The advisability of implanting a stent in carotid near-occlusion stenosis is a controversial topic. We have assessed procedural and clinical implications of stent implantation for carotid artery disease with near occlusion.
MethodsWe included 205 patients who underwent carotid artery revascularisation with a stent. The group of patients with near-occlusion stenosis (n=54) was compared to the rest of the population (n=151).
ResultsNo differences were found between groups for age, sex, and the percentage of symptomatic patients (three-quarters of the population). Carotid stent revascularisation for near-occlusion stenosis presented a high procedural success rate (96%) similar to that of revascularisation processes for other lesions (98%). Stenting in cases of near-occlusion stenosis required increased use of proximal protection (54% vs. 20.5%, P<0.001) and predilation (33% vs. 17%, P=0.01). The process to repair near-occlusion stenosis causes increased detachment of plaque, as shown by higher percentages of macroscopic plaque captured by protection devices (18.5% vs. 7%, P=0.01) and of perioperative ischaemic brain lesions (47% vs 31%, P=0.07). At 30 days of follow-up, the tendency towards adverse neurological events (death, major and minor stroke) was higher in the near-occlusion group (9.2% vs. 3.2%, P=0.08).
ConclusionsStent revascularisation for near-occlusion carotid stenosis has a high procedural success rate; however, its higher plaque load was responsible for the increased rate of ischaemic brain lesions and adverse neurovascular events at 30 days post-procedure.
La conveniencia del implante de stent en lesiones carotídeas suboclusivas es un tema controvertido. Nuestro trabajo valoró las implicaciones clínicas y de procedimiento de la revascularización de lesiones carotídeas suboclusivas.
MétodosSe incluyó a 205 pacientes con enfermedad carotídea revascularizados con stent: los pacientes con lesiones suboclusivas (n=54) fueron comparados con el resto de la población (n=151).
ResultadosNo hubo diferencias entre grupos para la edad, el sexo y la tasa de pacientes sintomáticos (que constituían 3 cuartas partes de la población). El implante de stent en lesiones suboclusivas cursó con una alta tasa de éxito (96%), similar al resto de las lesiones (98%). La revascularización de las lesiones suboclusivas condicionó un mayor uso de protección proximal (54% vs. 20,5%, p<0,001) y necesidad de predilatación (33% vs. 17%, p=0,01). El abordaje de lesiones suboclusivas ocasionó un mayor desprendimiento de placa, manifestado por una mayor tasa de material embólico extraído (18,5% vs. 7%, p=0,01) y de lesiones isquémicas cerebrales periprocedimiento (47% vs. 31%, p=0,07). A los 30 días de la revascularización, la tasa de eventos neurológicos (muerte, ictus mayor, ictus menor) mostró tendencia a ser mayor para el grupo con lesiones suboclusivas (9,2% vs. 3,2%, p=0,08).
ConclusionesLa revascularización con stent de lesiones carotídeas suboclusivas presenta una alta tasa de éxito de procedimiento; sin embargo, su mayor carga de placa ocasiona un superior porcentaje de lesiones isquémicas cerebrales, y de eventos neurovasculares en el primer mes.
Cerebrovascular diseases have a high incidence rate in Spain (166.9 strokes and 36.7 transient ischaemic events per 100000 inhabitants per year). They are the leading cause of death among Spanish women, and the second most common among men.1 Approximately a third of all strokes are due to extracranial carotid artery disease.2 Characteristics of the carotid plaque (severity of the obstruction, presence of an ulcer or thrombus, etc.), plus the extent of its symptoms, constitute the variables most closely related to the risk of having a cerebrovascular accident.3,4
Carotid stenosis near-occlusion (CSNO) still presents a treatment dilemma.5 On the one hand, critical carotid lesions may exhaust the intracranial vasoregulatory capacity, and this has been associated with an increased risk of cerebrovascular events.6 Nevertheless, the main mechanism of stroke due to carotid artery disease is embolus of atherothrombotic material that breaks off of plaque. The greater the severity of the obstruction, the greater the risk of embolism; however, risk is lower in near-occlusion stenosis because there is less blood flow to the brain.7
The purpose of this study is to investigate the clinical and procedural implications of treating near occlusion of the carotid artery with stent compared to other carotid conditions treated with percutaneous revascularisation.
MethodsThis descriptive single-centre study included, in consecutive order, patients who underwent carotid stenting between January 2008 and March 2012 at Hospital Virgen Macarena (Seville). We compared 2 populations, those with and without near occlusion of the carotid artery.
A multidisciplinary neurovascular team made up of neurologists, radiologists, and interventional cardiologists was responsible for carotid artery stenting in our hospital. The neurologist's role was to indicate the procedure, monitor the patient during the intervention, and review his or her neurological status at 24hours and at 30 days post-surgery. The carotid intervention was performed by 2 interventional cardiologists (R.R.S. and C.C.) who had completed structured training in this procedure (monitored theoretical training, viewing procedures in a high-volume centre, and rehearsing skills using a simulator). They were assisted in the first 5 cases by an expert interventional radiologist.
We assessed the success rate for the procedure (implantation of a stent in the internal carotid with no neurovascular events – minor stroke, major stroke, or death–) in the first 24hours and the cumulative rate of neurovascular events in 30 days. We also recorded presence of infarct shown by MR studies in the 10 days after the procedure.
Quantitative analysis of carotid lesions. Definition of near occlusion of the carotid arteryCarotid stenosis and the result of the revascularisation procedures were measured using Siemens Artis zee software (CAAS II, Pie Medical, The Netherlands). Stenosis severity was not measured according to NASCET criteria (the ratio of the minimum diameter of the stenosis to the reference value for the internal carotid). This is because in the cases of near-occlusion, the internal carotid artery frequently displays loss of calibre due to the decreased flow. For that reason, we have opted to use the diameter of the common carotid artery prior to the development of stenosis as the reference value.
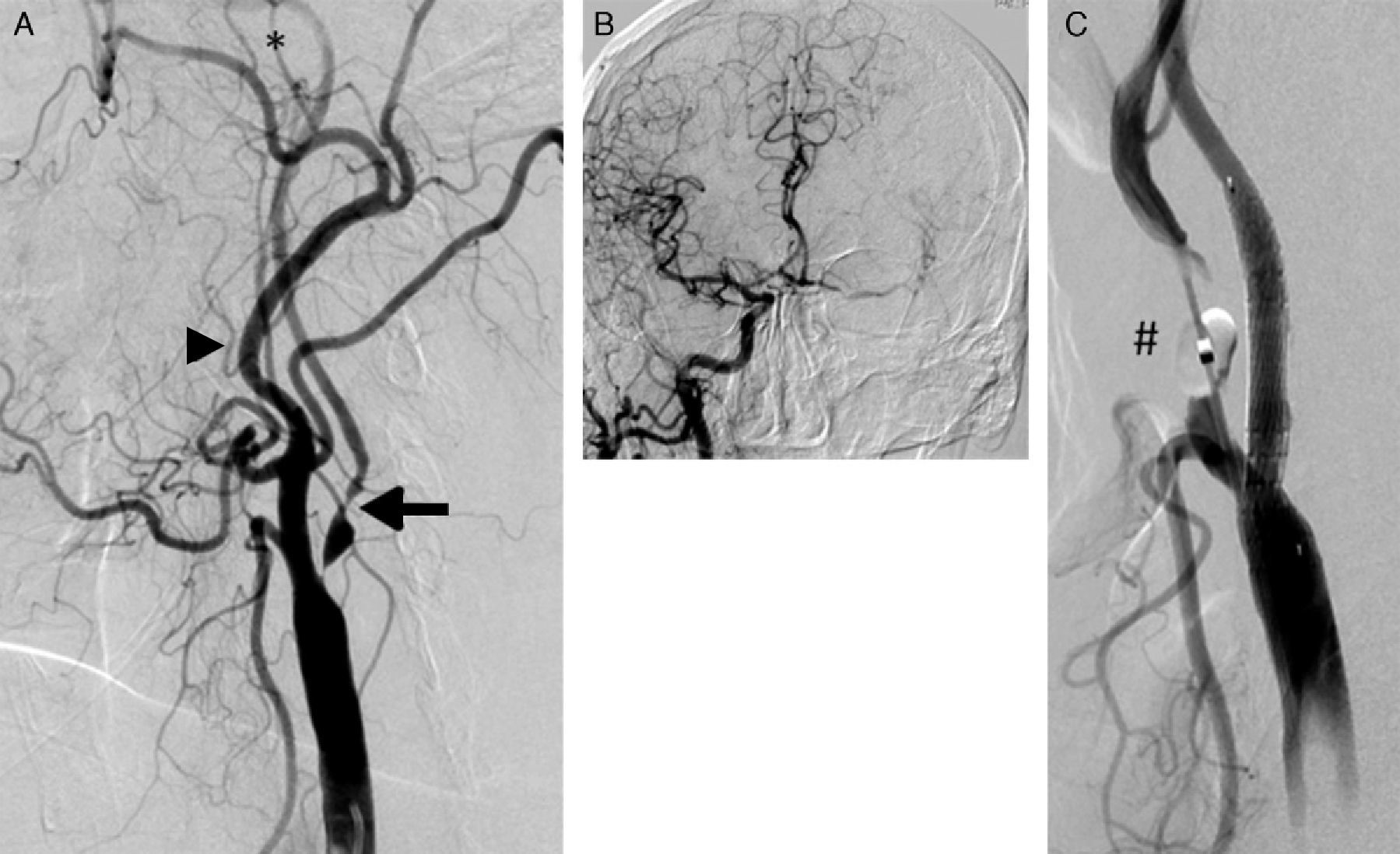
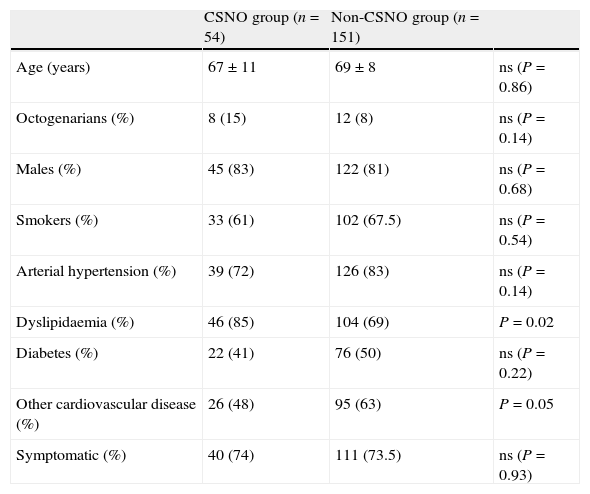
CSNO has been defined in early studies by applying a series of qualitative criteria7: decrease in the calibre of the internal carotid artery with respect to the common or internal contralateral artery, slower contrast transport in the internal carotid than in the external carotid, and/or evidence of intracranial collateral pathways. These criteria, which are indirect signs of the severity of plaque obstruction in the carotid, cannot be applied to the entire population with CSNO. For example, lack of continuity in the circle of Willis limits the presence of collateral pathways, while severe disease or contralateral carotid occlusion is an obstacle to evaluating collateral pathways and comparing flow and calibre between the two internal carotid arteries. On the other hand, the lesion may compromise the origin of the external carotid artery, and therefore its filling velocity. For this reason, we have added critical severity of obstruction (minimum luminal diameter less than 1mm) to the listed qualitative criteria (Fig. 1).
(A) Carotid artery near-occlusion meeting all qualitative criteria for that definition: internal carotid artery (arrow) with a diameter smaller than that of the external carotid (arrowhead); distal opacification in the territory of the internal carotid, which is smaller than the external carotid (*); compensation by collateral intracranial circulation (B). (C) Result after placement of Cristallo stent (Invatec-Medtronic). The inflated distal balloon (#) of the MoMa device (Invatec-Medtronic) can be seen at the origin of the external carotid artery.
Collateral intracranial circulation ipsilateral to the stenotic area was classified on a 3-point scale: absent, discrete (only filling from the anterior cerebral artery), and excellent (filling from the anterior and middle cerebral arteries ipsilateral to the stenotic lesion).
Revascularisation procedure for carotid artery with stent implantationOur unit indicated carotid revascularisation procedures based on the following criteria8: symptomatic patients (neurological signs in the preceding 6 months) with significant carotid stenosis (>50%) and asymptomatic patients with carotid stenosis>60% and occlusion of the contralateral carotid artery.
The only diagnostic test which patients underwent prior to the procedure was carotid Doppler ultrasonography to measure severity of the obstruction and plaque characteristics.
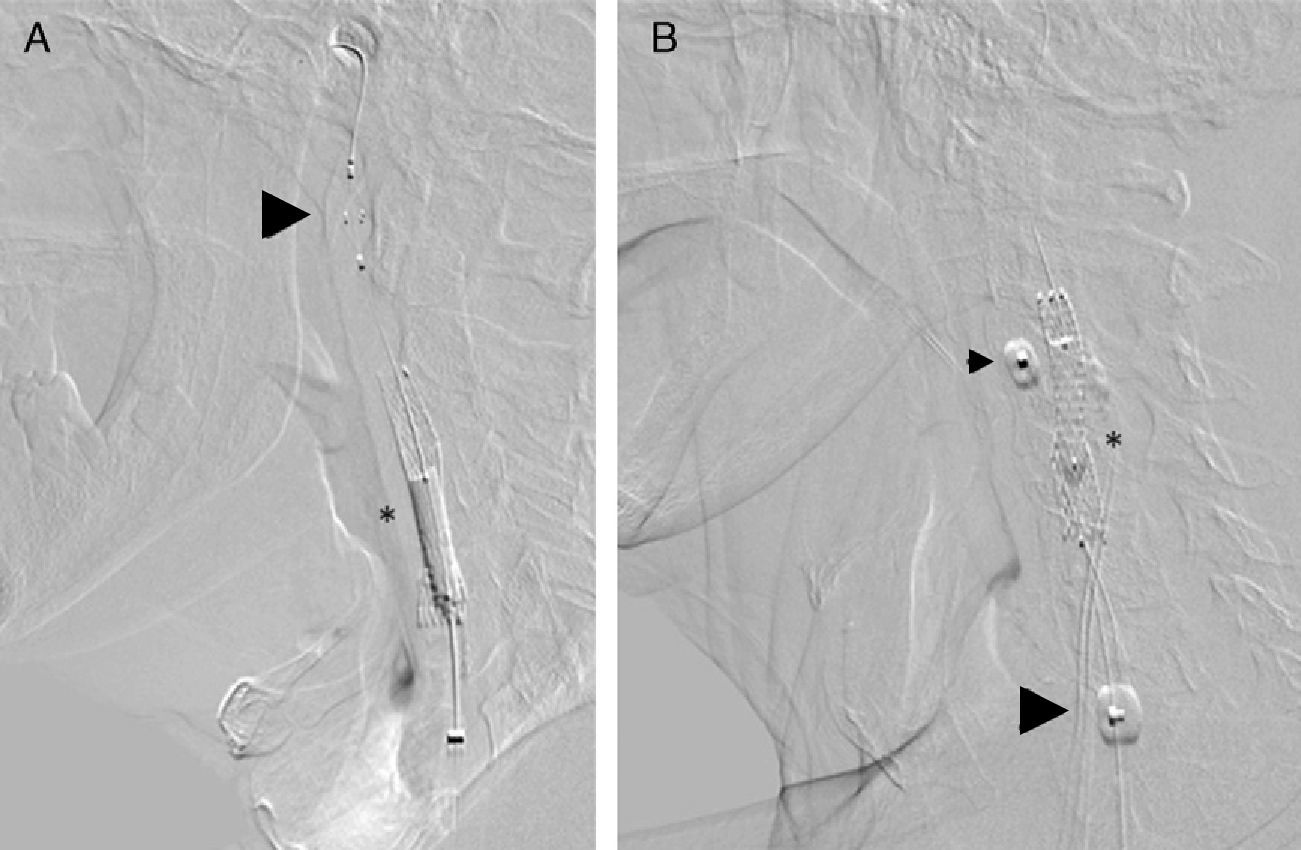
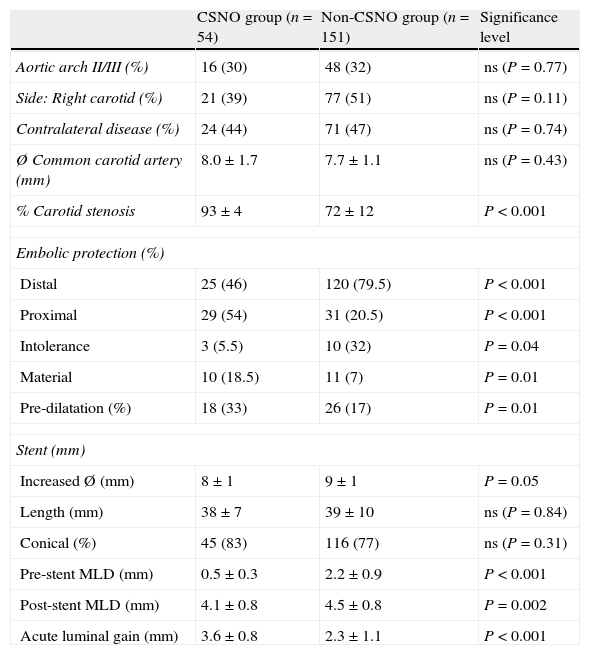
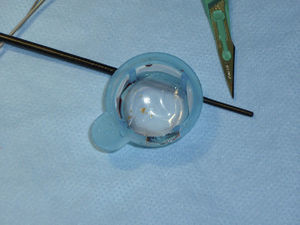
The procedure was performed using an embolic protection device as a routine safeguard. Our group used distal embolic filters (Angioguard, Cordis, USA) and proximal protection devices (MoMa, Medtronic, Italy) (Fig. 2). Protection was selected according to the lead interventionist's clinical judgement and depending on a series of factors: plaque severity and characteristics, vessel tortuosity, and presence of significant disease in the contralateral carotid.
Embolic protection devices used in the post-dilatation phase of the stent placement procedure (*). (A) Distal protection with an Angioguard filter (Cordis) that has been placed in the vertical part of the petrous segment of the cervical internal carotid artery (arrowhead). (B) Proximal protection using MoMa (Invatec-Medtonic) with the distal balloon inflated at the beginning of the external carotid artery (head of small arrow) and the proximal balloon in the common carotid artery (head of large arrow). This blocks anterograde flow through the stenotic area, with the appearance of contralateral retrograde flow protecting the patient from embolism.
Pre-dilatation was only performed when the stent would not pass through the stenosis, regardless of the embolic protection device employed. However, all patients underwent post-dilatation using a 5mm balloon. We used different types of open-cell stents (Protégé, eV3; Acculink, Abbott; Precise, Cordis, USA) and mixed-design stents (Cristallo, Medtronic, Italy) indiscriminately.
After an initial phase, we modified the intervention method in 2 ways. First, we suppressed routine administration of 1mg atropine prior to post-dilatation in order to achieve better control over arterial pressure during the phase just after the intervention. Additionally, we monitored patients for the 6hours following revascularisation, with particular attention to arterial pressure. Doses of antithrombotic agents were also changed. In the early phase of the study, patients were on dual antiplatelet treatment. During the procedure, they received 100U of heparin sodium/kg body weight, plus a set dose of 300mg clopidogrel after placement of the stent. In 2010, we decided to change the anti-thrombotic treatment so that the patient would receive 50U heparin sodium/kg body weight with no extra doses of clopidogrel. During the first month, the patient remained in treatment with dual antiplatelet drugs.
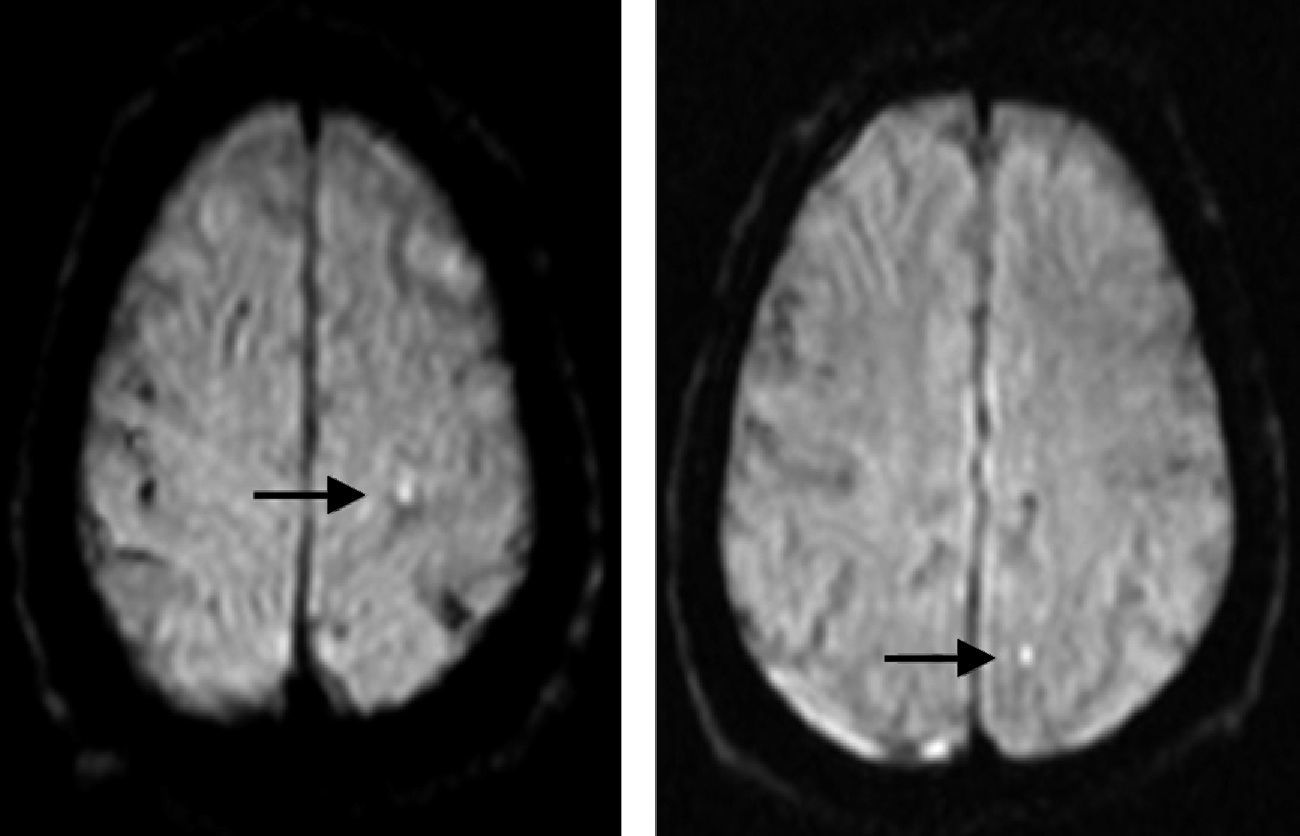
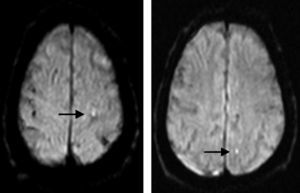
Periprocedural assessment of ischaemic brain lesionsDoctors ordered brain MRI studies (sagittal T1-weighted, axial T2-weighted, diffusion-weighted with ADC mapping, and coronal FLAIR images) for all patients in the 10 days following the procedure, except where contraindicated. In the end, 137 patients were evaluated (67% of the population); the main given for lack of an evaluation was logistical difficulty arranging the evaluation in the allotted time frame. MRI studies were completed using a Magnetom Symphony 1.5T unit (Siemens, Germany) with diffusion-weighted sequences. Areas that were hyperintense in diffusion-weighted sequences and hypointense in ADC maps (Fig. 3) were considered recent-onset ischaemic lesions.
Magnetic resonance image with diffusion-weighted sequence showing punctiform hyperintense features that indicate areas of restricted diffusion and therefore periprocedural infarct (arrows). These features appeared in the white matter of the left parietal lobe, ipsilateral to the revascularised carotid artery.
After being entered in an Excel spreadsheet (Microsoft Office 2007, USA), data were analysed using SPSS statistical software, version 20.0. Continuous variables were described as means±standard deviations; categorical variables were expressed as numerical values and percentages. We compared variables from 2 independent samples (group of patients with CSNO and the group of other patients with revascularisation procedures). The t-test was used for continuous variables and the chi-square test was used for categorical variables.
ResultsThese 4 years of activity have seen 205 carotid revascularisation procedures in our unit; 54 were performed to treat CSNO (26%), while the remaining 151 treated cases were significant but not near-occlusive lesions (non-CSNO, 74%).
Clinical characteristicsBaseline clinical characteristics for both groups are listed in Table 1. Both populations presented high and comparable percentages of symptomatic patients, who made up three-quarters of the total. There were no significant differences in cardiovascular risk factors with regard to smoking habits, arterial hypertension, and diabetes. Nevertheless, patients with CSNO lesions had higher dyslipidaemia rates (85% vs 69%, P=0.02). The 2 groups also differed with regard to non-carotid cardiovascular disease (coronary or peripheral vessel disease). Patients in the non-CSNO group showed a significantly higher frequency of non-carotid cardiovascular disease than the others (63% vs 48%, P=0.05).
Baseline clinical characteristics.
| CSNO group (n=54) | Non-CSNO group (n=151) | ||
| Age (years) | 67±11 | 69±8 | ns (P=0.86) |
| Octogenarians (%) | 8 (15) | 12 (8) | ns (P=0.14) |
| Males (%) | 45 (83) | 122 (81) | ns (P=0.68) |
| Smokers (%) | 33 (61) | 102 (67.5) | ns (P=0.54) |
| Arterial hypertension (%) | 39 (72) | 126 (83) | ns (P=0.14) |
| Dyslipidaemia (%) | 46 (85) | 104 (69) | P=0.02 |
| Diabetes (%) | 22 (41) | 76 (50) | ns (P=0.22) |
| Other cardiovascular disease (%) | 26 (48) | 95 (63) | P=0.05 |
| Symptomatic (%) | 40 (74) | 111 (73.5) | ns (P=0.93) |
±: mean±SD.
Table 2 summarises angiographic findings and the characteristics of the stent placement process. In the CSNO group, the minimum luminal diameter was 0.5±0.3mm. In this group, in addition to severity of the obstruction (minimum luminal diameter<1mm), we evaluated the presence and quality of the collateral intracranial circulation. In 38 patients (70% of the CSNO group), the CSNO lesion was accompanied by collateral intracranial circulation which was discrete in 14 patients (filling from the anterior cerebral artery only) and excellent in the other 24 (involvement by the anterior and medial cerebral arteries).
Angiographic and procedural data.
| CSNO group (n=54) | Non-CSNO group (n=151) | Significance level | |
| Aortic arch II/III (%) | 16 (30) | 48 (32) | ns (P=0.77) |
| Side: Right carotid (%) | 21 (39) | 77 (51) | ns (P=0.11) |
| Contralateral disease (%) | 24 (44) | 71 (47) | ns (P=0.74) |
| Ø Common carotid artery (mm) | 8.0±1.7 | 7.7±1.1 | ns (P=0.43) |
| % Carotid stenosis | 93±4 | 72±12 | P<0.001 |
| Embolic protection (%) | |||
| Distal | 25 (46) | 120 (79.5) | P<0.001 |
| Proximal | 29 (54) | 31 (20.5) | P<0.001 |
| Intolerance | 3 (5.5) | 10 (32) | P=0.04 |
| Material | 10 (18.5) | 11 (7) | P=0.01 |
| Pre-dilatation (%) | 18 (33) | 26 (17) | P=0.01 |
| Stent (mm) | |||
| Increased Ø (mm) | 8±1 | 9±1 | P=0.05 |
| Length (mm) | 38±7 | 39±10 | ns (P=0.84) |
| Conical (%) | 45 (83) | 116 (77) | ns (P=0.31) |
| Pre-stent MLD (mm) | 0.5±0.3 | 2.2±0.9 | P<0.001 |
| Post-stent MLD (mm) | 4.1±0.8 | 4.5±0.8 | P=0.002 |
| Acute luminal gain (mm) | 3.6±0.8 | 2.3±1.1 | P<0.001 |
±: mean±SD.
There were differences in the revascularisation procedures between the 2 groups. A larger percentage of the near-occlusion cases required pre-dilatation (33% vs 17%, P=0.01), although percentages remained low overall. The most noteworthy procedural differences had to do with the method of protection: use of a proximal protection device (MoMa) was significantly more frequent for near-occlusion cases than for other cases (54% vs 20.5%, P<0.001). We should point out that the intolerance rate for MoMa was higher in the non-CSNO group (5.5% vs 32%, P=0.04). Lastly, there were also significant differences between groups regarding capture of embolic material. More macroscopic material was present in revascularisation cases with CSNO lesions (18.5% vs 7%, P=0.01) (Fig. 4).
Carotid stent revascularisation according to stenosis severity: clinical implicationsThe success rate for procedures for CSNO cases was 96%. The procedure failed in 2 patients due to the appearance of a minor neurological deficit (1 case of amaurosis fugax, 1 case of mild upper limb paresis); both cases resolved completely. Success rate in the non-CSNO group was 98%, with 3 procedures being unsuccessful. In one case, the patient suffered a major stroke due to embolisation of material in the middle cerebral artery; the material was successfully extracted during the same procedure by thrombectomy with a Solitaire stent (eV3, USA). In the other 2 cases, patients suffered minor neurological disorders, which were transient in both cases.
The groups had identical percentages of cases with brain MRI studies (36 CSNO cases and 101 non-CSNO cases; 67% in both groups). Periprocedural ischaemic lesions were present in 47% of the patients with near-occlusive stenosis (88% silent); 31% of the non-CSNO patients had ischaemic lesions revealed by MR studies (90% silent) (P=0.07).
Three intracranial haemorrhages occurred in the first month after surgery, and they were fatal in patients with CSNO (mortality rate of 5.5%). In the other patient group, there was only 1 mortality due to intracranial haemorrhage in the month following surgery (0.6%). Hyperperfusion syndrome after revascularisation may have been the cause of the intracranial haemorrhage. All these cases occurred in symptomatic patients, 3 of whom had suffered a recent infarct in the territory of the ipsilateral middle cerebral artery.
The frequency of all neurological events within 1 month of the procedure was 9.2% in the CSNO group (3 deaths and 2 minor strokes) and 3.2% in the non-CSNO group (1 death, 1 major stroke, and 3 minor strokes; 10 days after the procedure, a patient experienced thrombosis of the central retinal artery). This reflects a tendency towards increased event frequency in the first group (P=0.08).
DiscussionThis study compiles experiences with treating near-occlusive carotid stenosis from a neurovascular team including neurologists, radiologists, and interventional cardiologists.
Revascularising CSNO lesions by stent placement was highly successful as a procedure, but it also entailed an increased number of neurological complications in the first month compared to revascularisation procedures for less severe stenosis. The larger plaque substrate in near-occlusive lesions resulted in a higher rate of intracranial ischaemic lesions after surgery. In addition, patients were more prone to intracranial bleeding due to hyperperfusion syndrome.
Population with carotid stenosis near-occlusionCSNO cases made up 26% of our population, which shows that the condition is common in daily practice. According to the literature, the frequency of CSNO lesions requiring revascularisation ranges from 14% to 29%,9,10 and the meta-analysis of the NASCET and ESCT studies showed a mean of 21.5%.7
The question of whether CSNO should be treated remains unanswered at present.5–8 The risk of suffering a neurological event due to carotid disease depends largely on the degree of obstruction. Plaques with the most risk of causing stroke are those involved in 90% to 99% stenosis. In this group, cases with near-occlusive plaque (95%–99%) present a lower risk of stroke than cases with stenosis in the 90% to 94% range. This occurs because most strokes are caused by embolisation of thrombotic material, not by low blood flow caused by the obstruction. To a certain extent, near-occlusion may ‘protect’ a patient from embolism and stroke. However, CSNO lesions evolve towards carotid occlusion, and if circumstances are favourable, the impact of this occlusion on the patient's cognitive abilities may be underestimated.
Angiography and treatment approach for carotid stenosis near-occlusionThe NASCET criteria for measuring carotid stenosis underestimate the degree of severity of CSNO lesions since the diameter of the internal carotid artery decreases as the degree of obstruction increases. With this in mind, other authors have identified CSNO based on exclusively qualitative angiography criteria.7 We used the same criteria, with the addition of the quantitative measurement of a minimum luminal diameter of less than 1mm. Indirect signs of the severity of the obstruction may not manifest clearly for a number of reasons (generally because of occlusion of the contralateral internal carotid artery).
Whether embolic protection devices should be deployed during stent revascularisation remains a controversial topic, and it has a very low recommendation level according to recent European clinical practice guidelines.8 Nevertheless, our group used distal or proximal protection devices during all procedures. Larger amounts of plaque, and possibly more unstable plaque composition, are factors explaining why the CSNO group had a higher rate of macroscopic material extracted by the embolic protection devices than the other group (18.5% vs 7%), as well as a higher frequency of cerebral ischaemia (47% vs 31%). Fortunately, this high percentage of ischaemic brain lesions was not correlated to clinical symptoms, except in 2 patients, each of whom suffered a minor stroke.
Stent revascularisation in near-occlusive stenosisThe success rate for the stent revascularisation procedure for CSNO was high and comparable to rates for less severe lesions (96% vs 98%). This tendency has been described in prior studies. Despite the high success rate, 3 patients with near-occlusive stenosis (5.5% of the population) presented symptoms of intracranial haemorrhage within a month of the procedure, and all died. Where no other factors are present, intracranial haemorrhage is due to hyperperfusion syndrome as a result of breaking the blood–brain barrier when revascularising a severely stenotic artery whose distal arteriolar bed has an exhausted vasodilator reserve. Factors predisposing an individual to hyperperfusion syndrome after carotid revascularisation include treatment of critical lesions, severe contralateral occlusion or stenosis, periprocedural hypertension,9 and excessive anticoagulant use.10 In our population, 4 patients developed hyperperfusion syndrome with intracranial haemorrhage resulting in death. Three were in the CSNO group (including 2 patients with no collateral intracranial circulation), and 1 in the other patient group. All deaths occurred in the first 2 years of our experience with the procedure (2008 and 2009). Since anti-thrombotic treatment was modified to reduce anticoagulant dosage and eliminate the loading dose of clopidogrel, and since doctors began prioritising control over arterial pressure during and after procedures, no other hyperperfusion events have occurred.
The cumulative 30-day fatality and stroke rate in the CSNO group was 9.2%, compared to 3.2% in the rest of the patients. This difference approached statistical significance. The higher rate of events in the CSNO group is due to deaths from intracranial haemorrhage. The 2 ischaemic strokes in this group were minor events and patients had recovered completely 30 days later.
LimitationsThis study has numerous limitations. First, its definition of near-occlusion stenosis contains an additional, and not yet validated, quantitative angiography measurement. If this were not the case, a third of the patients in the near-occlusion group, precisely those at the most risk for severe clinical complications from a revascularisation procedure, would not have been included in the group. Secondly, we did not analyse lesions according to their symptomatic repercussions, although there was a similar, and predominant, percentage of patients with symptoms in both groups. Lastly, clinical follow-up ended at 30 days, and more long-term evaluations will be needed.
ConclusionOur experience shows that stent revascularisation for CSNO lesions is an effective procedure, but its 30-day morbidity and mortality rates are higher than those for the treatment of less severe stenosis. On the one hand, stent implantation results in more plaque material being loosened. Despite the use of protective devices, this will result in a higher percentage of cerebral ischaemia. On the other hand, we find a higher rate of neurological events, mainly death due to intracranial haemorrhage, within 1 month of the revascularisation procedure. For these reasons, stent revascularisation for CSNO cases should be performed following an analysis of the patient's individual clinical and angiographic characteristics. Extreme caution must be used during the procedure (adjusting antithrombotic drug doses, using embolic protection devices, and monitoring blood pressure values).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ruiz-Salmerón RJ, Gamero MA, Carrascosa C, Pérez S, de Araujo D, Marcos F, et al. Revascularización carotídea con stent: implicaciones clínicas y para el procedimiento de la presencia de una lesión suboclusiva. Neurología. 2013;28:535–542.