Intracranial haemorrhages (ICHs) represent a severe and frequently lethal complication in patients treated with vitamin K antagonists (VKA). The purpose of our study is to describe the factors and clinical features associated with mortality in these patients.
MethodsWe conducted an observational, retrospective, multi-centre study based on prospective stroke registries in Spain. We included all patients admitted to neurology departments during a 1-year period who met the following inclusion criteria: being 18 or older, having a diagnosis of ICH, and receiving VKA. Clinical and radiological parameters and 3-month outcomes were analysed.
ResultsA total of 235 patients from 21 hospitals were included. Mortality rate at 90 days was 42.6%. Bivariate analysis showed a significant association between death and the following factors: median NIHSS score at admission (5 [IQR=9] vs 17 [IQR=14] points, P<.01) and presence of an extensive hemispheric haemorrhage (4.9% vs 35%, P<.01; χ2). Extensive hemispheric haemorrhages, in addition to being the most lethal type, were associated with a shorter time to death (mean of 16.5 days; 95% CI: 7.1-26). A logistic regression model showed that only baseline NIHSS scores independently predicted death (odds ratio=1.13 [95% CI: 1.08-1.17] for each point in the scale).
ConclusionICH in patients treated with VKA is associated with high mortality rates; mortality in these patients is mainly and independently associated with the clinical situation at stroke onset.
La hemorragia intracraneal (HIC) en pacientes tratados con anticoagulantes orales antagonistas de la vitamina K (AVK) es una complicación grave y frecuentemente letal; en este trabajo estudiamos las características clínicas y los factores que se relacionan con la mortalidad en este grupo de pacientes.
MétodosRealizamos un estudio observacional, multicéntrico y retrospectivo, de ámbito nacional, basado en registros prospectivos de pacientes con ictus. Se incluyó a los pacientes ingresados en servicios de Neurología durante un período de un año y que cumplieran los criterios de inclusión: pacientes mayores de 18 años con HIC que estuvieran en tratamiento con AVK y que ingresaron durante el periodo de estudio. Se analizaron las variables clínicas y radiológicas y su evolución a 3 meses.
ResultadosIncluimos a 235 pacientes provenientes de 21 hospitales. La mortalidad a los 90 días fue del 42,6%. En el modelo bivariante los factores asociados con defunción fueron: mediana en la puntuación de la escala NIHSS al ingreso (5 [RIQ=9] vs. 17 [RIQ=14] puntos, P<0,01) y la presencia de una hemorragia hemisférica extensa (4,9% vs. 35%, P<0,01; ×2). Las hemorragias hemisféricas extensas, además de ser las más letales, también presentaron el tiempo más corto hasta el fallecimiento (media 16,5 días; IC del 95%, 7,1-26). Realizamos un modelo de regresión logística que evidenció que solo la NIHSS basal predijo de forma independiente el fallecimiento (odds ratio=1,13 [IC del 95%, 1,08-1,17] por cada punto en la escala).
ConclusiónLa HIC en pacientes tratados con AVK conlleva una elevada mortalidad asociada principal e independientemente con la situación clínica al inicio del ictus.
Intracranial haemorrhage (ICH) represents 8%-15% of all strokes. The condition is associated with a short-term mortality rate of 20%-30%, which is higher than that of cerebral infarction or subarachnoid haemorrhage.1,2 Overall, incidence is considered to increase by 7%-10% in patients taking anticoagulants.3 Up to 23.4% of all cerebral haemorrhages occur in patients taking anticoagulants, with 3-month mortality rates between 52 and 63%.4,5 The higher rate of mortality in patients taking anticoagulants has been associated with multiple factors: the larger size of the haematoma in patients taking anticoagulants,6–8 the preference for the brainstem involvement,9 the growth of the haematoma,10,11 and the risk of intraventricular expansion of lobar and deep haemorrhages.10–12 Furthermore, no specific therapeutic measure has been demonstrated to be effective in reducing sequelae or mortality of this type of haemorrhage.
A recent descriptive, multi-centre study performed in Spain (the Traditional Anticoagulation Complications [TAC] Registry) shows an incidence of 2.9 cases/100 000 population for ICH associated with anticoagulant treatment with vitamin K antagonists (VKA-ICH); this represents 1.94% of all strokes and 13.6% of all cerebral haemorrhages. This study revealed a 3-month mortality rate of 42.6%.13 The main aims of the present study are to describe the mortality rate of VKA-ICH and to identify the clinical characteristics associated with death in the patients included in the TAC Registry.
Material and methodsThe TAC Registry study is an observational, retrospective, multi-centre study performed throughout Spain, based on registries and databases containing prospective data on consecutive stroke patients. Study patients were admitted to neurology departments (mainly to stroke units) between 1 September 2012 and 31 August 2013. Participating hospitals were general hospitals with a well-defined reference population, providing 24-h emergency care and with 24-h availability of brain CT or MRI, that treat patients in the acute phase of stroke and have a neurology department/unit, providing all stroke patients with specialised care (admission to the neurology department or to another department under supervision of a neurologist). Patient consent to participate in the study was also required. Inclusion criteria were: patients older than 18 years with ICH, who were being treated with VKA and were admitted during the study period. We excluded patients with diagnosis of epidural or subdural haematoma, subarachnoid haemorrhage, cerebral infarction with haemorrhagic transformation, or traumatic haemorrhage.
An electronic form was designed for hospitals to record participants’ clinical data: demographic data; vascular risk factors; type of oral anticoagulants (OACs) prescribed; reason for OAC prescription; International Normalised Ratio (INR) at admission; concomitant treatment; in-hospital or out-of-hospital death, date of death, stroke severity at admission and discharge (measured with the National Institutes of Health Stroke Scale, NIHSS); and outcome at 3 months (measured with the modified Rankin Scale, mRS). Haemorrhage location was classified into the following categories: lobar, deep (basal ganglia or thalamus), brainstem, cerebellar, extensive hemispheric (volume>100mL, regardless of the apparent source of bleeding, calculated using the formula ½[a×b×c]), and intraventricular (either primary or secondary to hemispheric or brainstem haemorrhages).
Since this study is retrospective and observational, we gathered no data which could be used to identify patients. Informed consent was therefore not necessary. The study was approved by the promoting hospital's ethics committee. Medians and 25th and 75th percentiles were calculated for discrete quantitative variables and for non-normally distributed continuous variables. Non-parametric statistics were used for data analysis. Categorical variables were studied using percentage rates; the chi-square-test was used to study their association with death. The association of several factors with time from stroke to death was studied using the Kaplan–Meier survival curves. Values of P<.05 were considered statistically significant. Data were analysed using the SPSS® v. 13.0, Microsoft Excel® 2010, and R v. 3.0.1 software.
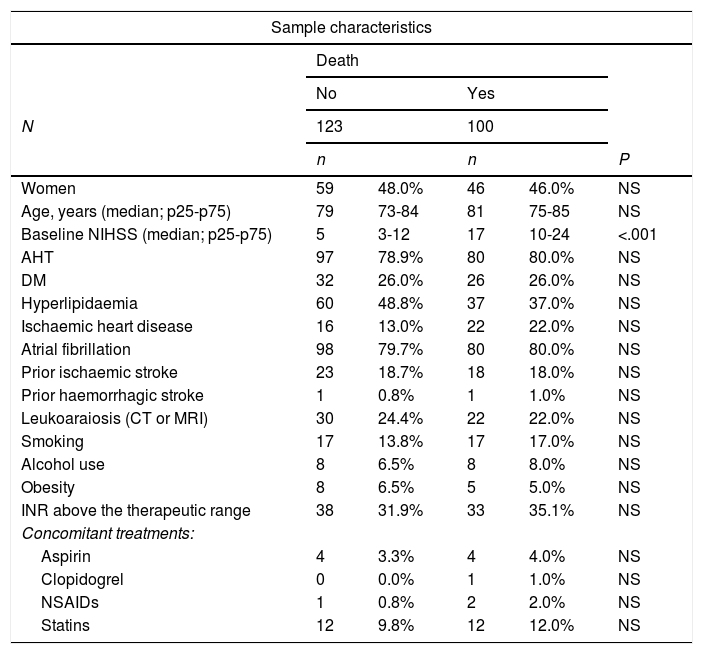
ResultsSample characteristicsWe found a total of 235 cases of VKA-ICH from 21 hospitals during the study period. Patients were followed up for 90 days in 223 cases, with 5.1% of participants lost to follow-up. The mortality rate during the first 90 days was 42.6%. Table 1 includes the baseline characteristics of the sample and compares the two study groups’ clinical characteristics, vascular risk factors, anticoagulation status at admission (measured with the INR), and concomitant medication. Median NIHSS score at admission was higher in the group of patients who died than in the group who survived (17 [IQR: 14] vs 5 [IQR: 9] points, P<.01); no statistically significant association with death was found for any other study variable.
Sample characteristics.
| Sample characteristics | |||||
|---|---|---|---|---|---|
| Death | |||||
| No | Yes | ||||
| N | 123 | 100 | |||
| n | n | P | |||
| Women | 59 | 48.0% | 46 | 46.0% | NS |
| Age, years (median; p25-p75) | 79 | 73-84 | 81 | 75-85 | NS |
| Baseline NIHSS (median; p25-p75) | 5 | 3-12 | 17 | 10-24 | <.001 |
| AHT | 97 | 78.9% | 80 | 80.0% | NS |
| DM | 32 | 26.0% | 26 | 26.0% | NS |
| Hyperlipidaemia | 60 | 48.8% | 37 | 37.0% | NS |
| Ischaemic heart disease | 16 | 13.0% | 22 | 22.0% | NS |
| Atrial fibrillation | 98 | 79.7% | 80 | 80.0% | NS |
| Prior ischaemic stroke | 23 | 18.7% | 18 | 18.0% | NS |
| Prior haemorrhagic stroke | 1 | 0.8% | 1 | 1.0% | NS |
| Leukoaraiosis (CT or MRI) | 30 | 24.4% | 22 | 22.0% | NS |
| Smoking | 17 | 13.8% | 17 | 17.0% | NS |
| Alcohol use | 8 | 6.5% | 8 | 8.0% | NS |
| Obesity | 8 | 6.5% | 5 | 5.0% | NS |
| INR above the therapeutic range | 38 | 31.9% | 33 | 35.1% | NS |
| Concomitant treatments: | |||||
| Aspirin | 4 | 3.3% | 4 | 4.0% | NS |
| Clopidogrel | 0 | 0.0% | 1 | 1.0% | NS |
| NSAIDs | 1 | 0.8% | 2 | 2.0% | NS |
| Statins | 12 | 9.8% | 12 | 12.0% | NS |
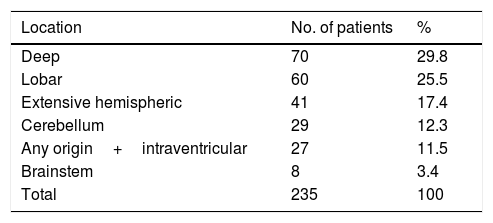
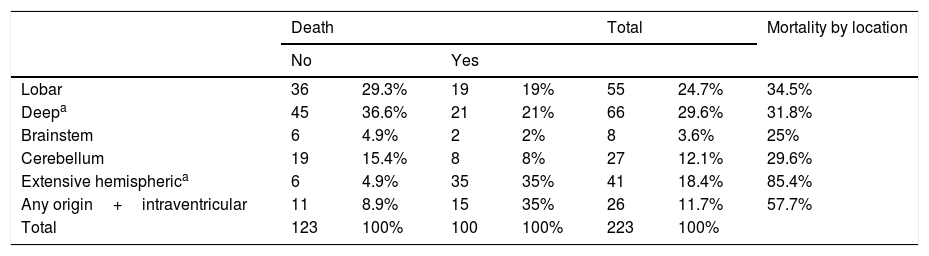
The most frequent location of the haemorrhage in the total sample was deep hemispheric regions (29.8%), followed by lobar regions (25.5%) (Table 2). Regarding the different topographic subgroups (Table 3), a statistically significant association was observed between extensive hemispheric haemorrhage (which may also be associated with an intraventricular haemorrhage) and progression to death (35% vs 4.9%, P<.01 [chi-square and Bonferroni correction]); intraventricular haemorrhages (regardless their origin) of <100mL volume were associated with the second highest mortality rate.
Lesion location and mortality.
| Death | Total | Mortality by location | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Lobar | 36 | 29.3% | 19 | 19% | 55 | 24.7% | 34.5% |
| Deepa | 45 | 36.6% | 21 | 21% | 66 | 29.6% | 31.8% |
| Brainstem | 6 | 4.9% | 2 | 2% | 8 | 3.6% | 25% |
| Cerebellum | 19 | 15.4% | 8 | 8% | 27 | 12.1% | 29.6% |
| Extensive hemispherica | 6 | 4.9% | 35 | 35% | 41 | 18.4% | 85.4% |
| Any origin+intraventricular | 11 | 8.9% | 15 | 35% | 26 | 11.7% | 57.7% |
| Total | 123 | 100% | 100 | 100% | 223 | 100% | |
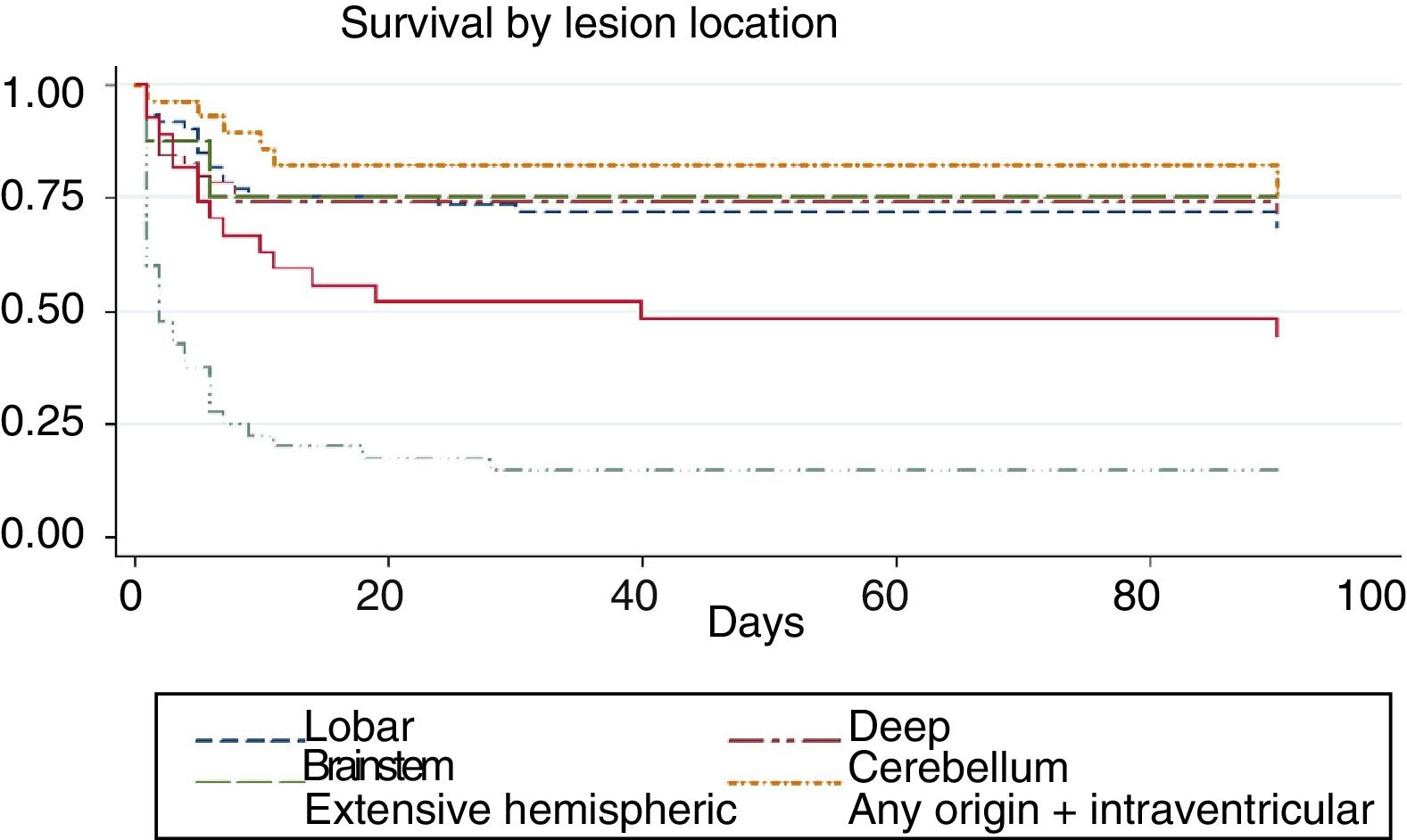
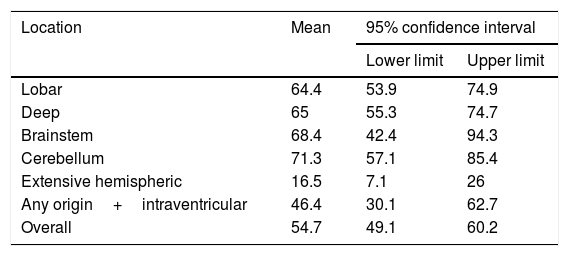
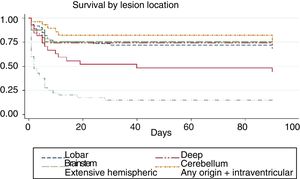
We conducted a survival analysis to assess the relationship between lesion location and time from stroke to death in the first 90 days. Fig. 1 shows the Kaplan–Meier curve displaying an association between lesion location and survival time (log rank, P<.01). Overall, the mean time from stroke to death in the first 90 days was 54.7 days (95% CI, 49.1-60.2 days) (Table 4). In addition to being the deadliest subtype, extensive hemispheric haemorrhages also presented shorter times to death (mean 16.5 days; 95% CI, 7.1-26).
Mean time from stroke to death, by lesion location.
| Location | Mean | 95% confidence interval | |
|---|---|---|---|
| Lower limit | Upper limit | ||
| Lobar | 64.4 | 53.9 | 74.9 |
| Deep | 65 | 55.3 | 74.7 |
| Brainstem | 68.4 | 42.4 | 94.3 |
| Cerebellum | 71.3 | 57.1 | 85.4 |
| Extensive hemispheric | 16.5 | 7.1 | 26 |
| Any origin+intraventricular | 46.4 | 30.1 | 62.7 |
| Overall | 54.7 | 49.1 | 60.2 |
To determine which variables were independently associated with progression to death, we used a logistic regression analysis where the maximum model included those variables associated with death in the bivariate analysis (baseline NIHSS score and extensive hemispheric haemorrhage). The model showed that only baseline NIHSS score independently predicted death (odds ratio: 1.13 [95% CI, 1.08-1.17] for each one-point increase in NIHSS score at admission).
DiscussionNIHSS score at admission and presence of extensive hemispheric haemorrhage (which also showed a shorter survival time) were significantly associated with progression to death in patients taking VKA. However, in the multivariate analysis, only NIHSS score was found to be an independent risk factor for death. Previous anticoagulation state, vascular risk factors, and concomitant medication were not associated with death.
The mortality rate for intracerebral haemorrhage associated with anticoagulant treatment is high (42.6% in our series). Due to the increased prescription of these drugs and the older age of the patients treated, complications derived from these treatments are becoming more frequent.6 A comparative study of patients taking anticoagulants with and without intracerebral haemorrhage has associated the risk of intracerebral bleeding with shorter duration of anticoagulant treatment, prothrombin time, previous cerebral infarction, and use of acenocoumarol; no association was found with age or indication for anticoagulation.8 Another recent Spanish study including over 20 000 patients treated with anticoagulants detected 190 severe haemorrhagic events (8.02%) out of a total 2369 haemorrhagic events. Forty-three presented severe brain haemorrhages, which have the highest risk of death (one in three cases)14; this has made VKA-ICH one of the most feared complications of anticoagulation therapy.15
Several previous studies have attempted to establish variables associated with prognosis and progression to death after ICH in patients taking anticoagulants. For example, patients’ age at admission has been proposed as prognostic maker16; in fact, patients aged older than 85 in our study presented a higher probability of death (51.7% vs 36.6%); this is similar to the rates described in the literature. Thus, statistically significant and independent associations have been described between intraventricular extension and haematoma volume5; haematoma volume and level of consciousness17; and intraventricular extension, diameter, and growth of the haematoma.4,18 Berwaerts et al.19 established a logistic regression model predicting the risk of death in patients with VKA-ICH as a function of the Glasgow Coma Scale score at admission, diameter of the haematoma, and signs of cerebrovascular disease on CT scan; other related factors were location in the posterior fossa, midline shift, and presence of intraventricular haemorrhage.
Thus, the prognostic factors most constantly associated with death in the different previous studies would be level of consciousness at admission and initial haemorrhage volume15; this coincides with the results of our study. A study conducted in Spain in 2013 also found that the only factor independently predicting death was baseline NIHSS score.20 We should highlight that the manifestation of cerebral haemorrhages as lacunar syndrome has been associated with favourable functional and vital outcomes, with a mortality rate of 0%.21
One of the most controversial aspects is the impact of the INR on outcomes. Approximately 68% of haemorrhages have been described in patients with an INR <3.04; in fact, in our series, 29.4% of patients were within the therapeutic range and only one-third were above the therapeutic range.13 According to our results, an INR above the therapeutic range is not associated with a higher risk of progression to death. A study by Alonso de Leciñana et al.20 did not associate haemorrhage volume with INR or establish a link between baseline INR, anticoagulation reversal, or haematoma enlargement and functional outcomes. However, other authors4,22 have established a higher mortality rate in patients with higher baseline INR, which may be due to the larger initial haemorrhage with an INR >3.11 This apparent discrepancy may be due to several factors, but mainly due to the fact that in our study, as in others, there was no follow-up image to assess the possible enlargement of the haematoma. Furthermore, we do not know the time since the last drug administration, the onset of the haemorrhage, and the INR measurement.
The influence of the concomitant use of antiplatelet treatment has also led to conflicting data in the literature. Some authors establish that concomitant treatment with antiplatelet drugs is not an independent risk factor for death,23 which is in line with our results. However, other authors have associated this treatment with a poorer short-term prognosis and increased mortality rate, possibly related to the increased volume of the haematoma.24
In any case, almost all studies report that the risk of death increases with higher numbers of poor prognostic factors, regardless of the surgical, or conservative treatment used.25 Several studies have reported that mortality and morbidity rates remain high, despite INR correction.12 Administration of vitamin K alone takes several hours to reverse anticoagulation26; therefore, additional measures are used in clinical practice, even in the absence of sufficient clinical evidence. A retrospective multi-centre study27 showed that absence of treatment with fresh frozen plasma and/or prothrombin complex is associated with more than twice the risk of death within 30 days, when compared to treated patients, especially those receiving both treatments. No statistically significant differences favouring one of the two treatments were found. Furthermore, anticoagulation reversal of INR <1.3 and systolic blood pressure <160mmHg in the first 24h are associated with a reduced rate of haematoma enlargement.28 These data do not coincide with those reported in a previous study, which did not establish a correlation between the baseline INR and/or the time to correction of INR and enlargement of the ICH.15
We should also mention that according to the corresponding pivotal clinical trials,29–32 direct-acting oral anticoagulants (DAOA), present equal or lower rates of intracranial bleeding than VKA. A mortality rate of 28% has been described in patients treated with DAOA who developed an ICH33; furthermore, ICH seems to present better functional outcomes and smaller volumes in patients treated with DAOA than in those treated with VKA.34
Our study does have some limitations. Since this was a retrospective analysis, we only have data for those patients examined by neurology departments at Spanish public hospitals. Also, we only have data on the first evaluation and no data on findings from follow-up neuroimaging (haematoma enlargement, intraventricular extension, etc.), which other studies have associated with final outcomes. Absolute values for haematoma volume were not recorded; instead, it was classified as larger or smaller than 100mL, which prevents us from making a more accurate correlation. One of the main limitations of our study is the lack of data on the treatment used in these patients, which meant that we were unable to analyse its possible impact on several radiological and laboratory parameters, as well as patients’ clinical progression. Neither was cause of death recorded.
In view of these results, we can conclude that VKA-ICH presents a high mortality rate, mainly and independently associated with clinical status at stroke onset.
FundingThis study was partially funded by an international research grant from Bristol Myers Squibb.
Conflicts of interestThe authors have no conflicts of interest to declare.
Asturias: Hospital Universitario Central de Asturias: Dr Sergio Calleja. Castile-La Mancha: Hospital Universitario de Albacete: Dr Eva Fernández and Dr Tomás Segura. Castile-Leon: Hospital Universitario de León: Dr Javier Tejada García and Dr Noelia González Nafría; Hospital Universitario de Salamanca: Dr José Carlos Gómez. Catalonia: Hospital del Mar: Dr Jaume Roquer; Hospital Universitari Arnau de Vilanova: Dr Francisco Purroy; Hospital de la Santa Creu i Sant Pau: Dr Alejandro Martínez Domeño and Dr Joan Martí Fábregas; Extremadura: Hospital San Pedro de Alcántara: Dr Ignacio Casado. Galicia: Hospital Clínico Universitario de Santiago de Compostela: Dr Manuel Rodríguez Yáñez. Balearic Islands: Hospital Universitari Son Espases: Dr Bárbara Vives, Dr Carmen Jiménez Martínez. Canary Islands: Hospital Universitario de Gran Canaria Dr. Negrín: Dr Juan Carlos López Fernández. Madrid: Hospital Universitario Ramón y Cajal: Dr Jaime Masjuán Vallejo; Hospital Universitario 12 de Octubre: Dr Jaime Díaz Guzmán; Hospital Universitario Clínico San Carlos: Dr Talía Liaño and Dr José Egido; Hospital Universitario La Paz: Dr Borja Enrique Sanz Cuesta and Dr Blanca Fuentes; Hospital General Universitario Gregorio Marañón: Dr Andrés García Pastor, Dr Raúl Domínguez, and Dr Antonio Gil; Hospital Universitario de La Princesa: Dr Gustavo Zapata Wainberg, Dr Álvaro Ximénez-Carrillo, Dr Sonia Quintas, and Dr José Vivancos. Navarra: Complejo Hospitalario de Navarra: Dr Jaime Gállego Culleré. Basque Country: Hospital Universitario Basurto: Dr Juan Luis Idro Montesa and Dr María del Mar Freijó. Valencia: Hospital Clínico Universitario de Valencia: Dr Anna Martín and Dr José Miguel Laínez; Hospital Universitario y Politécnico La Fe: Dr Aída Lago.
The members of the TAC Registry study group are listed in Appendix B.
Please cite this article as: Zapata-Wainberg G, Quintas S, Rico AX, Fernández LB, Vallejo JM, Culleré JG, et al. Factores pronósticos y análisis de la mortalidad de las hemorragias cerebrales asociadas a anticoagulantes orales antagonistas de la vitamina K. Resultados del Estudio TAC Registry. Neurología. 2018;33:419–426.