Leptomeningeal amyloidosis is a rare form of amyloidosis caused by a limited spectrum of mutations in the transthyretin gene (TTR), including the mutation in which threonine replaces alanine at codon 25. We describe the case of a patient with the A25T TTR variant. Only one report of such a case exists in published literature.1,2
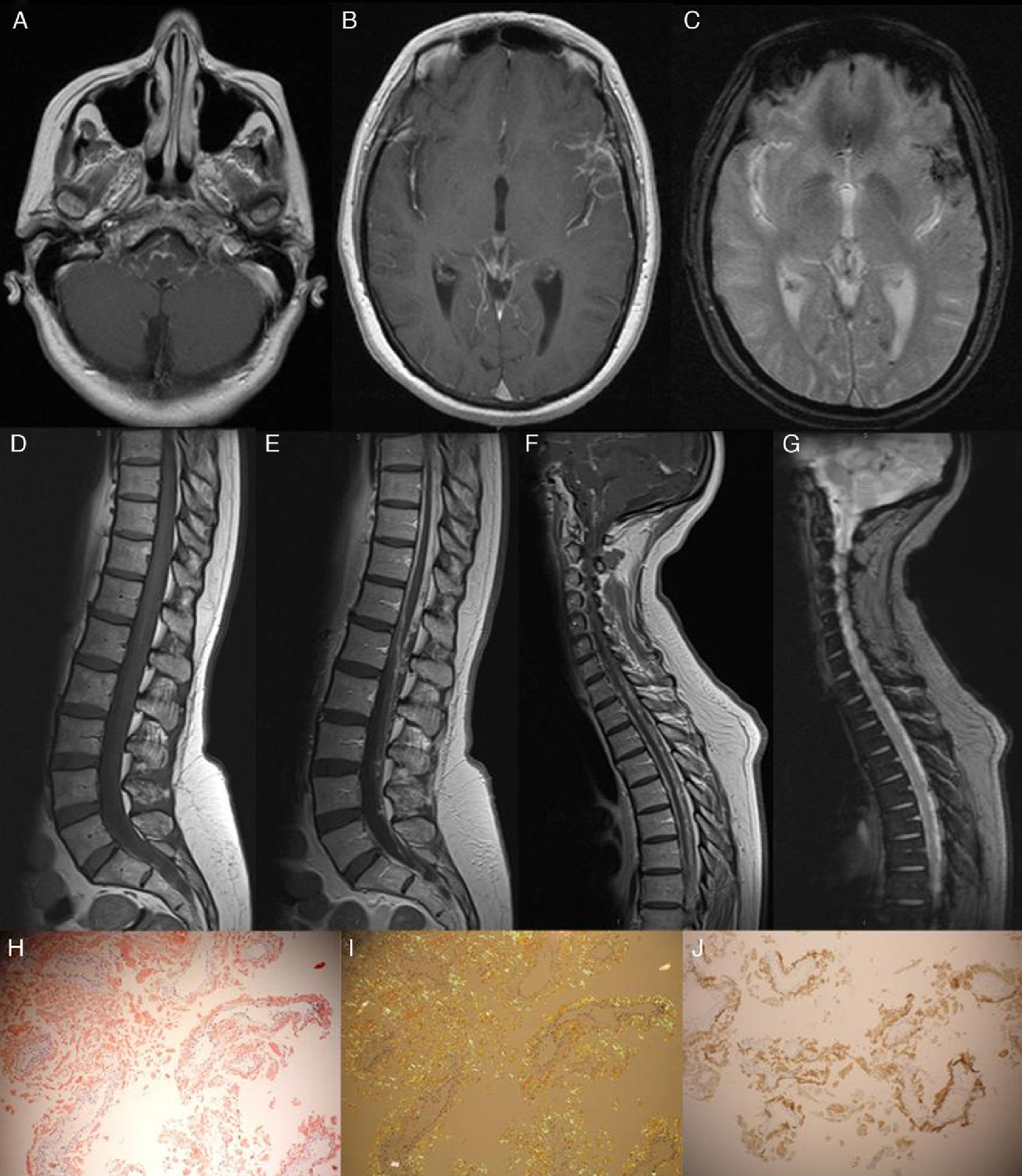
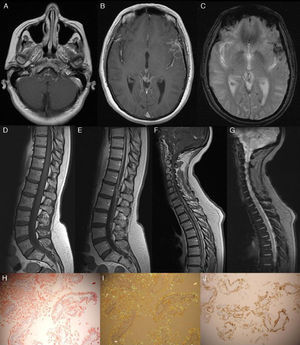
The patient was a 53-year-old woman with a 4-year history of progressive symptoms of vertigo, paraparesis, and ataxic gait. One month before, she had experienced sudden neurosensory hearing loss. A neurological examination revealed normal higher functions and right-sided hypoacusia. Deep tendon reflexes were present and generalised, with intact bilateral extensor plantar reflexes. We found moderate paresis of the right leg with tactile hypoaesthesia and hypopalaesthesia in both legs. Ataxic gait was also present. The CSF study revealed hyperproteinaemia (177mg/dL) and xanthochromia. The electromyography study ruled out peripheral neuropathy. Cranial and spinal cord magnetic resonance imaging revealed superficial siderosis in the left sylvian fissure and signs of chronic bleed in CSF (Fig. 1). Both studies found thickened meninges with gadolinium uptake (see Fig. 1). The meningeal biopsy revealed abundant interstitial and perivascular amyloid deposits that were positive for TTR (see Fig. 1). A genetic study confirmed presence of the A25T TTR mutation. The patient had two healthy children who declined to undergo a genetic study. The patient's mother had died of an undiagnosed disease with similar symptoms at the age of 60. The patient did not undergo studies to rule out the diagnosis of amyloidosis.
Gadolinium-enhanced axial T1-weighted MRI. Note the leptomeningeal contrast uptake on the surface of the brainstem and the left sylvian fissure (A and B). The T2*-weighted axial MRI shows superficial siderosis, principally in the sylvian fissure and occipital sulcus, as well as haemosiderin deposits in the left occipital horn (C). The sagittal T2*-weighted study revealed signs of chronic bleeding (G); the contrast-enhanced T1-weighted MRI showed meningeal thickening and contrast uptake (E and F). Meningeal biopsy: Under polarised light, the Congo red stain revealed abundant interstitial and perivascular amyloid deposits (H and I). Amyloid fibrils were revealed with the anti-TTR antibody (J).
Leptomeningeal amyloidosis associated with TTR mutations is a rare but fatal form of amyloidosis. The A25T TTR mutation found in our patient was previously reported in a Japanese patient who also developed superficial siderosis and died due to multiple intracranial haemorrhages. The autopsy confirmed selective amyloid deposition in the leptomeninges.1,2
Our patient presented both clinical signs and radiological findings compatible with superficial siderosis. Superficial siderosis of the central nervous system is caused by haemosiderin deposition in the leptomeninges and the surface of the brain. These deposits are the result of continuous or recurrent bleeding in the subarachnoid space which are due to multiple causes.3 This presentation was described in the published case of A25T TTR mutation, as well as in other cases of familial amyloidosis in which deposition predominantly affects the meninges.4,5 As a whole, these data stress the importance of considering leptomeningeal amyloidosis as an infrequent cause of superficial siderosis.
At present, there are no specific treatments for leptomeningeal amyloidosis. Liver transplantation, the treatment of choice for preventing the progression of neuropathy associated with most TTR mutations, is not effective in these cases; cells of the choroid plexus are the main producers of TTR in the brain.6 Tafamidis is an inhibitor that selectively binds to TTR in plasma and stabilises the tetrameric structure of amyloid, thereby preventing deposition.7,8 Unfortunately, there are no data suggesting that tafamidis would be able to cross the blood-brain barrier, and it is therefore not regarded as a means of slowing the course of the disease in cases of leptomeningeal amyloidosis (personal communication with Dr. Said). Intraventricular administration of a specific anti-sense oligonucleotide for TTR in the brains of transgenic mice with a mutated human TTR gene results in dose-dependent decreases in TTR expression in the choroid plexus. This is a compelling treatment strategy for these patients.6
We thank Dr. Solé for the images of the meningeal biopsy.
Please cite this article as: Llull L, Berenguer J, Yagüe J, Graus F. Amiloidosis leptomeníngea debida a la mutación A25T TTR. A propósito de un caso. Neurología. 2014;29:382–384.
This study was presented in poster format at the 64th Annual Meeting of the SEN.