Neuromyelitis optica is an inflammatory and usually relapsing demyelinating autoimmune disease of the central nervous system that targets the optic nerves and spinal cord. Rituximab has been used for different neurological diseases that are probably immune-mediated or involving humoral immunity. The objective of this study is to evaluate the efficacy and safety of rituximab as treatment for neuromyelitis optica in a tertiary hospital.
MethodsRetrospective study of patients with neuromyelitis optica treated with rituximab 1000mg on days 1 and 15, repeated every 6–8 months. We recorded EDSS score, relapse rate, overall condition, CD19+ count, presence of anti-NMO antibodies, and possible adverse reactions.
ResultsSix patients were treated; all were women with a median age of 46 years (range 38–58). Anti-NMO antibodies were detected in 3 patients (50%). Baseline EDSS was 4 (range 2.0–5.5). Two patients had previously been treated with an immunomodulatory drug. Median time from the first rituximab infusion to first relapse was 3.7 years (range 1.7–6.9). Two patients had infusion reactions after the first dose of rituximab. Four patients remained relapse-free and their EDSS score did not progress during rituximab treatment, one patient showed no clinical improvement, and one patient could not be evaluated.
ConclusionRituximab can be considered an attractive therapeutic alternative for patients with neuromyelitis optica as there are no approved treatments for this disease. Further studies with rituximab are needed to establish the role of this drug in treating neuromyelitis optica.
La neuromielitis óptica es un trastorno autoinmune, inflamatorio y desmielinizante del sistema nervioso central que afecta a la médula espinal y al nervio óptico, de carácter recurrente. Rituximab ha sido utilizado en el tratamiento de varias enfermedades neurológicas de probable naturaleza autoinmune o donde la inmunidad humoral estaba implicada. El objetivo de este estudio es evaluar la eficacia y seguridad de rituximab en el tratamiento de la neuromielitis óptica en un hospital terciario.
MétodosEstudio retrospectivo de pacientes con neuromielitis óptica tratadas con 2 infusiones de rituximab 1.000mg separadas por 15 días, cada 6–8 meses. Se evaluó la puntuación EDSS, la existencia de brotes, el estado general, el recuento de CD19+, la presencia de anticuerpos anti-NMO y las reacciones adversas.
ResultadosSe trataron 6 pacientes, todas mujeres (mediana de edad 46 años; rango 38–58). En 3 de ellas (50%) se detectó la presencia de anticuerpos anti-NMO. La EDSS basal fue de 4 (rango 2–5,5). Dos pacientes recibieron previamente algún fármaco inmumodulador. La mediana desde el primer brote hasta el tratamiento con rituximab fue de 3,7 años (rango 1,7–6,9); 2 pacientes presentaron alguna reacción adversa tras la primera infusión del fármaco. En cuanto a la respuesta, 4 pacientes no volvieron a tener brotes y su EDSS no progresó, en una paciente no se observó mejoría clínica y otra no pudo evaluarse.
ConclusionesEl uso de rituximab en pacientes con neuromielitis óptica puede considerarse una alternativa terapéutica interesante, ya que no existen tratamientos autorizados para esta enfermedad. Son necesarios más estudios con rituximab para establecer el lugar de este fármaco en la terapéutica de la neuromielitis óptica.
Neuromyelitis optica (NMO) is an inflammatory and demyelinating relapsing autoimmune disease of the central nervous system that affects the spinal cord and the optic nerve.1 According to the 2006 criteria for a diagnosis of NMO, there must be at least one episode of optic neuritis, one of myelitis, and at least 2 or 3 supportive criteria: demyelinating spinal cord lesion affecting 3 or more segments, MRI not meeting Paty criteria for diagnosis of multiple sclerosis (MS), and presence of anti-aquaporin-4 antibodies (anti-NMO antibody).2 There are no clinical trials evaluating treatment for this entity at present.3 Treatment consists of immunosuppressants/immunomodulatory drugs, and glucocorticoids and plasmapheresis are used to treat relapses. In the context of autoimmunity, T cell-dependent autoreactivity depends on B-cells as antigen presenting cells or co-stimulatory cells.4 Since pathological production of antibodies is a trait common to numerous autoimmune diseases, rituximab (RTX) has been used over the last few years as treatment for various neurological diseases that are probably autoimmune, and in which humoral immune response is impaired.5 RTX is an antibody which acts against CD20+ B cells by depleting circulating cells for 6 to 8 months.5 The aim of this study is to assess effectiveness and safety of RTX as treatment for NMO in a tertiary care hospital.
MethodsThis is a retrospective study of patients with NMO diagnosed according to criteria by Wingerchuk et al.2 and treated with RTX. Treatment cycles consisted of 2 infusions of 1000mg 15 days apart, repeated every 6 to 8 months (to maintain a level of CD19+ lymphocyte expression>1%); in one patient we used a schedule of 4 doses of 375mg/m2. Patients were examined on the day of infusion and at 3-month intervals. We recorded EDSS (Kurtzke scale), relapse rate, overall condition, CD19+ count, and presence of anti-NMO antibodies. Adverse reactions during treatment with RTX were systematically recorded. All patients were informed about the possible risks and benefits of the treatment with RTX, with specific emphasis on the possibility of developing progressive multifocal leukoencephalopathy, cytokine release syndrome, and allergic reactions. We obtained informed consent from all patients. The follow-up period covered at least 6 months after the last administration of RTX.
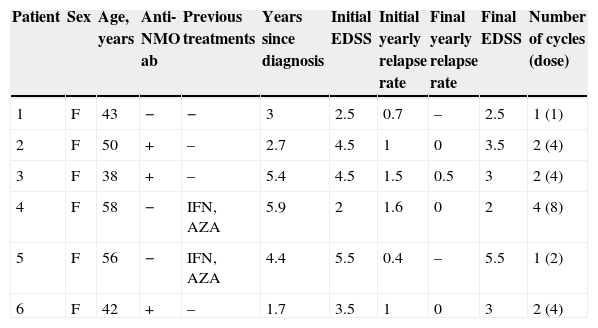
ResultsPatients’ clinical characteristics are listed in Table 1. Two patients had previously been treated with an immunomodulator and all received glucocorticoids for relapses and plasmapheresis for refractory relapses. Two patients presented some type of infusion-related adverse effect after the first dose of RTX; one case resolved with administration of 80mg methylprednisolone and an antihistamine, but treatment was interrupted in the other case when symptoms persisted. Regarding patient's responses, 4 patients show a lower yearly relapse rate and their EDSS score has not progressed; one patient showed no clinical improvement after 2 doses of RTX, and her treatment was changed to cyclophosphamide. Response to RTX could not be evaluated in one patient who received only one dose because she experienced a severe adverse effect related to the first infusion. In this case, the alternative therapy provided was also cyclophosphamide. Median follow-up time in the 4 patients who responded was 34 months (range, 15–56 months). We monitored anti-NMO antibodies titres in all seropositive patients to assess their status after treatment with RTX; one patient tested negative at a later date. We repeated the anti-NMO antibody count in patients with negative results and their results remained the same.
Clinical characteristics of patients.
| Patient | Sex | Age, years | Anti-NMO ab | Previous treatments | Years since diagnosis | Initial EDSS | Initial yearly relapse rate | Final yearly relapse rate | Final EDSS | Number of cycles (dose) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 43 | − | − | 3 | 2.5 | 0.7 | – | 2.5 | 1 (1) |
| 2 | F | 50 | + | – | 2.7 | 4.5 | 1 | 0 | 3.5 | 2 (4) |
| 3 | F | 38 | + | – | 5.4 | 4.5 | 1.5 | 0.5 | 3 | 2 (4) |
| 4 | F | 58 | − | IFN, AZA | 5.9 | 2 | 1.6 | 0 | 2 | 4 (8) |
| 5 | F | 56 | − | IFN, AZA | 4.4 | 5.5 | 0.4 | – | 5.5 | 1 (2) |
| 6 | F | 42 | + | – | 1.7 | 3.5 | 1 | 0 | 3 | 2 (4) |
Anti-NMO ab: anti-aquaporin 4 antibodies; AZA: azathioprine; EDSS: Expanded Disability Status Scale; F: female; IFN: interferon beta.
To date, there is no standard therapy for this condition, which is usually relapsing and tends to have a grave prognosis. Some 30% to 40% of patients in some series died from respiratory failure within 5 years, which is a highly infrequent outcome in patients with MS.6 Over the last few years, different dosing schedules of RTX for treating NMO have been used; this has also been tried to treat MS and other autoimmune diseases.4,5,7 One patient was started on a schedule of 4 doses of 375mg/m2; since then, all other patients have received 2 doses of 1000mg 15 days apart. Repeating the RTX schedule at 6 to 9 months is supported by the evidence obtained in patients with MS. This schedule is based on the time it takes for CD20+ B-cells to be detected in blood again.8 Effectiveness in our patients is slightly lower than that reported in other studies. For example, the series by Bedi et al. showed that all patients stabilised or improved their EDSS score9; the series by Jacob et al.,10 one of the largest series published, showed that a large percentage of patients improved after RTX treatment; and the series by Kim et al.11 found an 88% reduction in relapse rate. In our series, 80% of the patients treated with RTX present a reduction in their yearly relapse rates. One of our patients suffered a relapse one month after beginning RTX treatment, but remained relapse-free later. The other 3 patients have been relapse-free since beginning RTX treatment. Our low number of patients limits the possibility of comparing our results to those from the studies previously mentioned. In the series by Pellkofer et al.,12 8 out of the 10 patients studied presented reduced relapse rates, and relapses were correlated with the reappearance of B-cells. This study also highlights that although anti-NMO antibodies are involved in disease pathogenesis, their titres are not reduced with the administration of RTX.12 Kim et al.13 recently published the results after 5 years of follow-up on 30 patients with NMO spectrum disorders and treated with RTX; as much as 87% of their patients showed a decreased annual relapse rate, and 60% were relapse-free. Blood CD19 count indicates that B-cell depletion persists, and therefore, effects of RTX remain over time.10,12 These authors monitored CD27 B-cells instead of CD19+, since they consider CD27+ measurements to be more sensitive than CD19+ measurements. They used these data to support their decision to repeat RTX treatment. Most of the patients treated in these studies presented anti-NMO antibodies; in our case, the presence of only 3 patients with anti-NMO antibodies could explain our more limited results, which are in line with those reported by other authors.14 Seropositive results vary among different populations; for example, in patients with optic-spinal MS (a variant of NMO), this value ranges from 27% to 63%.15 The limited number of cases and the short follow-up periods for certain patients make it difficult to determine the role of RTX in treatment of this condition at our centre.
ConclusionPositive results for RTX treatment in other autoimmune diseases, together with the degree of severity that NMO sometimes presents, support this therapeutic option. Using RTX in patients with NMO should be considered an interesting therapeutic alternative, since there are no authorised treatments for this condition. Further studies on the use of RTX should be performed to determine the role of this drug in the treatment of NMO.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández-Megía MJ, Casanova-Estruch B, Pérez-Miralles F, Ruiz-Ramos J, Alcalá-Vicente C, Poveda-Andrés JL. Evaluación del uso de rituximab en la neuromielitis óptica. Neurología. 2015;30:461–464.