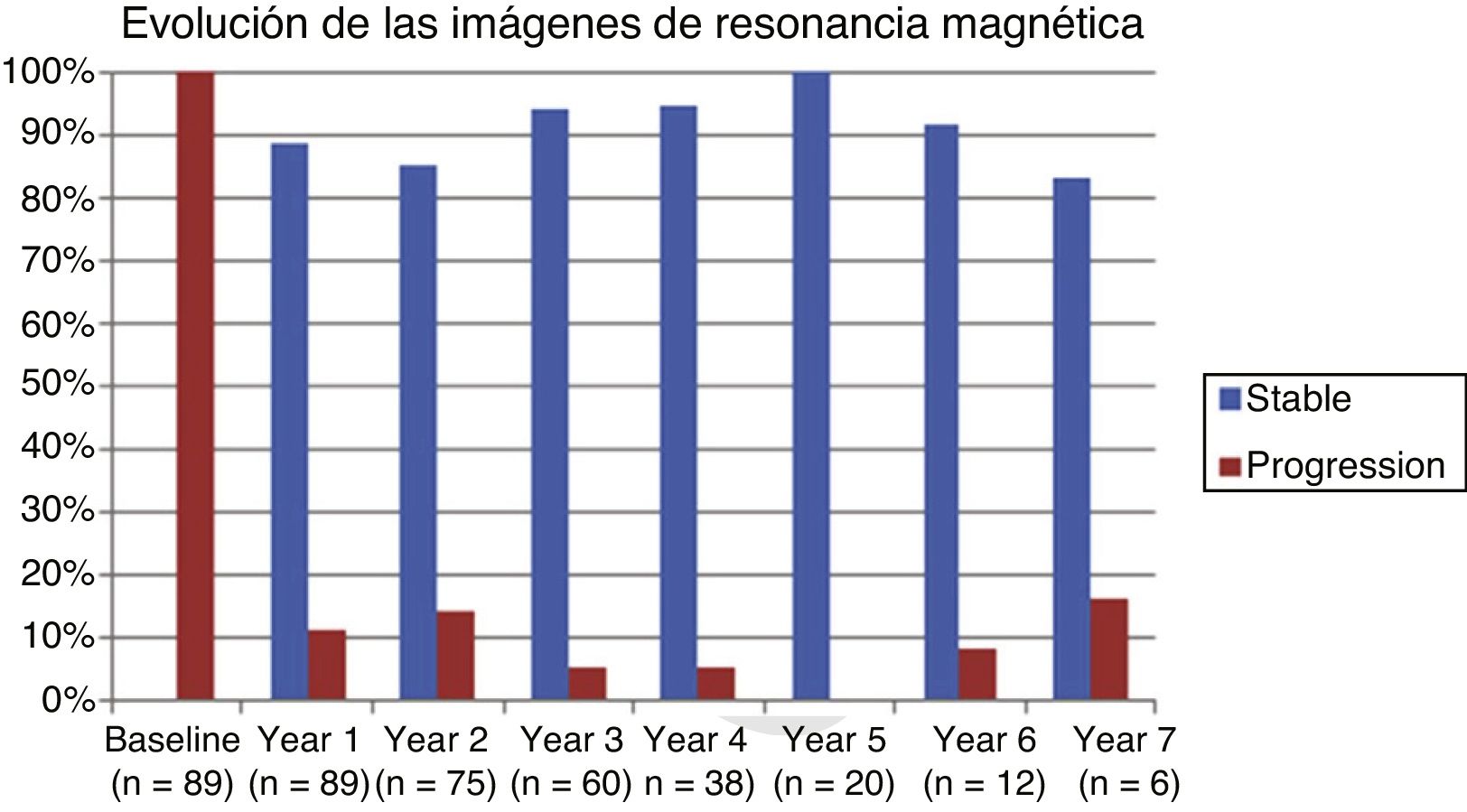
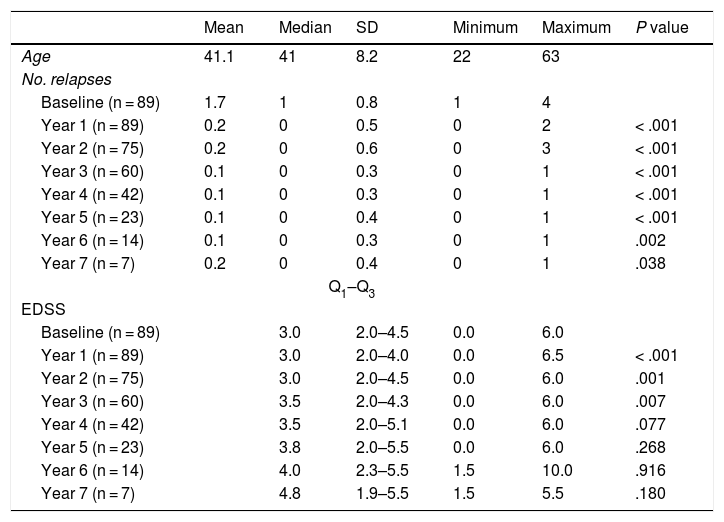
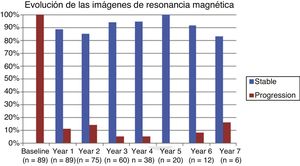
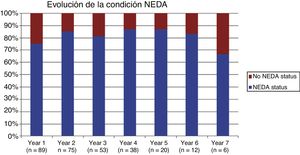
Table 2 shows the progression of the different parameters analysed each year in our sample. Fig. 1 shows the progression of EDSS scores, Fig. 2 shows disease progression based on MRI data, and Fig. 3 shows the percentage of patients with NEDA status over the study period. No statistically significant differences were observed between men and women or between age groups in the number of relapses or in radiological progression (P > .05). The only significant differences were in progression of disability at follow-up years 2 and 7 (P < .05), with men showing more marked progression than women.
Age, number of relapses, and Expanded Disability Status Scale scores in our sample, by year. Statistical significance (P < .05) is reported for the comparison between annual and baseline values.
| Mean | Median | SD | Minimum | Maximum | P value | |
|---|---|---|---|---|---|---|
| Age | 41.1 | 41 | 8.2 | 22 | 63 | |
| No. relapses | ||||||
| Baseline (n = 89) | 1.7 | 1 | 0.8 | 1 | 4 | |
| Year 1 (n = 89) | 0.2 | 0 | 0.5 | 0 | 2 | < .001 |
| Year 2 (n = 75) | 0.2 | 0 | 0.6 | 0 | 3 | < .001 |
| Year 3 (n = 60) | 0.1 | 0 | 0.3 | 0 | 1 | < .001 |
| Year 4 (n = 42) | 0.1 | 0 | 0.3 | 0 | 1 | < .001 |
| Year 5 (n = 23) | 0.1 | 0 | 0.4 | 0 | 1 | < .001 |
| Year 6 (n = 14) | 0.1 | 0 | 0.3 | 0 | 1 | .002 |
| Year 7 (n = 7) | 0.2 | 0 | 0.4 | 0 | 1 | .038 |
| Q1–Q3 | ||||||
| EDSS | ||||||
| Baseline (n = 89) | 3.0 | 2.0–4.5 | 0.0 | 6.0 | ||
| Year 1 (n = 89) | 3.0 | 2.0–4.0 | 0.0 | 6.5 | < .001 | |
| Year 2 (n = 75) | 3.0 | 2.0–4.5 | 0.0 | 6.0 | .001 | |
| Year 3 (n = 60) | 3.5 | 2.0–4.3 | 0.0 | 6.0 | .007 | |
| Year 4 (n = 42) | 3.5 | 2.0–5.1 | 0.0 | 6.0 | .077 | |
| Year 5 (n = 23) | 3.8 | 2.0–5.5 | 0.0 | 6.0 | .268 | |
| Year 6 (n = 14) | 4.0 | 2.3–5.5 | 1.5 | 10.0 | .916 | |
| Year 7 (n = 7) | 4.8 | 1.9–5.5 | 1.5 | 5.5 | .180 | |
EDSS: Expanded Disability Status Scale.
We found no significant differences in NEDA status between the 15 patients who discontinued treatment due to presence of anti–JC virus antibodies and those who did not discontinue treatment (P = .584): at the end of the follow-up period, 73.3% of the patients with anti–JC virus antibodies reached NEDA status (including the patient who died due to PML).
We also studied the association between NEDA status and EDSS scores before treatment, finding no statistically significant differences between patients scoring 0 to 3 (n = 45) and those scoring 3 to 6 (n = 44).
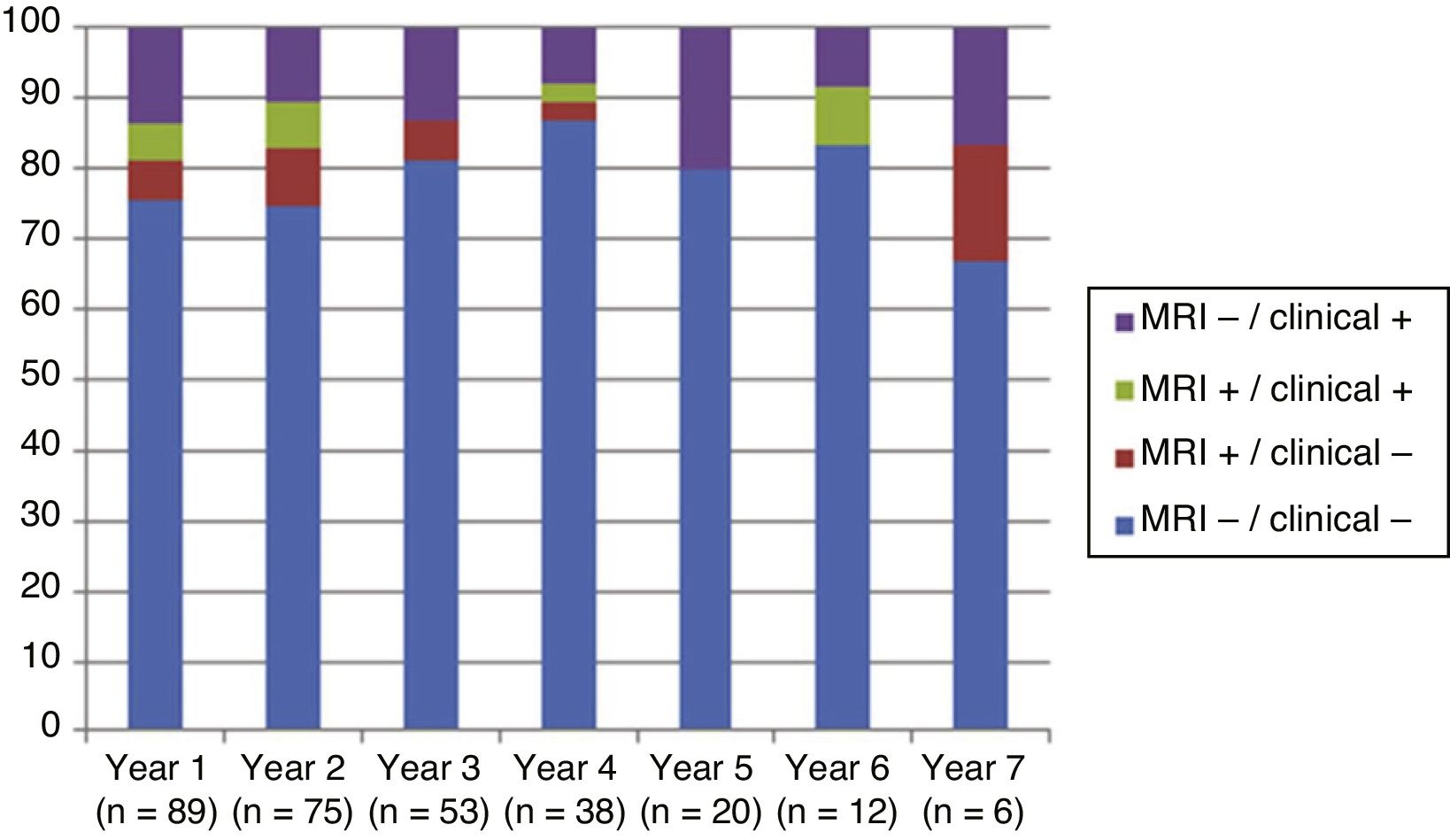
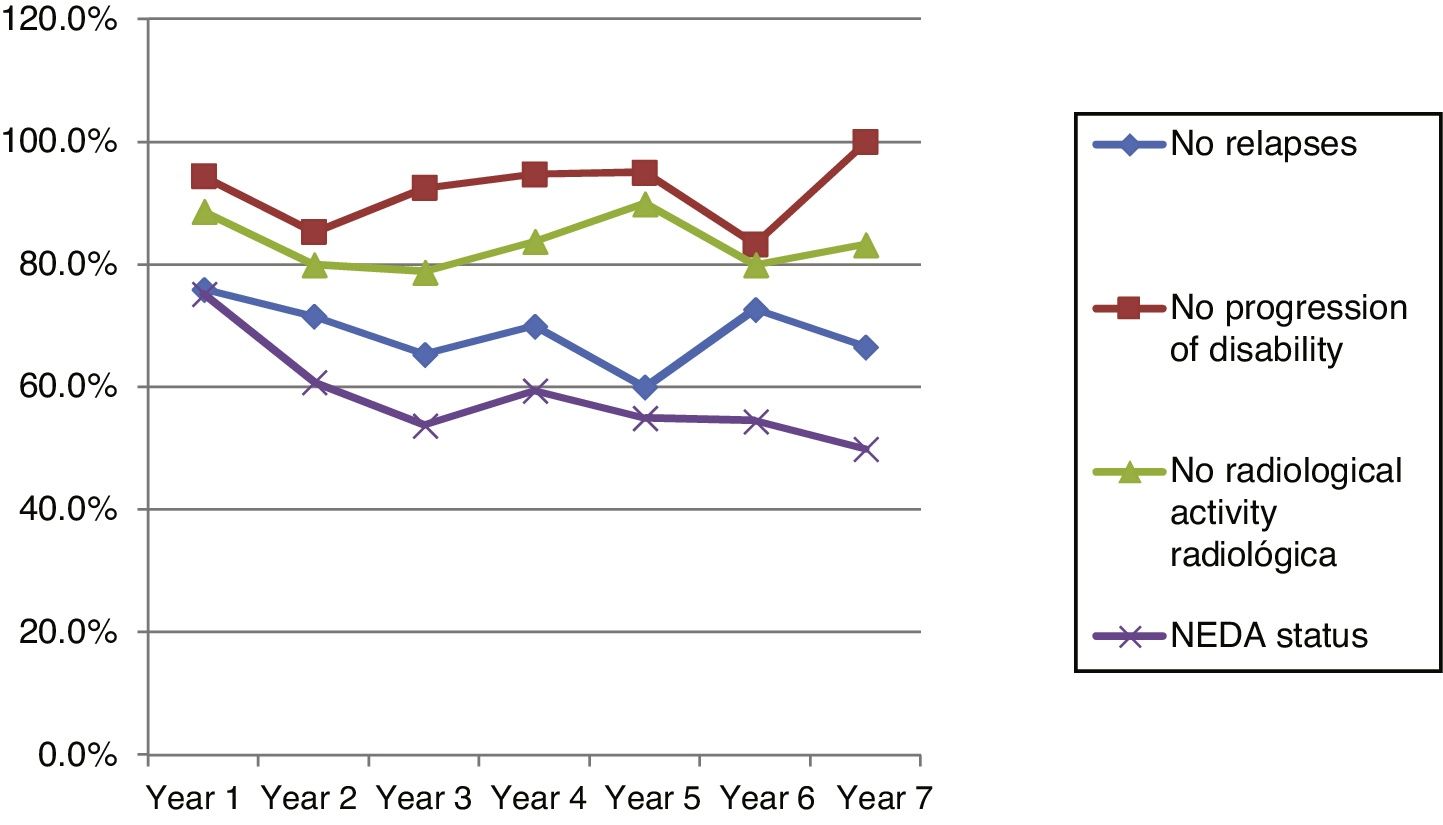
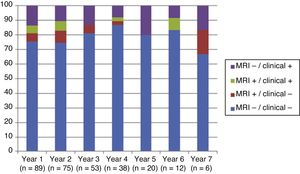
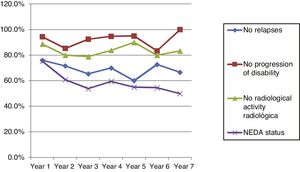
Fig. 4 shows our sample’s clinical and radiological progression over the treatment period. Our data show that in almost every year, loss of NEDA status was due to clinical progression not associated with radiological alterations; the proportion of patients losing NEDA status was relatively stable (ranging from 13.48% in year 1 to 16.67% in year 7), and only 4 of 53 patients presented neuroimaging alterations from follow-up year 3. Fig. 5 shows the percentage of patients each year who were free from clinical and radiological activity.
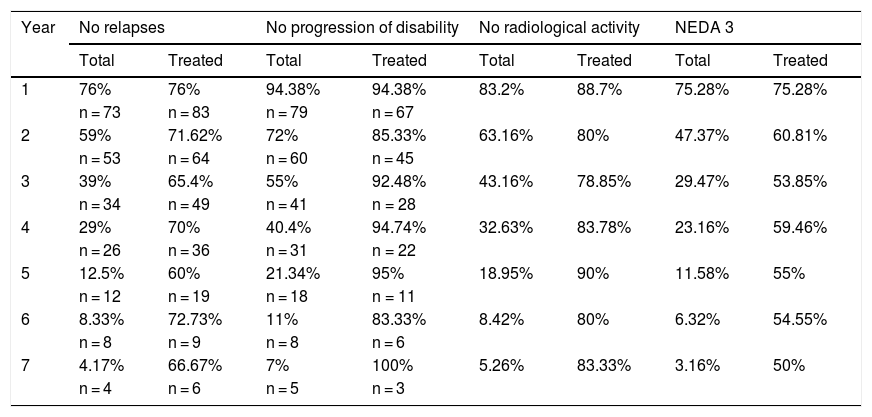
Cumulative NEDA rates decreased progressively over the 7-year treatment period, although they always remained over 50% (Table 3).
Cumulative proportion of NEDA patients in each year of treatment.
| Year | No relapses | No progression of disability | No radiological activity | NEDA 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Treated | Total | Treated | Total | Treated | Total | Treated | |
| 1 | 76% | 76% | 94.38% | 94.38% | 83.2% | 88.7% | 75.28% | 75.28% |
| n = 73 | n = 83 | n = 79 | n = 67 | |||||
| 2 | 59% | 71.62% | 72% | 85.33% | 63.16% | 80% | 47.37% | 60.81% |
| n = 53 | n = 64 | n = 60 | n = 45 | |||||
| 3 | 39% | 65.4% | 55% | 92.48% | 43.16% | 78.85% | 29.47% | 53.85% |
| n = 34 | n = 49 | n = 41 | n = 28 | |||||
| 4 | 29% | 70% | 40.4% | 94.74% | 32.63% | 83.78% | 23.16% | 59.46% |
| n = 26 | n = 36 | n = 31 | n = 22 | |||||
| 5 | 12.5% | 60% | 21.34% | 95% | 18.95% | 90% | 11.58% | 55% |
| n = 12 | n = 19 | n = 18 | n = 11 | |||||
| 6 | 8.33% | 72.73% | 11% | 83.33% | 8.42% | 80% | 6.32% | 54.55% |
| n = 8 | n = 9 | n = 8 | n = 6 | |||||
| 7 | 4.17% | 66.67% | 7% | 100% | 5.26% | 83.33% | 3.16% | 50% |
| n = 4 | n = 6 | n = 5 | n = 3 | |||||
The table presents the cumulative number of patients with NEDA status and meeting each of the parameters included in this measure for each follow-up year, for all patients (total) and the patients who completed treatment each year (treated).
Treatments for MS currently aim to maintain clinical and radiological remission for as long as possible; this has led to the creation of the NEDA treatment parameter.
The demographic characteristics of our sample meet the criteria for prescribing natalizumab8; the median EDSS score of 3 indicates mild-to-moderate disability at treatment onset.
The 7-year follow-up period allowed us to study the medium- and long-term safety and effectiveness of the drug. One patient died due to PML, and 3 patients presented adverse reactions requiring treatment discontinuation (one due to anaphylaxis and 2 due to infections [tonsillar abscess and spondylodiscitis]), which led to hospitalisation and were thought to be linked to the drug’s immunosuppressive activity. However, the high drop-out rate due to JC virus seropositivity (56% of patients) in follow-up years 2 and 7 (median, year 4) may have led us to underestimate other potential complications of treatment. Our results should therefore be interpreted with caution. Only 12 patients completed 6 years of treatment, and only 6 completed the 7-year treatment period. Interestingly, we observed no significant differences in clinical progression between patients with different treatment durations, which suggests that patients discontinuing treatment due to risk of PML may be forgoing the possibility of good response to natalizumab.
Our sample showed a significant reduction in the number of relapses and in progression of disability during the first 4 years of treatment compared to baseline. However, from follow-up year 4 patients did not show more relapses but disability did progress, which may be attributed to degeneration associated with the disease.
After one year of treatment, 67 of 89 patients (75.28%) presented NEDA status; this percentage remained stable throughout the 7 years of follow-up (ranging from 74.67% to 86.84%), although the number of patients continuing with the treatment decreased progressively, mainly due to the risk of PML (few patients discontinued treatment due to lack of effectiveness).
Cumulative NEDA rate decreased over the study period but remained high, reaching 50% after 7 years of treatment.
As reported in the literature, 72% of patients continued with treatment at follow-up year 4; after 7 years, however, fewer than half of the patients continued with the treatment, mainly due to positive test results for anti–JC virus antibodies and risk-benefit management. We found no significant differences in NEDA status at the end of the follow-up period between patients dropping out of the study due to JC virus seropositivity and the remaining patients, which suggests that most drop-outs were due to risk-benefit management decisions rather than loss of treatment effectiveness.
The progression of disability was more marked in men than in women during follow-up years 2 and 7; this may be explained by the fact that the disease tends to be more disabling in men during the early years, with disability rates equalising after 5 years of follow-up.9
Natalizumab appears to be more effective in clinical practice than in experimental studies,10,11 as shown by several open-label trials. The GEXNE study,12 conducted in Spain, retrospectively analysed the effectiveness of natalizumab in 825 patients treated for a year; 63% of patients reached NEDA status, with relapses decreasing by 88%. Horga et al.13 followed up a sample of 112 patients treated with natalizumab for one year; 33% met NEDA criteria, and 76% remained clinically stable. Both studies used samples with more severe disability and a higher relapse rate than our patients, which may explain the differences in effectiveness.
Very few studies have analysed the long-term effectiveness of natalizumab. Prosperini et al.14 studied 152 patients for 7 years. By the end of the study, only 34% of patients met NEDA criteria and 41% showed no progression of disability, compared to 79.7% in our study. Both series show similar baseline clinical characteristics; therefore, discrepancies may be partially explained by differences in follow-up parameters: Prosperini’s study group used brain and spinal cord MRI, increasing the sensitivity to progression (they detected a 10% increase in the number of lesions).
We analysed clinical and radiological progression separately, and observed that for nearly every year, loss of NEDA status was mainly due to clinical progression not associated with radiological alterations (after follow-up year 3, only 4 of 53 patients presented neuroimaging alterations). Furthermore, the rate of loss of NEDA status was fairly constant (ranging from 13.48% in year 1 to 16.67% in year 7). This may be explained by disparities in clinical and radiological findings in patients with MS, due to the difficulty of detecting small lesions,15 particularly in the cortex, and the fact that MRI scans were performed in the absence of clinical relapses in some cases. Our results are consistent with those of Rotstein et al.,16 who report that most patients worsened due to clinical progression, with no previous evidence of changes in MR images. Spinal imaging studies should be included in future studies to better characterise disease stability.14,16
Although NEDA status is difficult to achieve in the long term, future studies should include measurement of brain atrophy (NEDA-4), which is correlated with the degree of neurodegeneration17 and constitutes an important prognostic factor.
Conflicts of interestIsabel Muñoz Pousa and Lucía Naya Ríos have no conflicts of interest to declare.
The remaining authors have received travel expenses for attending congresses and courses, and have participated in studies funded by Biogen, Novartis, Genzyme, Serono, and Bayer-Schering.
None of the authors have conflicts of interest to declare in relation to this study.
Please cite this article as: Pato Pato A, Costa Arpín E, Rodríguez Regal A, Rodríguez Constenla I, Cimas Hernando I, Muñoz Pousa I, et al. Evolución de una serie de pacientes con esclerosis múltiple remitente-recurrente tratados con natalizumab durante 7 años mediante el parámetro no evidencia enfermedad activa. Neurología. 2021;36:346–352.