Cerebral ischaemia is one of the most common neurological diseases worldwide. Its many sequelae range from motor and sensory symptoms to cognitive decline and dementia. Animal models of cerebral ischaemia/hypoperfusion elicit effects on long term memory; however, the effects of these procedures on short term memory are not clearly understood and effects induced by alternative hypoperfusion models are completely unknown.
MethodsWe evaluated the effects of 2 cerebral hyperperfusion models on memory in 3-month-old male rats. Episodic memory and working memory were assessed using the new object recognition test and the spontaneous alteration test, respectively. Neurological assessment was also performed, along with an open field test to evaluate locomotor activity.
ResultsRats in both hyperperfusion models displayed no cognitive changes. Rats with unilateral left-sided ligation plus temporary ligation of the right carotid tended to show slightly impaired performance on the new object recognition test on the second day after the procedure. In contrast, the group with permanent unilateral ligation tended to display alterations in working and episodic memory 9 days after the procedure, but they subsequently recovered.
ConclusionDespite these differences, both hypoperfusion groups displayed clear signs of motor impairment 2 days after the procedure, as reflected by their decreased locomotor activity during the open field test.
La isquemia cerebral es una de las enfermedades neurológicas más frecuentes a nivel mundial. Las múltiples secuelas abarcan desde lo motor y la sensibilidad hasta un deterioro cognitivo y la demencia. Los modelos animales de isquemia-hipoperfusión cerebral tienen su efecto sobre la memoria a largo plazo, sin embargo, aún no están claros sus efectos a corto plazo y, en el caso de algunos modelos de hipoperfusión, se desconocen por completo.
MétodosSe valoraron los efectos de 2 modelos de hipoperfusión cerebral sobre la memoria en ratas macho de 3 meses de edad. Se evaluó la memoria episódica y de trabajo empleando las pruebas de reconocimiento de objetos nuevos y alternancia espontánea, respectivamente, así como una valoración neurológica y una prueba de locomoción en una arena para campo abierto.
ResultadosSe encontró que en los 2 modelos de hipoperfusión las ratas no presentan una alteración cognitiva. A pesar de que existe una tendencia en las ratas con ligadura unilateral izquierda más ligadura transitoria de la carótida derecha a presentar una leve alteración negativa en la prueba de reconocimiento de objetos nuevos al segundo día después de la oclusión, mientras que el grupo con ligadura unilateral permanente tiende a presentar alteraciones en la memoria de trabajo y episódica a los 9 días luego de la isquemia, con una posterior recuperación.
ConclusiónSin embargo hay una clara afectación motora 2 días después de la cirugía, reflejada por una disminución en la actividad locomotora en la prueba de campo abierto, en ambos grupos con hipoperfusión.
Stroke is the most common neurological disease in the world. It results in approximately 5million deaths a year,1 and the World Health Organization (WHO) lists stroke as the second most frequent cause of death worldwide, accounting for 9.7% of all deaths; 4.95million deaths occur in middle- to low-income countries.2,3
Cognitive deficit associated with cerebral ischaemiaCognitive deficit is frequently present after stroke, and it has a severe impact on the quality of life of both the patient and his or her family members. Some of the most frequent cognitive deficits include attention deficit disorder, spatial neglect, visual inattention, apraxia, amnestic syndrome, and vascular dementia.4
The above cognitive changes are caused by the death of groups of neurons secondary to either haemorrhagic or ischaemic stroke.5
Strokes leading to volumes of brain softening greater than 100mL cause dementia, whereas volumes of less than 50mL are not associated with that entity. When the volume of brain softening measures between 50 and 100mL, the effect is unknown, but dementia may occur. Nevertheless, if a lesion measuring less than 50mL affects a strategic area, such as a single thalamic lesion in the basal ganglia, it can cause such cognitive changes as attention deficit disorder, spatial neglect, visual inattention, and apraxia.6,7
It is estimated that some 25% to 50% of all people with stroke develop vascular dementia, which either provokes deficiencies, or else reveals the presence of prior cognitive deficiencies that it may also exacerbate.8 This condition tends to appear suddenly following infarct, thrombosis, or cerebral haemorrhage. The decline occurs gradually, manifesting as focal neurological signs, occasional partial recoveries, and exacerbations secondary to stroke recurrence. In cases of small and nearly silent infarcts, the disease progression is nearly indistinguishable from that seen in Alzheimer disease.9
Amnestic syndrome differs from dementia in that patients do not show overall cognitive impairment and only memory is affected. The syndrome is typified by the inability to acquire new knowledge, retain it, and retrieve information; this may affect either working or episodic memory.
Working and episodic memoryWorking memory refers to the type used to store information for short periods of time while it is being used to perform a specific task. It includes short-term memory which, according to Baddeley's theory, is limited to passive storage of information for short time periods. Working memory has a control system with a limited capacity for attention, known as the central executive component. Its purpose is to process information and it is assisted by 3 different storage components: the phonological circuit (based on sound and language), the visuospatial circuit (similar to the phonological circuit except that it stores visual and spatial information instead of acoustic data), and the episodic buffer (a short-term mechanism for storing information of different types that can be retrieved from the long-term episodic memory). Tests of working memory feature tasks requiring information storage and processing in the short term.10
Episodic memory is active in the recall of concrete events in space and time, background cognitive processes, and in the neuronal mechanisms that participate in recalling these events. It responds to the questions ‘what?’, ‘when?’ and ‘where?’ with regard to a specific occurrence in a person's life.10–12 It works as an association between different systems of information (sensory, temporal, and spatial) that form a complex array known as an ‘event’.12
Animal models are used to research the relationship between cerebral hypoperfusion and locomotor and cognitive changes, especially those affecting working and episodic memory.13–18
The two-vessel occlusion model of hypoperfusion (occlusion of both carotid arteries) has been thoroughly studied with regard to learning and memory disorders, especially those affecting spatial memory since the above procedure severely affects the hippocampus. This has been demonstrated in water maze tests and the 8-arm radial maze.19–21 Studies have clearly established that experimental cerebral hypoperfusion impairs spatial learning ability in rats. Episodic memory in rats has been evaluated using the object recognition test, whereas working memory is evaluated with the Y-maze. This test has shown that permanent occlusion of both carotid arteries affects working memory.22
Seen as a whole, these data show that severe cerebral hypoperfusion has a deleterious effect on the hippocampus, which leads to impaired visuospatial learning and poorer episodic and working memory. Impairment may be time-dependent; some long-term studies reported no differences between the sham group and the double carotid occlusion group at one week after hyperperfusion, and found that frank cognitive impairment only appeared 16 months later.23
Nevertheless, the one-vessel carotid occlusion model has not been studied to determine its effects on locomotion or memory, even though the technique is easy to perform and results in a pathophysiological situation similar to that occurring in atherosclerotic carotid stenosis.
Materials and methodsResearchers used 25 male Wistar rats weighing between 270 and 370g and aged approximately 3 months. All experiments were undertaken with approval and followed applicable Mexican regulations for the care and use of research animals (NOM-062-ZOO-1999) and the guidelines published by the Society for Neuroscience.
Study groupsRats underwent the following treatments: the LLCC or left lesion group had surgery to produce cerebral hypoperfusion by means of permanent ligation of the left common carotid (n=6). The DLCC or dual lesion group experienced hypoperfusion by permanent ligation of the left common carotid in addition to temporary occlusion (10min) of the right common carotid (n=6). The sham group underwent surgery that exposed but did not ligate both carotid arteries (n=6). All subjects underwent testing prior to surgery in order to be able to compare baseline performance with that at different times after the surgery. Tests included the open field test, new object recognition test, and the spontaneous alternation test in the T-maze. Each animal was handled 5minutes per day for 5 days before beginning behavioural tests to become accustomed to handling.
Open field testThe open field test, developed by Calvin Hall in 1934, was used to evaluate spontaneous locomotor activity. Each rat was placed alone in a translucent acrylic box (75cm×75cm×50cm high) with sand in the bottom.24 The box had been modified to include a grid drawn on the bottom forming 25 identical 15-cm squares.
The open field test was performed in 2 sessions. In the initial or habituation session, the rat was placed in the centre of the box and permitted to explore the space freely for 5minutes. This session was not included in the statistical analysis as its sole function was to allow the animal to acclimate to new surroundings. In the second session, performed 24hours later, the subject was returned to the acrylic box for 5minutes, under the same conditions as those described for the habituation session.25
During this second session, researchers counted the number of squares the rat crossed (a square was counted once three-quarters of the rat's body had entered the next square) and the total time the animal remained in each of the 2 areas defined below. Measurements of these 2 variables took into account immobility and travel time, and specific behaviours in each area. The 2 areas were divided as follows: the central area (corresponding to the 9 central squares) and the peripheral area (the 16 squares arranged around the central area). Researchers also recorded vertical activity (periods in which the rat adopts a standing position). The number of squares crossed was used to assess the degree of spontaneous activity; time spent immobile or exploring in the central or peripheral areas, as well as any vertical activity, were markers used to evaluate interest in exploring and motor coordination.
Novel object recognition testEpisodic memory was assessed using the novel object recognition test.26,27 This test is based on rats’ natural tendency to spend more time examining unfamiliar objects than examining recognised or familiar items. We used 2 identical objects plus a different novel object on each testing day for a total of 4 pairs of identical objects (plastic bottles measuring between 10 and 15cm) and 4 novel objects also made of plastic and approximately the same size as the bottles.
The test was divided into 3 phases: a habituation phase, a familiarisation phase with 2 identical objects, and a novel object recognition phase. Each phase lasted 5minutes. The habituation phase was performed as in the open field test; it was not included in the statistical analysis for the novel object recognition test since the sole purpose of this phase was to allow the animal to acclimate to its new surroundings.
After the 5-minute habituation period, the animal was returned to its home cage where it remained during 20minutes for the familiarisation phase. At this point, 2 identical objects (A1 and A2) had been placed in the home cage, 15cm from the walls at positions 1 and 2.
Spontaneous alternation test in a T-mazeWorking memory was evaluated using the spontaneous alternation test in a T-maze. The test is based on a rodent's tendency to alternate its choice of arms of a T-maze during different sessions. Rats depend on their working memory to recall which arm they have already visited and will therefore explore the other arm on the second attempt.
The spontaneous alternation test was performed according to the Deacon and Rawlins protocol (2006). There was no habituation phase. At the start of each trial, all maze doors were raised and the rat was placed in the starting box. After remaining in the starting box for 20seconds, the rat was permitted to choose one of the arms of the maze. After having made its choice, the animal was shut inside the arm by closing the corresponding guillotine door for 30seconds. The researcher then lifted the guillotine door to the familiar arm and gently returned the rat to the start area with its back to the goal arms. It was then given free choice of one arm or the other once again. After this, the rat was placed in the starting box where it remained for 1minute between trials.
We performed 5 trials per subject on day 7 prior to surgery and on post-surgery days 2, 9, and 15. Researchers evaluated the percentage of correct alternations between goal arms per animal; correct alternation consisted of a rat visiting the arm it had not yet explored.
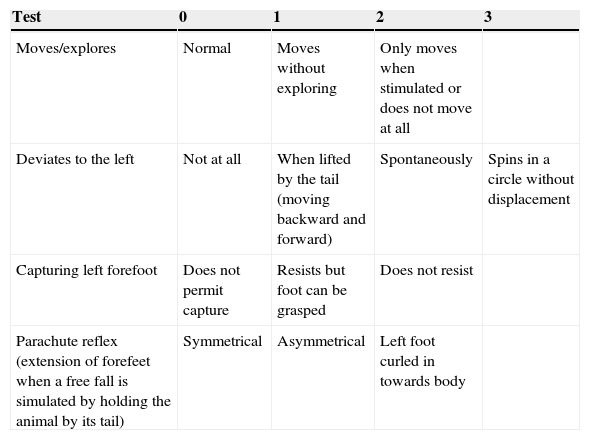
Neurological assessmentA rapid neurological assessment was performed for all 3 rat groups (the sham, LLCC, and DLCC groups) 7 days prior to surgery, just after recovery from anaesthesia, after 24 and 48hours, and at 8, 9, 14, and 15 days after surgery. The assessment was performed using a test allowing researchers to measure the degree of neurological damage on a scale of 0 to 9, where 0 indicates lack of neurological impairment and 9 indicates maximum impairment.28
The assessment drew from a series of tests and was based on observation of the responses listed in Table 1.
Neurological assessment in rats.
| Test | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Moves/explores | Normal | Moves without exploring | Only moves when stimulated or does not move at all | |
| Deviates to the left | Not at all | When lifted by the tail (moving backward and forward) | Spontaneously | Spins in a circle without displacement |
| Capturing left forefoot | Does not permit capture | Resists but foot can be grasped | Does not resist | |
| Parachute reflex (extension of forefeet when a free fall is simulated by holding the animal by its tail) | Symmetrical | Asymmetrical | Left foot curled in towards body |
Additional behavioural tests were performed on days 2, 9, and 15 (post-surgery) in all 3 groups to repeat the evaluations of episodic and working memory. Likewise, the open field test was repeated to evaluate locomotor activity and in later neurological assessments.
Statistical methodSurvival in the different surgical groups is shown as a percentage, as are results from the neurological assessment. Results from other tests (open field test, novel object recognition, spontaneous alternation in T-maze) are expressed as mean±standard deviation. These results were processed using the two-way repeated measures ANOVA method. The 2 ways, or factors, were the days elapsed post-surgery and the surgical procedure completed. We later performed the Student–Newman–Keuls test for significance levels of P<.05. Data are expressed as percentages of change.
ResultsNeurological assessmentAnimals in the sham and LLCC groups scored 0 on the neurological evaluations they completed before and after the surgical procedure (at 7 days before surgery, after recovering from anaesthesia, and at 24hours, 48hours, and days 8, 9, 14, and 15 after surgery). In the DLCC group, only 2 animals had a score of 1 and one had a score of 2 at 24hours and 48hours after surgery. Animals scored 0 on all other evaluations.
The animal with a score of 2 was excluded from further testing since its performance on day 2 was poor and it displayed severe hypomotility. However, it began to recover on day 9. The physical examination recorded drooping left eyelid or ptosis in several animals. This finding was observed in the LLCC group in one rat (16.6%) and in 3 of the rats surviving induced hypoperfusion in the DLCC group (42.8%). There were no cases in the sham group.
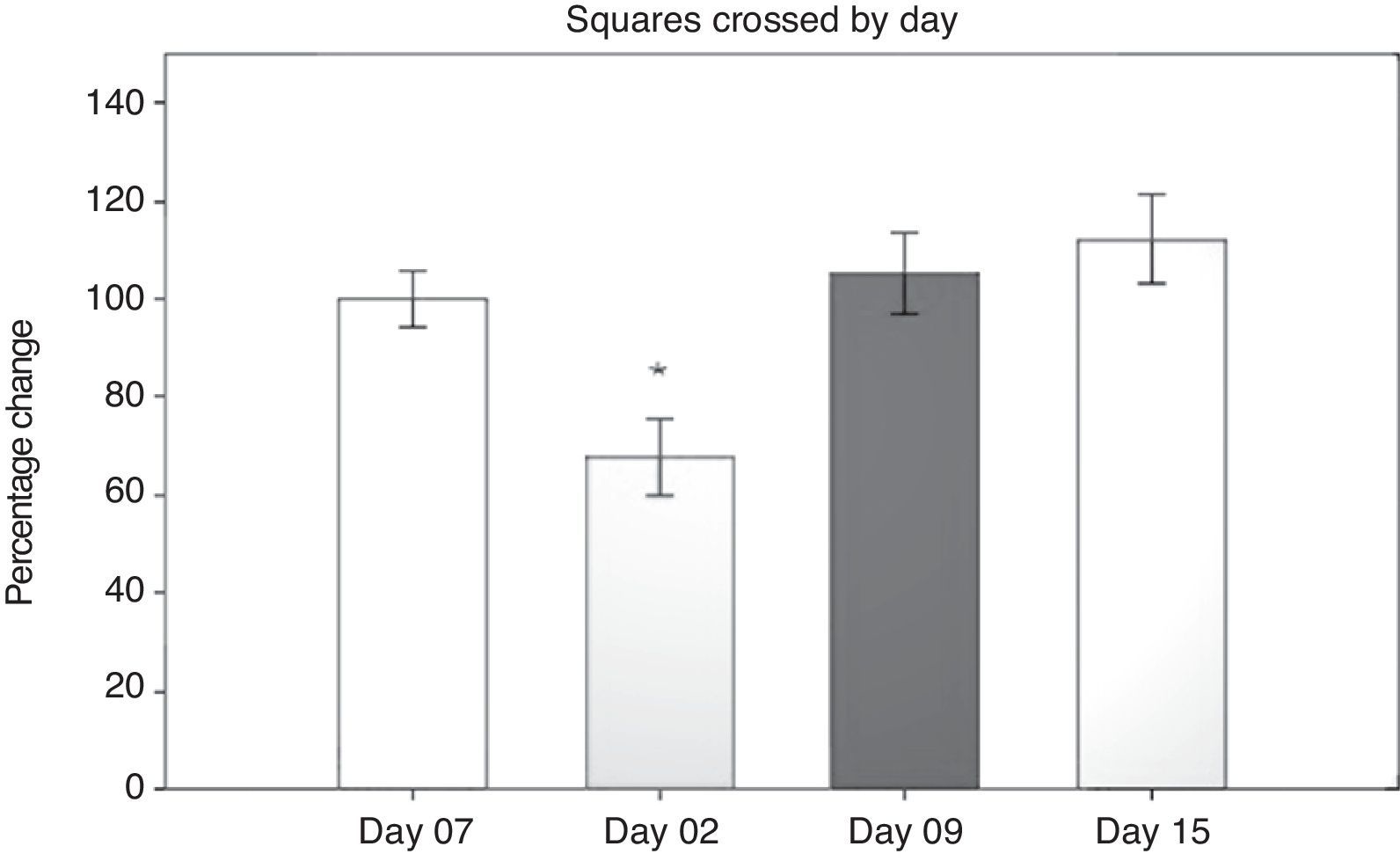
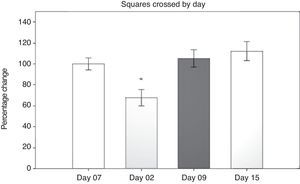
Open field test: number of squares crossedResults from the open field test were first analysed using a 2-way repeated measures ANOVA of the number of squares crossed in 5minutes. The 2 ways were number of days post-surgery and the type of surgery that had been performed. The hypothesis of equality of variances was found to be true (P=.499). The analysis found a difference for the factor ‘days’ (F[3,45]=13.558; P<.001) (Fig. 1). The post hoc Student–Newman–Keuls test (Fig. 1) demonstrated a significant difference in the time factor between day 2 and days 9 and 15 post-surgery, and with day 7 before surgery (P<.001).
On day 2 post-surgery, groups subjected to cerebral hypoperfusion showed decreased locomotor activity, but activity returned to normal beginning on day 9 post-surgery. Hypomotility was also observed in the sham group on day 2, but the effect was less pronounced than in the other groups, and activity did not differ significantly from that on day 7 before surgery (day −7). The sham group in particular demonstrated differences in performance between day 2 and days 9 and 15 (P=.038). The LLCC group displayed differences between day 7 before surgery and day 2 post-surgery (P=.015), between days 2 and 15 (P=.008), and between days 2 and 9 (P=.006). The DLCC group displayed differences between days 2 and 15, (P=.001), days 2 and minus 7, (P=.003), and days 2 and 9 (P=.008).
Open field test: total time in the peripheral areaUsing 2-way repeated measures ANOVA, researchers analysed the total time in which the subject remained in the peripheral area during the open field test. The hypothesis of equality of variances was found to be true (P=.865). The analysis revealed differences with regard to the factor ‘days’ (F[3,45]=3.906; P=.015), but not with respect to the factor ‘surgery type’ (F[2,45]=0.265; P=.771) or the interaction of both factors (surgery type×days) (F[6,45]=0.418; P=.863). The post hoc test reported a difference in the factor ‘time’ between days 2 and 15 (P=.009) and a tendency towards significance for days minus 7 and 2 (P=.077). We did not observe intergroup differences on different days. This indicates that rats tended to spend less time in the peripheral area on later days than on day 2, the day on which all groups spent the most time in the peripheral area on average.
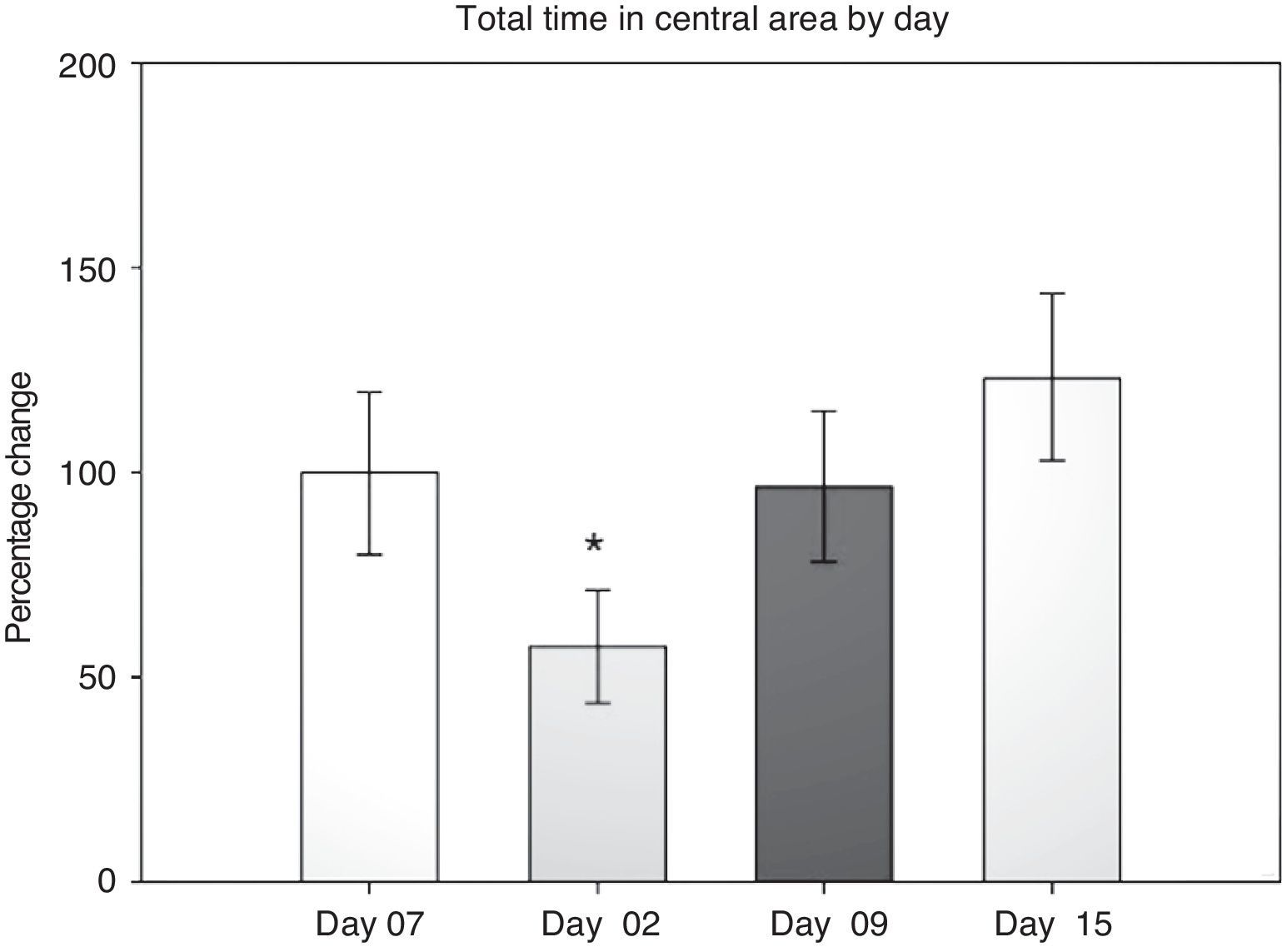
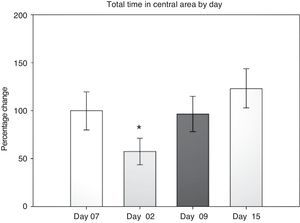
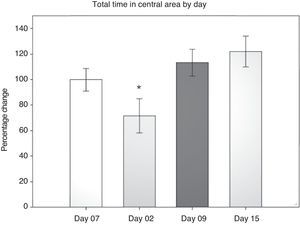
Open field test: total time in the central areaIn evaluating this variable, we measured the time spent grooming, displaying vertical behaviour, exploring, and resting in the central area during the 5-minute testing period. Data were analysed using 2-way repeated measures ANOVA. The hypothesis of equality of variances was found to be true (P=.911). The analysis identified a difference for the factor ‘day’ (F[3,45]=3.894; P=.015), but not for the factor ‘surgery type’ (F[2,45]=0.231; P=.797), or for the interaction of both (surgery type×day) (F[6,45]=0.408; P=.870). The post hoc Student–Newman–Keuls test (Fig. 2) identified a significant difference for the factor ‘day’ between days 2 and 15 (P=.008) for the analysis of time spent in the peripheral area. This shows that while rats spent less time exploring the centre on day 2 post-surgery, they spent increasing amounts of time in the centre in subsequent sessions up to day 15. There were no intergroup differences apart from a tendency in the DLCC group (P=.078 do not test) between post-surgery days 2 and 15.
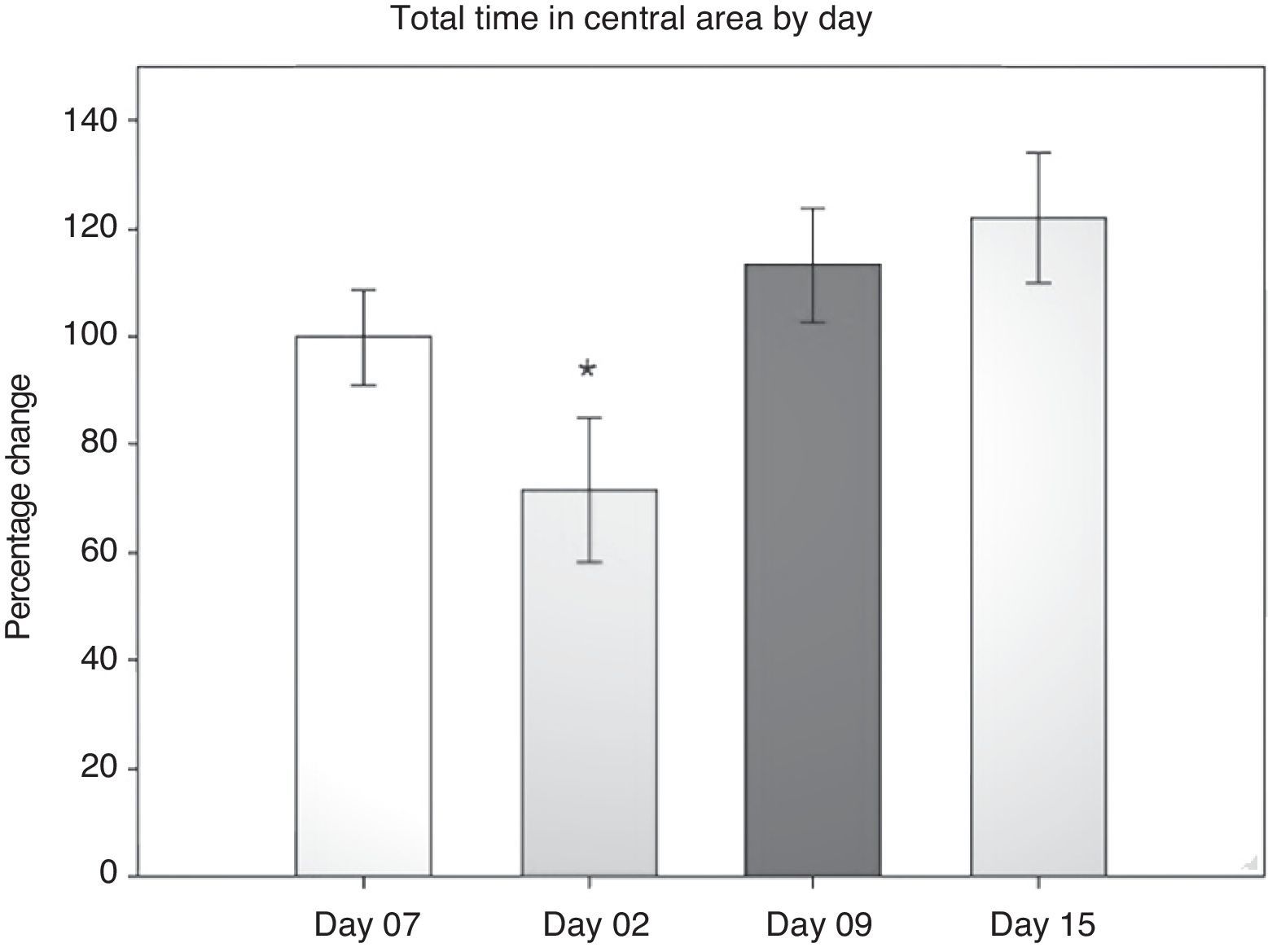
Open field test: total vertical behaviour timeResearchers timed the rats’ vertical behaviour in the entire field (central and peripheral areas) during each 5-minute session. Data were analysed using two-way repeated measures ANOVA. The hypothesis of equality of variances was found to be true (P=.224). The analysis found highly significant differences for the factor ‘day’ (F[3,45]=7.932; P=.001) and for the interaction ‘surgery type’בday’ (F[6,45]=2.628; P=.029). Differences for the factor ‘surgery type’ were not significant (F[2,45]=2.775; P=.094). The post hoc Student–Newman–Keuls analysis (Fig. 3) found a difference between days 2 and 9 (P=.001), days 2 and 15 (P<.001) and days 2 and minus 7 (P=.014). The LLCC group demonstrated a tendency towards significance for days minus 7 and day 2 (P=.071). In contrast, the DLCC group showed a significant difference between day 2 and day minus 7 (P=.007), day 9 (P=.003), and day 15 (P<.001). Comparing the factors ‘surgery type’ and ‘day’ also revealed differences, on day 2, between the sham and DLCC groups (P=.023) and the sham and LLCC groups (P=.015). On day 15, differences were also present between the sham and LLCC groups (P=.050) and the DLCC and LLCC groups (P=.026).
Open field test: total frequency of vertical behavioursTotal frequency of vertical behaviours was measured by counting each time the animal exhibited vertical behaviour, whether in the central or the peripheral area, during the 5-minute open field test. Data were analysed using 2-way repeated measures ANOVA. The hypothesis of equality of variances was found to be false (P<.050). The analysis found a highly significant difference for the factor ‘day’ (F[3,45]=9.674; P<.001), but not for surgery type (F[2,45]=0.391; P<.683) or for the interaction of both factors (surgery type×day) (F[6,45]=1.344; P<.258). The post hoc Student–Newman–Keuls test indicated a significant difference for the factor ‘day’ between day 2 and day 7 before surgery, and between day 2 and days 9 and 15 post-surgery (P<.001). In the LLCC group, we observed a difference between day 2 and days minus 7 (P=.018), day 9 (P=.019), and day 15 (P=.015). Likewise, the DLCC group displayed differences between day 2 and day minus 7 (P=.003), day 9 (P=.003), and day 15 (P<.001).
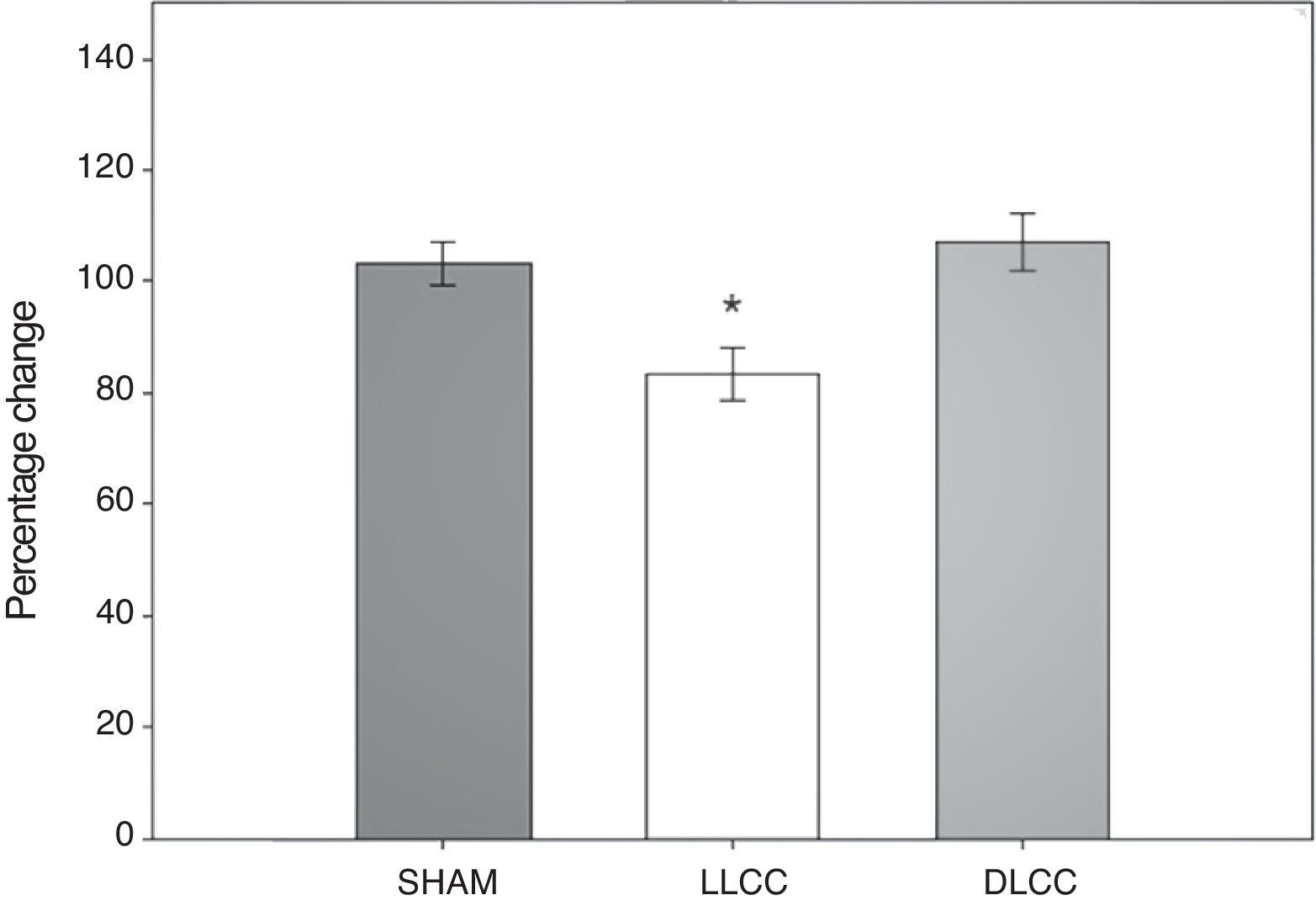
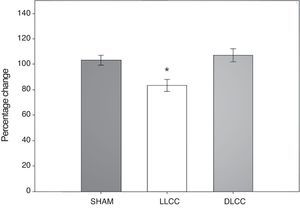
T-maze spontaneous alteration testResults from the spontaneous alternation test in the T-maze were analysed using the percentage of correct alternations displayed by subjects undertaking that test. Data were analysed using 20-way repeated measures ANOVA. The hypothesis of equality of variances was found to be true (P=.320). This analysis found significant differences for surgery type (F[2,45]=5.399; P=.017) and for the interaction of surgery type×day (F[6,45]=3.015; P=.015), but not for the factor ‘day’ alone (F[3,45]=0.205; P=.892) (Fig. 4).
The post hoc Student–Newman–Keuls test demonstrated a statistically significant difference for the factor ‘surgery type’ between the LLCC group and the sham group (P=.022) and the DLCC group (P=.020). The LLCC group also showed a nearly significant difference between day minus 7 and day 9 (P=.061). Similarly, the DLCC group displayed a tendency towards a lower percentage of correct alternation on day 2 compared to day 15 (P=.075). Regarding interaction between factors (surgery×day), there was a difference between the LLCC and sham groups on day 2 (P=.04), between the LLCC group and the sham and DLCC groups on day 9 (P=.009, P≤.001 respectively), and between the LLCC and DLCC groups on day 15 (P=.012). The difference between the sham and DLCC groups tended towards significance (P=.069).
DiscussionThis study assesses the effects of 2 hypoperfusion models on episodic and working memory, as well as on spontaneous locomotor activity. These models consisted of permanent ligation of the left common carotid artery in the first case, and in the second, the same procedure with the addition of a temporary (10min) occlusion of the right common carotid to achieve double occlusion.
Rat model of common carotid artery occlusion has been widely studied to determine its effects on both unilateral17,29 and bilateral blood flow.30–32 However, only the bilateral model has been used to study effects on behaviour, particularly long-term effects on spatial, episodic, and working memory.19–23 In this study, we evaluated episodic and working memory in a model featuring permanent unilateral common carotid occlusion and a new model combining that procedure with temporary occlusion of the other carotid artery. Subjects were tested within a relatively short time period.
The rodents did not display significant changes in working and episodic memory.
In contrast, the open field test revealed a considerable decrease in spontaneous locomotor activity on day 2 post-surgery in groups experiencing hypoperfusion (LLCC and DLCC) compared to the sham group. Based on the above, we deduce that carotid artery occlusion probably had a greater effect on the motor cortex than on the hippocampus in the first days after onset of hypoperfusion. We also observed that the decrease in locomotor activity was even more marked in the DLCC group. This result is logical if we consider the changes in cerebral blood flow that other authors have described: after occlusion of the common carotid artery, cerebral blood flow decreases from 1.41+0.17 to 0.99+0.08mL/g/minute. Blood flow is reduced to about 75% of the normal level and remains at 1.15+0.08 during 24hours before returning to normal levels at day 5 post-surgery.33 This state was well tolerated by the rats; there were no mortalities in the LLCC group, and their memory loss appeared at a later point.
In contrast, the high mortality rates in the DLCC group shows that double occlusion resulted in severe ischaemic injury in some of these animals. Some authors studying bilateral occlusion in Wistar rats have reported blood flow dropping to 33.6% in the cerebral cortex, to 58.3% in the hippocampus, and to 69.5% in the thalamus34; another study in Wistar rats reported decreases of 62% to 69% after 30minutes of hypoperfusion.35 In many cases, this practice results in irreparable neural damage and even death in some subjects; the surviving rodents have a higher probability of presenting sequelae in locomotor activity. These findings are also supported by the decrease in vertical behaviour and the neurological evaluations in which DLCC rats displayed higher scores than rats in the other 2 groups. Nevertheless, they did exhibit signs of recovery beginning on day 9, which seems to point to mechanisms mediating plasticity and neurogenesis which would thus contribute to compensation and recovery in the medium term.
Another result that corresponds to the severity of hypoperfusion is the presence of signs similar to those observed in Horner syndrome.
Some authors have stated that these signs in rats are caused by lesion to the vagus nerve during neck surgery.36 However, based on our own study, we believe that these signs occur as a result of hypoperfusion itself since they affected a higher percentage of individuals in the group undergoing double ligation of the carotid (42.8%) than in the group with permanent ligation of the left carotid artery only. In all cases, the signs appeared ipsilateral to the permanent ligation on the left side. Meanwhile, none of the animals in the sham group displayed ptosis although both common carotid arteries had been dissected and both vagus nerves had been manipulated. Likewise, none of the animals in the double ligation group presented ptosis on the right side even though the vagus nerve had been touched and they had experienced right-sided ischaemia lasting 10minutes. Ptosis therefore seems to be secondary to hypoperfusion due to an infarct at the hippocampal level, as occurs in humans with temporal lobe damage.5,37 Another possibility is that the basilar artery could be affected by a thrombus caused by the carotid occlusion.38
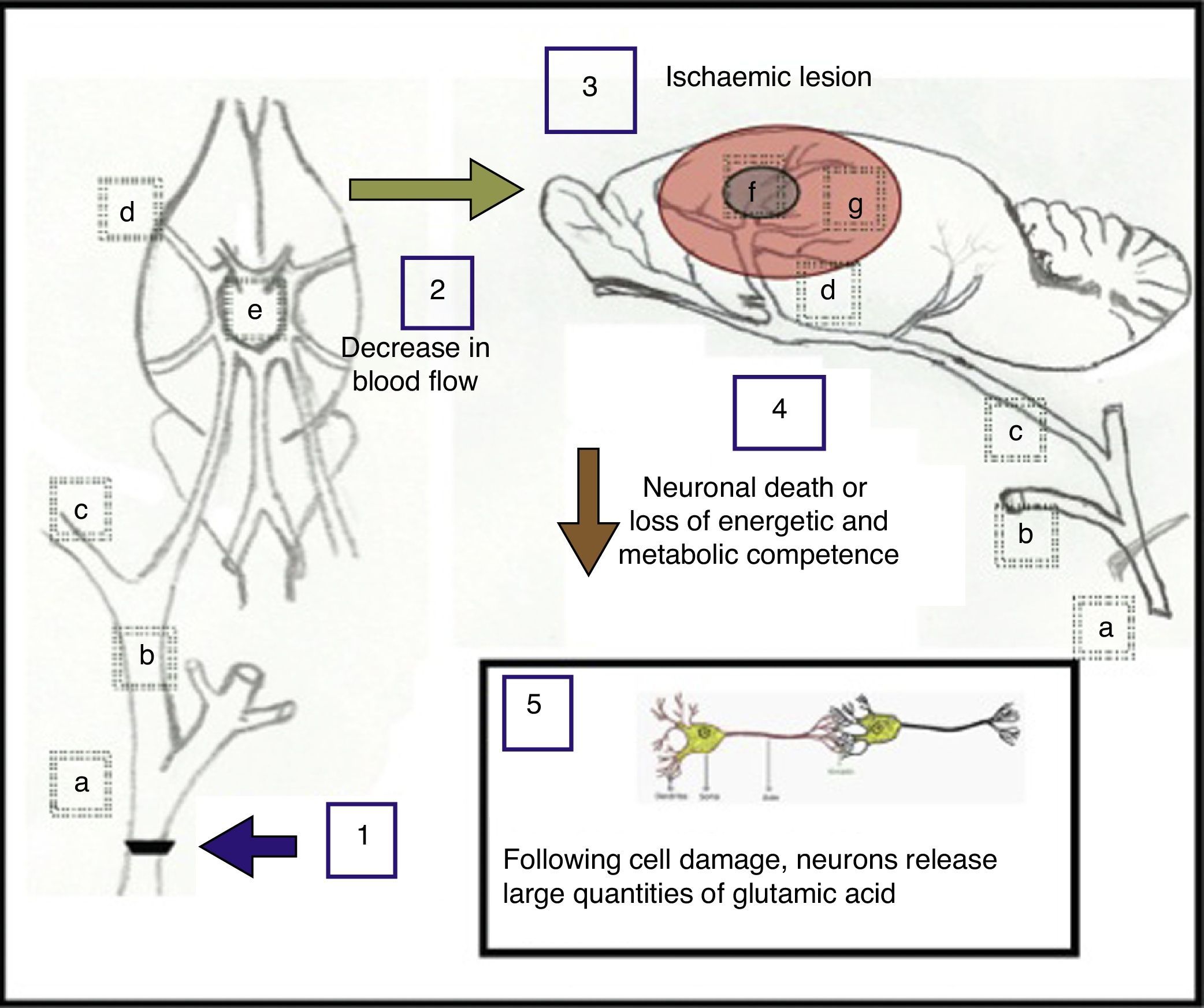
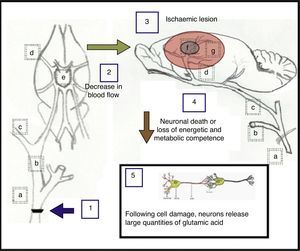
One possible explanation for the results observed after carotid artery occlusion is shown in Fig. 5 and also described below.
Proposed explanation of the effects caused by unilateral and bilateral occlusion at the fork of the common carotid artery. (a) Common carotid artery; (b) external carotid artery; (c) internal carotid artery; (d) middle cerebral artery; (e) circle of Willis; (f) ischaemic nucleus and (g) ischaemic penumbra.
Unilateral occlusion of the common carotid artery (1), performed to simulate the consequences arising from atherosclerotic carotid stenosis, gives rise to a decrease in cerebral blood flow (2) of approximately 25%.33 In turn, occlusion of both carotid arteries elicits a decrease in blood flow of approximately 66.4%,34 causing one or more ischaemic lesions (3) in which neuronal death occurs, or where neurons lose energetic and metabolic competence (4). As a result of this damage to cells, neurons may release large quantities of glutamic acid (5).28
The main areas found to be affected after unilateral ligation of a carotid artery display decreased blood flow as follows: 75% of the former capacity in the medial cortex, 86% in the dorsolateral cortex, 90% in the lateral cortex, 85% in the hippocampus, 56% in the medial thalamus, 62% in the lateral thalamus, 78% in the medial striatum, and 85% in the lateral striatum in Wistar rats.17 With double ligation in Wistar rats, blood flow has been reported to drop to 33.6% in the cerebral cortex, 58.3% in the hippocampus, and 69.5% in the thalamus. These are the main areas supplied by the middle cerebral artery, which arises from the terminal end of the internal carotid artery and is the most affected by an occlusion at the bifurcation of the common carotid artery. At the same time, these areas are involved in locomotion and memory. Nevertheless, our double ligation model showed more impairment of locomotion than of memory.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martínez-Díaz JA, García LI, Hernández ME, Aranda-Abreu. Efectos sobre la locomoción y la memoria de 2 modelos de hipoperfusión cerebral en ratas Wistar macho. Neurología. 2015;30:407–415.