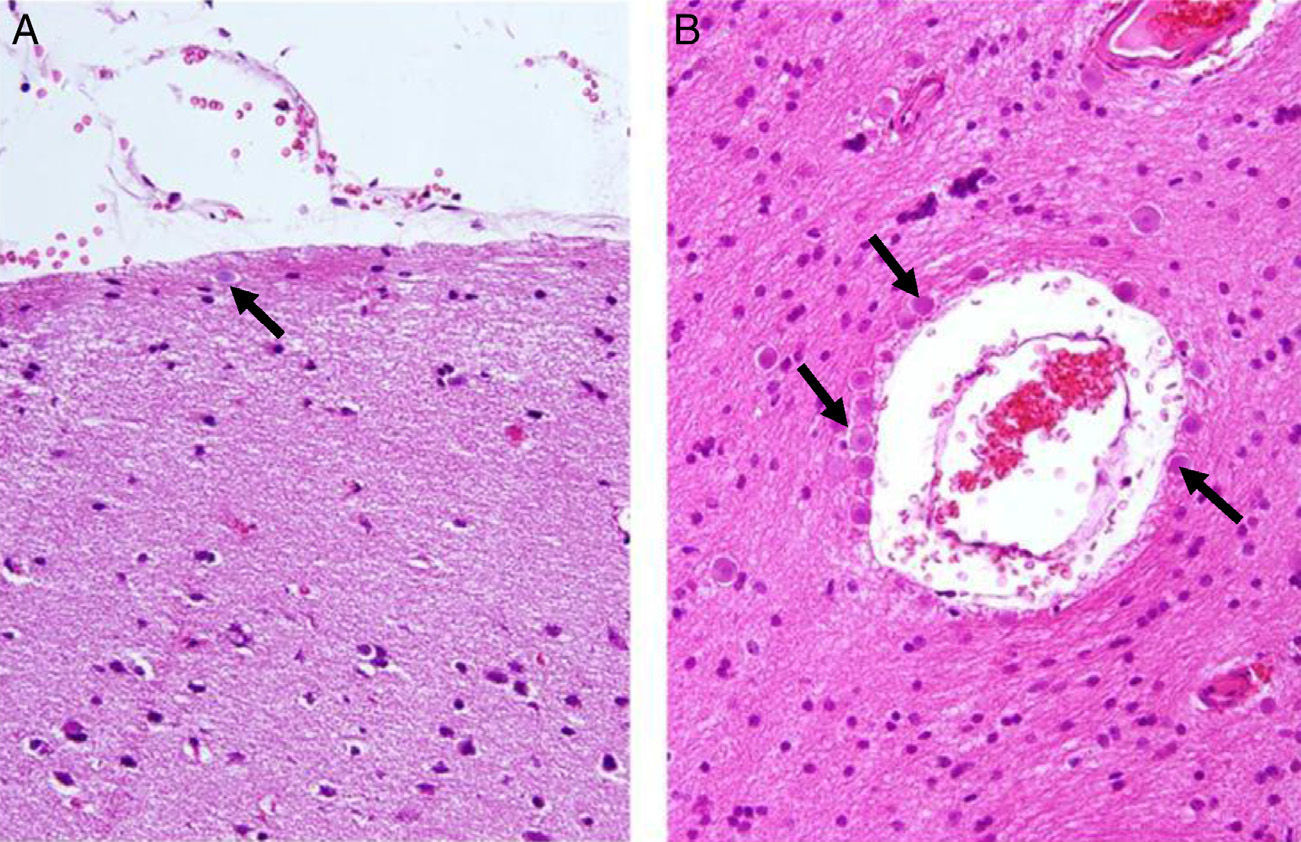
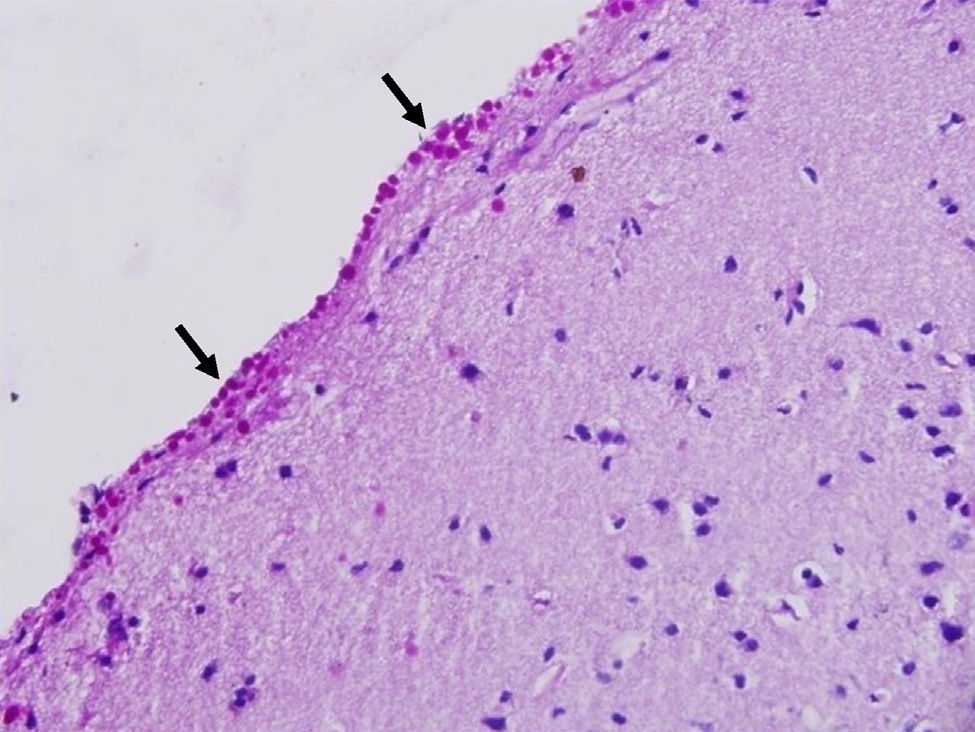
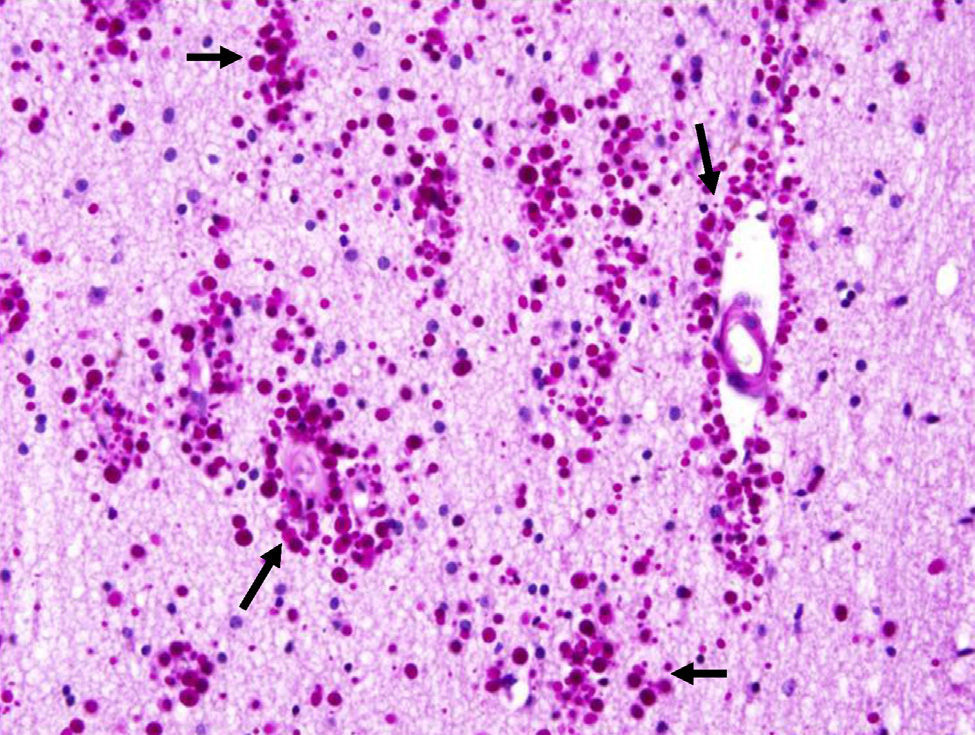
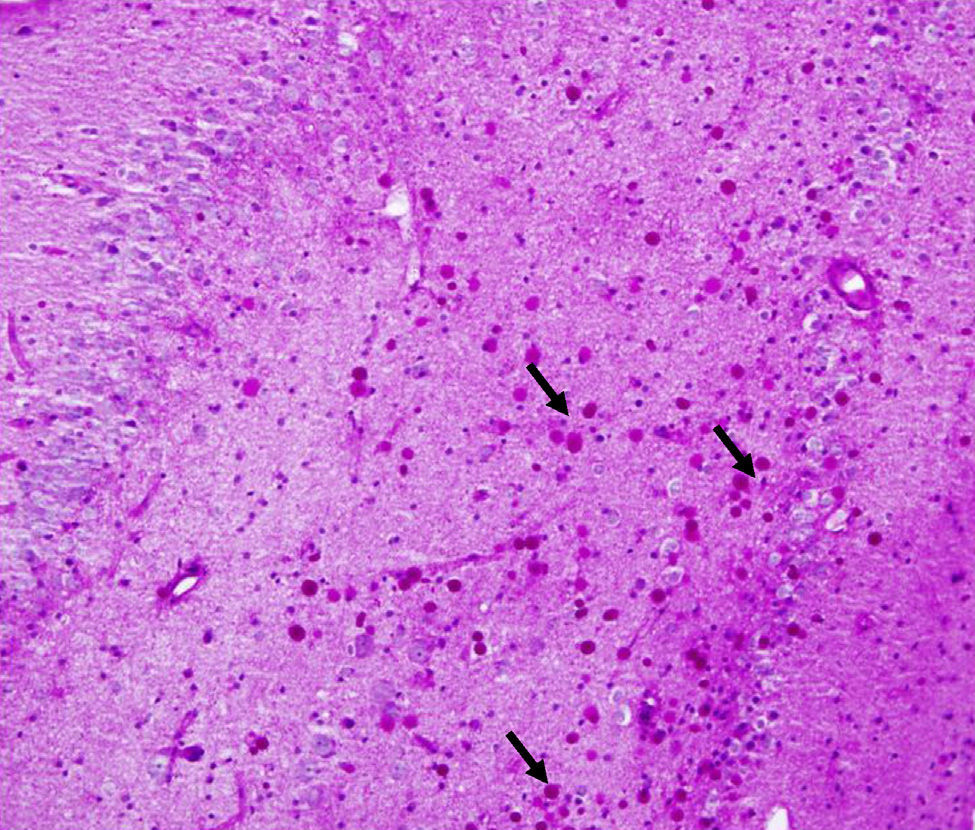
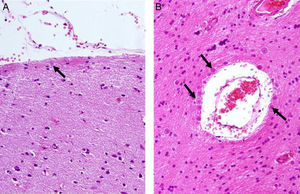
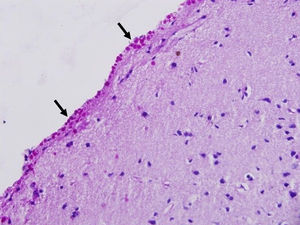
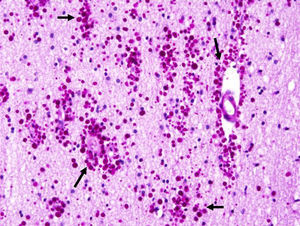
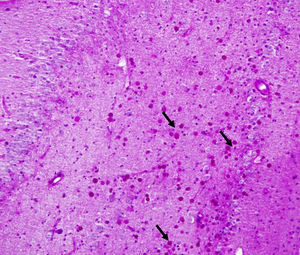
Corpora amylacea (CoA) are present in about 60% of atrophic hippocampi resected from patients with drug resistant temporal lobe epilepsy (DRTLE). They have also been described in the lateral temporal neocortex, although less frequently.
ObjectiveThe objective is to measure the presence, distribution and density of CoA in the lateral temporal lobes of patients with DRTLE and focal cortical dysplasia (FCD), also examining how CoA density may be linked to demographic and clinical traits.
MethodsResected tissue from 35 patients was analysed. CoA density was assessed with a semi-quantitative scale according to the criteria established by Cherian et al.
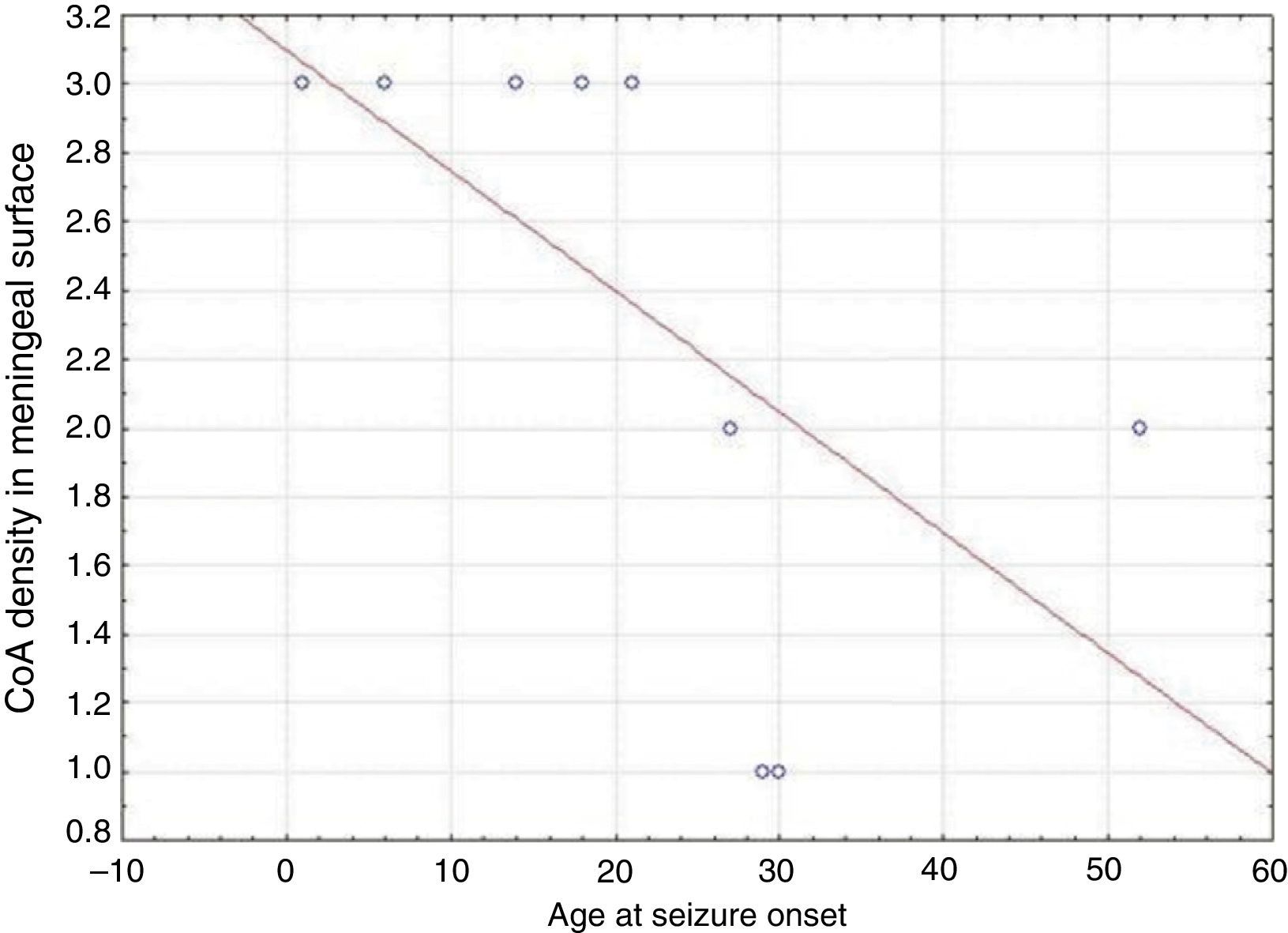
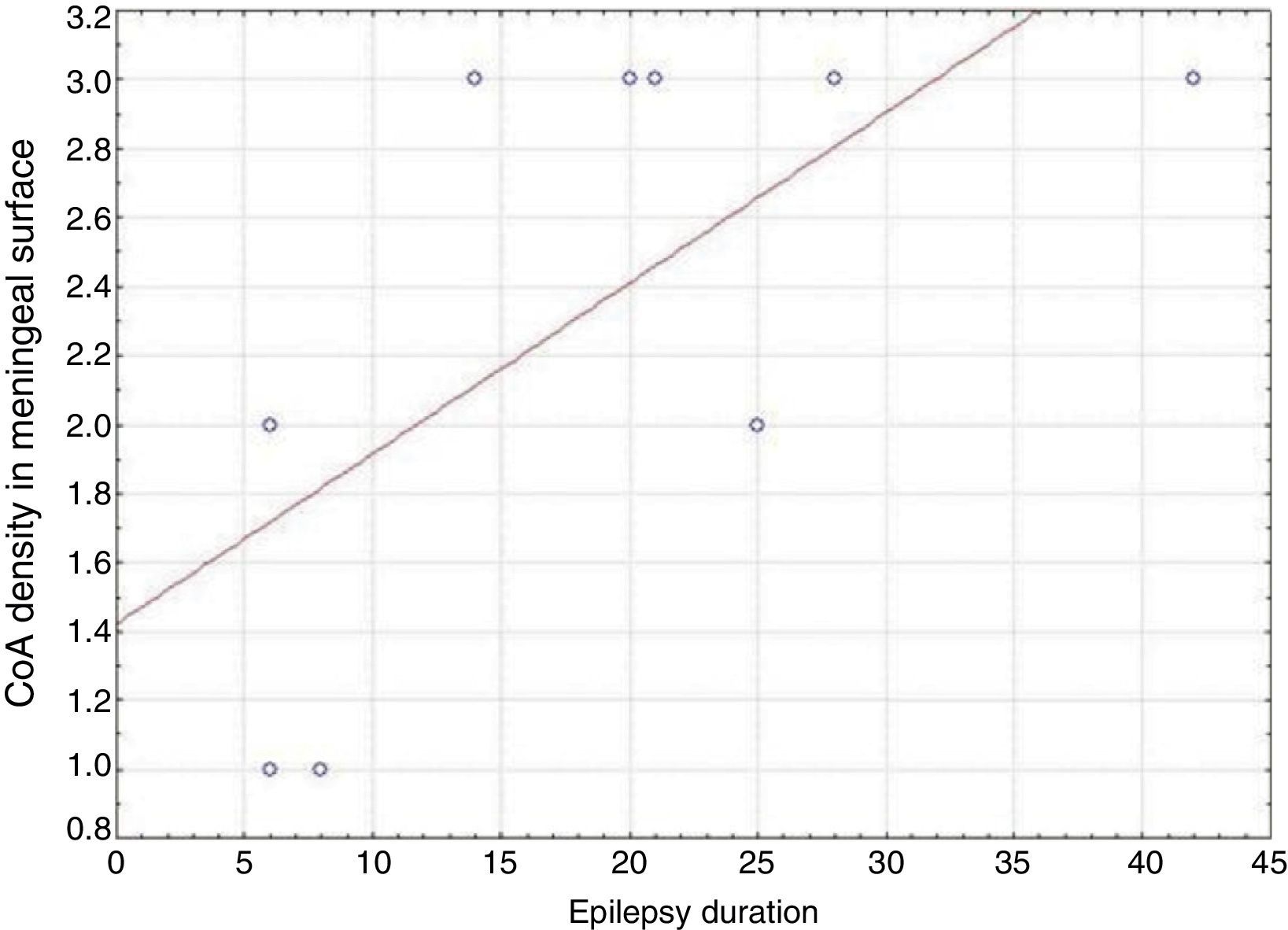
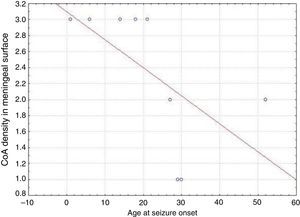
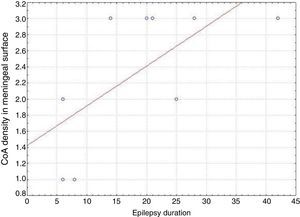
ResultsPresence of CoA in the neocortex of nine patients was associated with hippocampal sclerosis (FCD type IIIa, seven cases), dysembryoplastic neuroepithelial tumour (FCD type IIIb, one case), and cavernous angioma (FCD type IIIC, 1 case). The meningeal surface (MS) was involved in all cases, and eight cases displayed CoA in the cerebral parenchyma (white matter) and around blood vessels. CoA density on the MS showed a negative correlation with age at seizure onset (r=−0.828, P<.05) and a positive correlation with disease duration (r=0.678, P<.05) but not with postoperative clinical outcome.
ConclusionsPatients with DRTLE and a primary lesion (hippocampal sclerosis, tumour, vascular malformation) associated with mild FCD were shown to have CoA deposits in the neocortex. No association was found between presence of CoA and clinical outcome 1 year after surgery.
Los cuerpos amiláceos (CoA) se presentan en aproximadamente el 60% de los hipocampos atróficos resecados de pacientes con epilepsia del lóbulo temporal farmacorresistente (ELTFR). Su presencia en la neocorteza temporal lateral ha sido observada con menor frecuencia.
ObjetivoEl objetivo es evaluar la presencia, la distribución y la densidad de CoA en el lóbulo temporal lateral de pacientes con ELTFR y displasia cortical focal (DCF) y la relación de su densidad con variables demográficas y clínicas.
MétodosAnalizamos histológicamente el tejido resecado de 35 pacientes con ELTFR. La densidad de los CoA fue evaluada con una escala semicuantitativa según los criterios de Cherian et al.
ResultadosLa presencia de CoA en la neocorteza de 9 pacientes estuvo asociada a esclerosis hipocampal (DCF tipo iiia, 7 casos), tumor neuroepitelial disembrioplásico (DCF tipo iiib, un caso) y angioma cavernoso (DCF tipo iiic, un caso). Todos los pacientes tuvieron afectación de la superficie meníngea (SM) y en 8 casos se localizaron en el parénquima cerebral (sustancia blanca) y alrededor de los vasos sanguíneos. La densidad de los CoA en SM tuvo una correlación negativa con la edad de inicio de las crisis (r=-0,828, p<0,05) y positiva con la duración de la enfermedad (r=0,678, p<0,05) pero no con la evolución clínica postquirúrgica.
ConclusionesEn pacientes con ELTFR con lesión principal (EH, tumor, malformación vascular) asociada a DCF ligeras se constata la acumulación de CoA en la neocorteza. No se encontró una asociación entre la presencia de CoA y la evolución clínica al año de la cirugía.
Surgery constitutes a valid treatment alternative in patients with drug-resistant temporal lobe epilepsy (DRTLE). Its most frequent neuropathological substrate is hippocampal sclerosis (HS), also known as mesial temporal sclerosis,1 and it is detected by MRI in 87% to 89% of all patients.2
Robitaille et al. proposed the umbrella term ‘polyglucosan bodies’ (PB) to refer to Lafora bodies, Lafora-like bodies, Bielschowsky bodies, and corpora amylacea (CoA), since all of these structures show biochemical similarities with no histochemical or ultrastructural differences. PBs are rounded, amorphous, laminated, and basophilic. They range from 10 to 50μm in diameter and they are composed of glucose polymers.3
PBs are considered non-specific structures.3 When present in greater quantities, they are considered a pathognomonic symptom in several different diseases, and the specific term describing them will depend on the coexisting symptoms. For example, PBs are called Lafora bodies in Lafora disease,4 Bielschowsky bodies in choreoathetosis and cerebral palsy,5 and CoA in normal ageing and neurodegenerative diseases.6
Focal cortical dysplasia (FCD) is a specific subtype among the malformations of cortical development. It is highly epileptogenic and a frequent cause of drug-resistant epilepsy.7
CoA serve as a marker of HS when neuronal loss and gliosis are difficult to assess due to surgical resection of hippocampal tissue.8
CoA can be seen in the hippocampus and lateral neocortical tissue in DRTLE. However, there are few published studies describing them at either of these locations.9–11
In light of the above, our study aims to assess the presence, distribution, and density of CoA in the lateral temporal lobes of surgically treated patients with DRTLE and FCD, as well as to determine the connection between CoA density and demographic and clinical variables (age at seizure onset, epilepsy duration, and post-operative results).
Subjects and methodsWe consulted the Centro Internacional de Restauración Neurológica (CIRN) database for patients with DRTLE who had undergone temporal lobectomy with electrocorticography and shown no response to any antiepileptic drugs (AED) for at least 2 years before the surgery. Of these surgically treated patients, we performed histological studies on those with FCD and CoA.
All participants underwent a thorough pre-surgical evaluation, including clinical and neurological evaluation, according to protocol. We assessed seizure history and performed a video-electroencephalography (EEG) study, neuropsychological assessment, and structural and functional MRI studies.
Imaging criteria required for a diagnosis of HS were hippocampal atrophy (evidence of neuronal loss), loss of the inner structure of the hippocampus, hyperintensity in T2-weighted FLAIR images (gliosis), and hypointensity in T1-weighted IR images.12,13
We used the classification system proposed by Blümcke et al.14 for histological diagnosis of FCD.
Neocortical tissue was resected en bloc and immediately fixed with paraformaldehyde for 72hours. It was later sectioned in 3mm portions and embedded in paraffin. Portions were sectioned into 6μm slices and stained with haematoxylin-eosin (H&E), Klüver-Barrera, and Periodic Acid-Schiff (PAS) stains.
CoA density was assessed on a semi-quantitative scale15: >10 CoA per high-power field (HPF) (grade 3), 6-10 CoA per HPF (grade 2), and <6 CoA per HPF (grade 1). We also analysed CoA location within the specimens under study.
We used a modified version of the Engel Epilepsy Surgery Outcome Scale (EESOS) criteria to classify outcome 1 year after surgery,16 and SAS version 6 for statistical data analysis. Non-parametric tests were applied. For the correlation analysis, we used Spearman rho and Mann-Whitney U test to compare differences between independent groups. Results with a P-value<.05 were considered statistically significant.
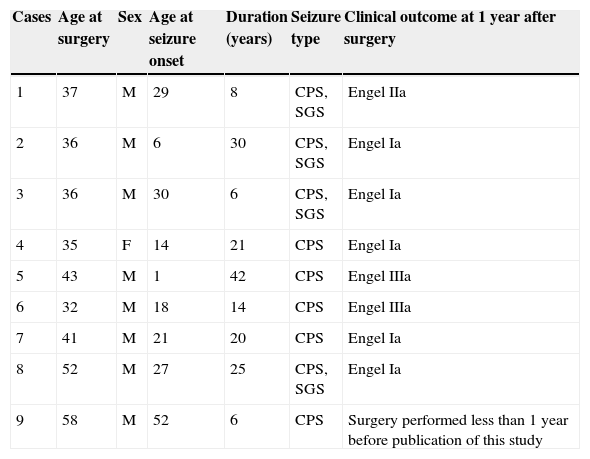
ResultsDemographic data from patients with CoA are summarised in Table 1. The temporal lobe epilepsy database included 35 patients, nine of whom presented CoA (25.71%). Mean age at surgery was 41.11 years and mean duration of the disease before surgery was 19.11 years.
Clinical and demographical data of patients with corpora amylacea and drug-resistant temporal lobe epilepsy. Clinical outcome
| Cases | Age at surgery | Sex | Age at seizure onset | Duration (years) | Seizure type | Clinical outcome at 1 year after surgery |
|---|---|---|---|---|---|---|
| 1 | 37 | M | 29 | 8 | CPS, SGS | Engel IIa |
| 2 | 36 | M | 6 | 30 | CPS, SGS | Engel Ia |
| 3 | 36 | M | 30 | 6 | CPS, SGS | Engel Ia |
| 4 | 35 | F | 14 | 21 | CPS | Engel Ia |
| 5 | 43 | M | 1 | 42 | CPS | Engel IIIa |
| 6 | 32 | M | 18 | 14 | CPS | Engel IIIa |
| 7 | 41 | M | 21 | 20 | CPS | Engel Ia |
| 8 | 52 | M | 27 | 25 | CPS, SGS | Engel Ia |
| 9 | 58 | M | 52 | 6 | CPS | Surgery performed less than 1 year before publication of this study |
CPS, complex partial seizures; SGS, secondarily generalised seizures.
Structural MRI studies corroborated the presence of HS in 7 out of these 9 patients. Diagnosis in two patients was confirmed histologically based on the selective neuronal loss observed in fields CA1 (Sommer's sector), CA4 (end folium), and the granule cell layer (GCL) of the dentate gyrus, associated with diffuse gliosis. Mesial structures could not be evaluated by microscope in the remaining five patients due to the surgical procedures they had undergone. MRI in one patient showed a leptomeningeal cystic mass in the right temporal region with a discrete expansive pattern. The remaining patients displayed no neocortical abnormalities.
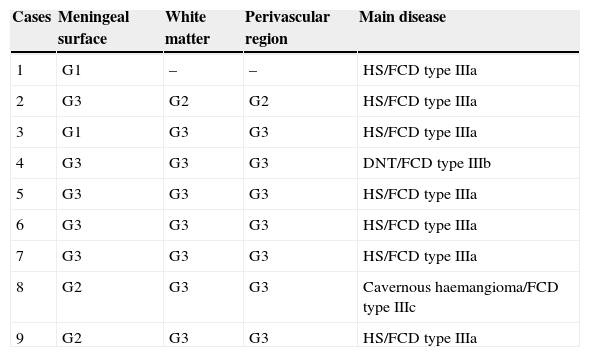
CoA were rendered visible by H&E (Fig. 1), and even more so with the PAS stain due to its deep red colour (Figs. 2–4). These stains enabled use of the semi-quantitative scale. Table 2 shows CoA location, density (grades), and diagnosis of the main disease for each of the nine patients. A more detailed histological analysis of the lateral neocortex, performed for all nine patients, showed abnormal cortical lamination (FCD) associated with HS (FCD type III, seven patients); dysembryoplastic neuroepithelial tumour (FCD type IIIb, one patient), and cavernous angioma (FCD type IIIc, one patient). CoA were observed on the meningeal surface in all nine patients, eight of whom also presented CoA in the white matter and around blood vessels. Four patients presented the maximum CoA density at these three locations: however, there were no statistically significant differences in CoA density with regard to location. Both of the patients to undergo hippocampal histological study displayed CoA (density grades 1 and 3). CoA were located in field CA4 and the GCL (Fig. 4) in one patient, and in field CA4 in the other.
Distribution and density of corpora amylacea (in grades), and main disease
| Cases | Meningeal surface | White matter | Perivascular region | Main disease |
|---|---|---|---|---|
| 1 | G1 | – | – | HS/FCD type IIIa |
| 2 | G3 | G2 | G2 | HS/FCD type IIIa |
| 3 | G1 | G3 | G3 | HS/FCD type IIIa |
| 4 | G3 | G3 | G3 | DNT/FCD type IIIb |
| 5 | G3 | G3 | G3 | HS/FCD type IIIa |
| 6 | G3 | G3 | G3 | HS/FCD type IIIa |
| 7 | G3 | G3 | G3 | HS/FCD type IIIa |
| 8 | G2 | G3 | G3 | Cavernous haemangioma/FCD type IIIc |
| 9 | G2 | G3 | G3 | HS/FCD type IIIa |
FCD, focal cortical dysplasia; HS, hippocampal sclerosis; G, grade; DNT, dysembryoplastic neuroepithelial tumour.
Table 1 shows post-operative outcomes according to EESOS. One year after surgery, five patients with CoA remained seizure-free, whereas three patients experienced seizures.
In our series, we found that patients who were younger at seizure onset presented a higher density of CoA in the meningeal surface (negative correlation, r=−0.828) (Fig. 5). In addition, longer history of epilepsy was associated with a higher CoA density in the meningeal surface (positive correlation, r=0.678) (Fig. 6). No correlations were found between age at surgery and CoA density, or between presence of CoA and clinical outcome at 1 year after surgery (P=.18).
As previously stated, few published studies address the presence of CoA in the lateral temporal neocortex in patients with drug-resistant epilepsy. Two of these studies11,17 are case reports, and the rest of them also address associated microscopic alterations, including neuronal abnormalities, perivascular glial satellitosis, reactive gliosis, and FCD.9,18,19
Keller proposes that accumulation of CoA is associated with ageing.6 However, this premise is not applicable to our patients for two reasons: mean age at surgery was 41.11, and no correlations were identified between CoA density in the different studied locations and age at surgery. However, we found that distribution of CoA in our study was somewhat similar to that in cerebral ageing (subpial, subependymal, and perivascular regions).17
In addition to cerebral ageing, other conditions that favour CoA development include chronic hypoxia, diabetes mellitus, and multiple sclerosis.20 None of our patients presented any of these conditions, and only two of them had experienced perinatal insult.
Previous studies10,11,17 and case series8,9,15,18,19,21–26 have described abundant CoA in the neocortex and hippocampus of patients with DRTLE and HS. CoA have been documented in the following regions of resected hippocampi: CA1, CA3, CA4, end folium and dentate gyrus, and to a lesser extent, the parahippocampal cortex.8,15,25 Of the two hippocampi we examined, only one was assigned a grade of 3 in field CA4 and the GCL.
Our series demonstrated a negative correlation between age at seizure onset and CoA density on the meningeal surface, as well as a positive correlation between duration of epilepsy and CoA density at that location. Both Ribeiro et al. and Abubakr et al. suggest that the association between epilepsy duration and presence of CoA limited to the hippocampus is due to the epileptogenic process.22,23 Other authors postulate that development of CoA is associated with processes induced by insult.27 Seizures might therefore contribute to CoA development in the hippocampus and neocortex.
Abubakr et al. postulate that seizures can promote PB or CoA development due to the increased demand on the glycolytic pathway, which leads to the formation of stable conjugates of ubiquitin with polyglucosan. Polyglucosan levels increase due to induction of heat shock proteins.23 This hypothesis may also explain the presence of CoA in the lateral neocortex in our patients. On the other hand, some authors state that astrocyte proliferation in response to epileptic seizures may lead to CoA accumulation.17
Several studies reporting contradictory results have described associations between presence of CoA in the hippocampus and clinical outcomes in patients with DRTLE who underwent surgery.
The study by Van Paesschen et al. compared clinical features and post-operative outcomes between 26 patients with HS and CoA and 15 patients with HS without CoA. No significant differences were found between the two groups. These authors also suggested that the inverse correlation between CoA density and neuronal density supports the hypothesis of neuronal loss as the cause of CoA, structures that would constitute an epiphenomenon in the pathogenic processes involved in HS.25
Cherian et al. found no significant differences in clinical outcome in 58 patients with HS, including cases with and without CoA (33 vs 25 patients), after 2 years of follow-up.15 The study by Erdamar et al. did not identify a correlation between CoA count and neuronal loss.28
Likewise, Kawamura et al. could not demonstrate any significant differences in age at seizure onset, disease duration, and clinical outcome between patients with HS and CoA (18 patients) and those with HS but no CoA (11 patients).26
Radhakrishnan et al. compared clinical features, EEG results, and post-operative outcome between patients with DRTLE and HS with CoA (n=129; 34.5%) and those without CoA (n=244; 65.5%). Age at surgery and duration of epilepsy were higher in the patient group with CoA than in the group without CoA. Despite clinical outcomes being similar in both groups, the authors found a greater number of patients under treatment with AEDs in the CoA group.24
Patients remained seizure-free after surgery in both of the studies describing CoA in the neocortex.11,17 Our small series could not demonstrate an association between presence of CoA and clinical outcome at 1 year after surgery. We must be mindful in any case that CoA is not the main histological diagnosis, but rather a consequence of DRTLE (see Table 2).
In summary, accumulations of CoA in the neocortex can be seen in patients with DRTLE whose main lesion (HS, tumour, vascular malformation) is associated with mild FCD. We found no connection between presence of CoA and clinical outcome at 1 year after surgery.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Estupiñán-Díaz BO, Morales-Chacón LM, García-Maeso I, Lorigados-Pedre L, Báez-Martín M, García-Navarro ME, et al. Cuerpos amiláceos en la neocorteza de pacientes con epilepsia del lóbulo temporal y displasia cortical focal. Neurología. 2015;30:90–6.