In ancient and current traditional medicine in Mexico, extracts from the leaves or whole plant of ‘life leaf’ (Kalanchoe pinnata [K. pinnata] Lam) have been used to treat an entity known locally as ‘yellow epilepsy’ (alferecía amarilla) when it is accompanied by seizures. However, the anticonvulsive activity of its stems and roots remains unexplored.
MethodsThe anticonvulsant activity of the methanolic root extract (MER) or stem (MES) of K. pinnata Lam. was evaluated in a pentylenetetrazol-induced seizure model in BALB/c mice, and effects were compared to those of diazepam. The stem extract fractions that produced anticonvulsant activity were subsequently evaluated using the pentylenetetrazol-induced seizure model.
ResultsWe observed increased latency of tonic–clonic seizures that was inversely proportional to the dose of MRE, with a similar impact on the lethal effects of pentylenetetrazol. Different doses of the MSE showed a dose-dependent increase in latency to myoclonus, clonus, and tonic–clonic seizures, acting similarly to diazepam and offering 100% protection against the lethal effects of pentylenetetrazol. Fractioning MSE decreased its effectiveness, but when fractions were mixed with fractions of chloroform and ethyl acetate, anticonvulsive activity was restored. The preliminary phytochemical analysis identified alkaloids and sterols in MRE, and sterols and terpenes in MSE.
ConclusionsThe anticonvulsant activity of K. pinnata Lam. decreases with increased doses of MRE, whereas the effect of MSE is dose-dependent and preserved in the mixture chloroform and ethyl acetate. We suggest that the metabolites responsible for these effects are sterols in MRE, and sterols and terpenes in MSE.
En México, la medicina tradicional emplea extractos de hojas o de la planta completa de «siempre viva» (Kalanchoe pinnata [K. pinnata] Lam.) para tratar la alferecía amarilla cuando presenta convulsiones. La actividad anticonvulsivante del tallo o de la raíz sigue sin explorarse.
MétodosEl extracto metanólico de la raíz (EMR) y el del tallo (EMT) de K. pinnata Lam., fueron evaluados con el modelo de inducción de convulsiones con pentilentetrazol en ratones de la cepa Balb/C, comparado con diazepam. Las fracciones del EMT fueron subsecuentemente evaluadas.
ResultadosEl EMR incrementó la latencia a las crisis clónico-tónicas de forma inversamente proporcional a la dosis, observándose el mismo patrón sobre los efectos letales del pentilentetrazol. Todas las dosis evaluadas del EMT aumentaron la latencia a las mioclonías y a las crisis clónicas de forma dosis-dependiente e incrementaron la latencia a las crisis clónico-tónicas de manera semejante al diazepam con una protección del 100% contra los efectos letales del pentilentetrazol. El fraccionamiento del EMT redujo su eficacia. Al mezclar las fracciones de cloroformo y acetato de etilo, se recuperó la actividad anticonvulsivante y la protección contra los efectos letales. El análisis fitoquímico preliminar identificó alcaloides y esteroles en el EMR; esteroles y terpenos en el EMT.
ConclusiónLa actividad anticonvulsivante de EMR de K. pinnata Lam. disminuye aumentando la dosis y en el EMT se presenta de forma dosis-dependiente, conservándose en la mezcla de cloroformo y acetato de etilo. Se sugiere que los metabolitos responsables de estos efectos son esteroles en el EMR; esteroles y terpenos presentes en el EMT.
Kalanchoe pinnata (Lam.) (K. pinnata; syn. Bryophyllum pinnatum) is a member of the Crassulaceae family endemic to Madagascar which is currently widely disseminated around the world.1,2 In Mexico it is popularly known as ‘siempre viva’, or ‘life leaf’, and it is found from 20 to 2600m above sea level.3
In some areas of the state of Veracruz, Mexico, the entire plant is used in traditional medicine to make infusions to treat ‘yellow epilepsy’ seizures.4 Some studies have shown that a liquid extract of K. pinnata leaves (50-200mg/kg) has anticonvulsant and sedative properties, with greater efficacy against the effects of picrotoxin than those of strychnine.5 Using pentylenetetrazole (PTZ) seizure models is the most common procedure for identifying potential anticonvulsant substances,6,7 such as plant extracts.8 This technique identifies PTZ-induced seizures (75-90mg/kg, i.p., 6-7) and establishes 5 levels: 0 (no convulsions), 1 (progressive decrease in motor activity), 2 (myoclonic seizures, localised movements), 3 (clonic seizures, loss of upright posture), 4 (tonic–clonic seizures), and 5 (death).6,9,10 The mechanism by which PTZ induces seizures has not yet been well established. However, some studies suggest that it acts on the picrotoxin-sensitive site of the GABAA receptor.6–11 Various GABAA agonists, such as diazepam (DZP), protect against PTZ-induced seizures.12,13
In this study, the potential anticonvulsant effects of different doses of methanolic root (MRE) and stem (MSE) extracts of K. pinnata were evaluated using a PTZ-induced seizure model in BALB/c mice and compared to the effects produced by DZP, a clinically effective anticonvulsant drug.
Material and methodsPlant materialK. pinnata was collected in the city of Xalapa (Veracruz, Mexico) and authenticated by phytologists from the Biological Research Centre at the Universidad Veracruzana (CIB-UV), where a specimen was kept (reference number CIB 90901, 9092).
K. pinnata (Lam.) extract preparationK. pinnata stem (598.06g) and root (495g) were dried for 7 days at 50°C, resulting in 88.11g of stem and 56.04g of root, which were separated and chopped. The plant material was extracted with methanol (MeOH) for 5-7 days and concentrated using a rotary evaporator (BUCHI, Flawil, Switzerland) at 65°C, which resulted in 32.60g of stem extract and 5.90g of root extract. These extracts were then used in the PTZ model to determine their potential as anticonvulsants. Based on the results obtained, only the stem extract was fractionated. One gram of the stem extract was purified using column chromatography. The column was packed with silica gel (70-230 mesh, Merck). The eluents used were chloroform (CHCl3), ethyl acetate (AcOEt), and MeOH. Four fractions were collected: CHCl3 (8.2%), AcOEt (8.3%), MeOH (35.2%), and an inorganic salt (IS, 48.3%).
AnimalsOne hundred and fourteen young BALB/c mice13 of both sexes weighing from 22 to 25g at the beginning of the experiments were used. They were acquired from the Biotechnology Institute Animal Services Department of the Universidad Nacional Autónoma de México (IBTUNAM), in Cuernavaca (Morelos, Mexico). They were kept in acrylic cages under controlled conditions of light (12:12 light/darkness cycles) and temperature (25±1°C) and had ad libitum access to purified water and food (Harlan México, S.A. de C.V.). All the animals were maintained in accordance with the ethical protocol established in the ‘Guide for the care and use of laboratory animals’ (National Research Council, 199614) and the official Mexican guidelines (NOM-062-ZOO-1999).
Drugs and reagentsPTZ, propylene glycol, and Tween 80 were procured from Sigma (St. Louis, MO, USA). DZP was obtained from Laboratorios Cryopharma (Cryopharma S.A. de C.V., Mexico). The solvents CHCl3, AcOEt, and MeOH were purchased from PFQ (PFQ S.A. de C.V., Mexico) and purified by fractional distillation and using rectification columns.
Preliminary phytochemical testsThe following qualitative preliminary tests were used: Dragendorff and Wagner reagents for alkaloids, Liebermann–Burchard and Salkowski tests for sterols and terpenes, and Shinoda test for flavonoids. We used the flame test to determine the chemical profile of the IS, the Griess reagent for nitrates (NO3), and AgNO3 for chlorides (Cl−).15,16
Experimental seriesAnticonvulsant activity of MSEThirty-six juvenile mice were assigned to 6 independent groups (n=6 per group). The first group (VEH) was given 10mL/kg of the vehicle (cosolvent, composed of propylene glycol, Tween 80, and saline solution; 4:1:5, v:v:v) orally.17 The second group received the reference treatment DZP (5mg/kg; i.p.).13 The remaining 4 groups were given oral doses of 100, 200, 400, or 800mg/kg of the stem extract respectively. All the treatments were administered 30minutes before the convulsant PTZ (75mg/kg, i.p.). After the PTZ was delivered, the mice were placed in individual acrylic cages (11cm×11cm×11cm) for observation.
There were 2 observation phases. During the first phase, which lasted 30minutes, we evaluated the latency to onset of (a) myoclonic, (b) clonic, and (c) tonic–clonic seizures. The moment PTZ was administered was considered time zero. The second phase involved assessing protection against the lethal effects of PTZ, with total protection being survival of the experimental animal 24hours after PTZ administration.
Anticonvulsant activity of MREThirty-six additional mice were divided into 6 individual groups (n=6 per group) and used to test the effects of MRE. One group was given the vehicle (VEH, 10mL/kg orally), a second received DZP (5mg/kg, i.p.), and the remaining 4 received 100, 200, 400, and 800mg/kg doses of MRE by oral route, respectively. The experimental process remained the same as in the case of the MSE.
Anticonvulsant activity of MSE fractionsForty-two mice were assigned to 7 independent groups (n=6 per group). One was administered the vehicle (10mL/kg orally), another one DZP (5mg/kg, i.p.), and the 5 remaining received MSE fractions orally (100mg/kg): CHCl3, AcOEt, MeOH, CHCl3–AcOEt (1:1), and the IS. Treatment effects were evaluated as described in the previous protocols.
All the experimental sessions were recorded with a video camera (Sony, DCR-DVD101, Carl-Zeiss lens) and subsequently analysed.
Statistical analysisOne-way ANOVA for independent groups was used to analyse the data. When P-values≤.05, we used the Student–Newman–Keuls post hoc test. Results were expressed as the mean±SD.
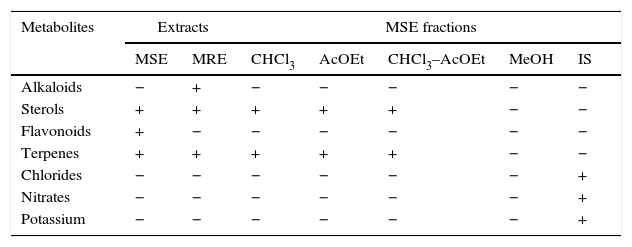
ResultsPreliminary phytochemical analysisThe results of the preliminary phytochemical analysis of the K. pinnata extracts are presented in Table 1. Alkaloids and sterols were identified in the MRE. Terpenes and sterols were detected in the CHCl3, AcOEt, and CHCl3–AcOEt MSE fractions. Flavonoids were distinguished in the MSE but not in its fractions. Chlorides, nitrates, and potassium were identified in the IS fraction of the MSE.
Preliminary phytochemical analysis.
| Metabolites | Extracts | MSE fractions | |||||
|---|---|---|---|---|---|---|---|
| MSE | MRE | CHCl3 | AcOEt | CHCl3–AcOEt | MeOH | IS | |
| Alkaloids | − | + | − | − | − | − | − |
| Sterols | + | + | + | + | + | − | − |
| Flavonoids | + | − | − | − | − | − | − |
| Terpenes | + | + | + | + | + | − | − |
| Chlorides | − | − | − | − | − | − | + |
| Nitrates | − | − | − | − | − | − | + |
| Potassium | − | − | − | − | − | − | + |
AcOEt, ethyl acetate; CHCl3, chloroform; CHCl3–AcOEt, chloroform–ethyl acetate; MRE, methanolic root extract; MSE, methanolic stem extract; MeOH, methanol; IS, inorganic salt; +, presence; −, absence.
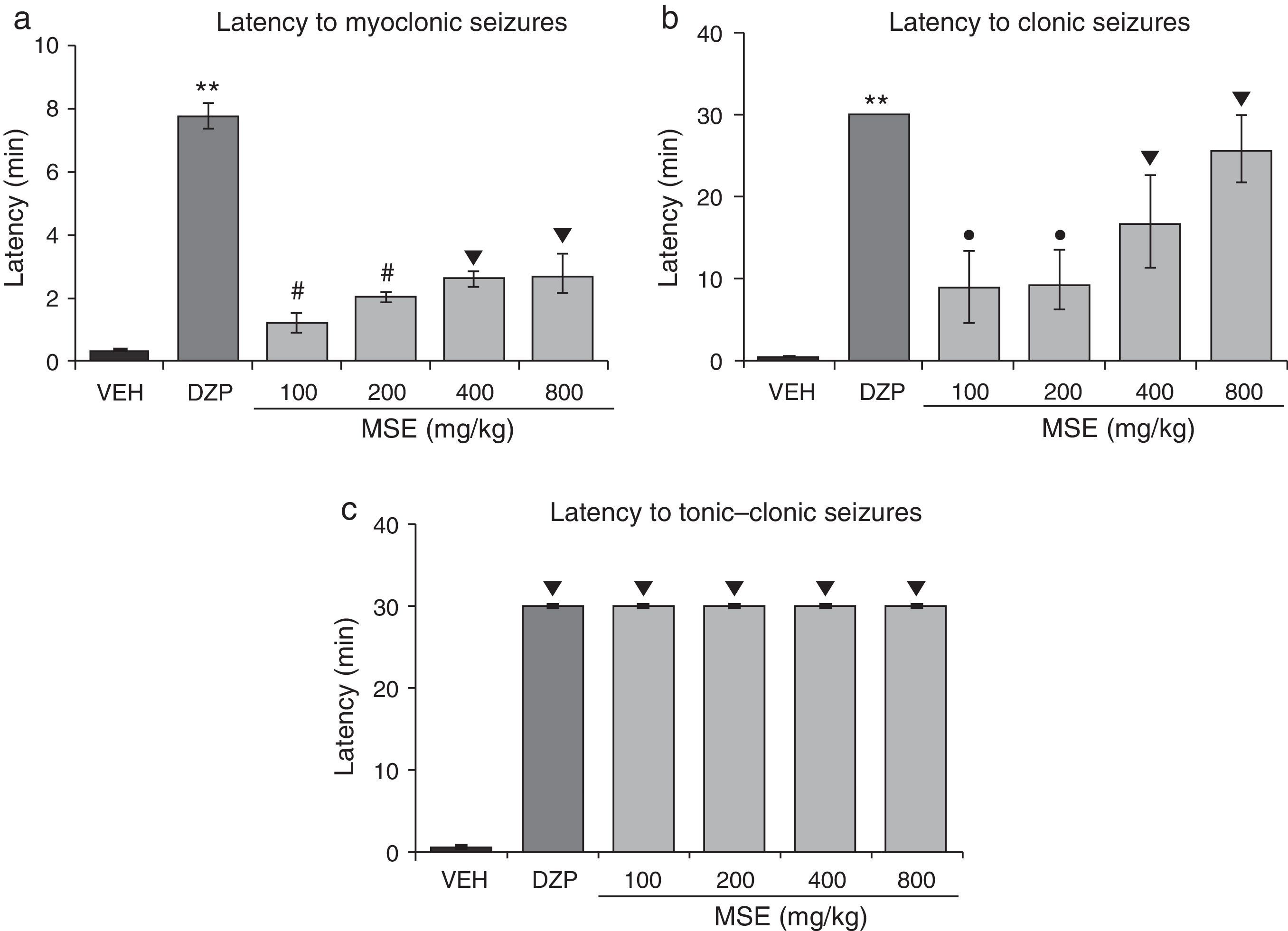
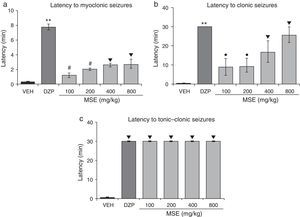
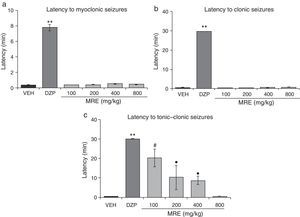
Statistical analysis demonstrated significant differences in latency to myoclonic seizures (F5.30=48.02; P<.001). The post hoc test showed increased latency to myoclonic seizures for all doses of MSE compared to the vehicle. However, MSE showed less efficacy than DZP, and the 100mg/kg and 200mg/kg doses were less efficacious than the 400mg/kg and 800mg/kg doses (Fig. 1a). The statistical analysis also demonstrated significant differences in latency to clonic seizures (F5.30=8.19; P<.001). Latency to clonic seizures also increased in a dose-dependent manner; MSE was less efficacious than DZP, and the 100mg/kg and 200mg/kg doses were less efficacious than the doses of 400mg/kg and 800mg/kg (Fig. 1b). Latency to tonic–clonic seizures varied significantly among treatments (F5.30=62765.40; P<.001). According to the post hoc test, all the doses of MSE significantly increased this variable with respect to the vehicle and showed the same efficacy as DZP (Fig. 1c).
Model of PTZ-induced seizures. The stem extract showed anticonvulsant activity similar to that of DZP. (a) **P<.001 vs all groups; #P<.001 vs VEH, MSE 400, and MSE 800; ▾P<.001 vs VEH. (b) **P<.001 vs all groups; ●P<.001 vs VEH and MSE 800; P<.001 vs VEH. (c) P<.001 vs VEH. DZP: diazepam group; MSE: methanolic stem extract group; VEH: vehicle group.
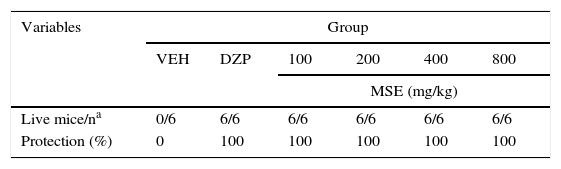
Lastly, all MSE doses and DZP provided 100% protection against the lethal effects of PTZ (Table 2).
Protective effects of MSE against PTZ lethality.
| Variables | Group | |||||
|---|---|---|---|---|---|---|
| VEH | DZP | 100 | 200 | 400 | 800 | |
| MSE (mg/kg) | ||||||
| Live mice/na | 0/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| Protection (%) | 0 | 100 | 100 | 100 | 100 | 100 |
DZP, diazepam; MSE, methanolic stem extract; VEH, vehicle.
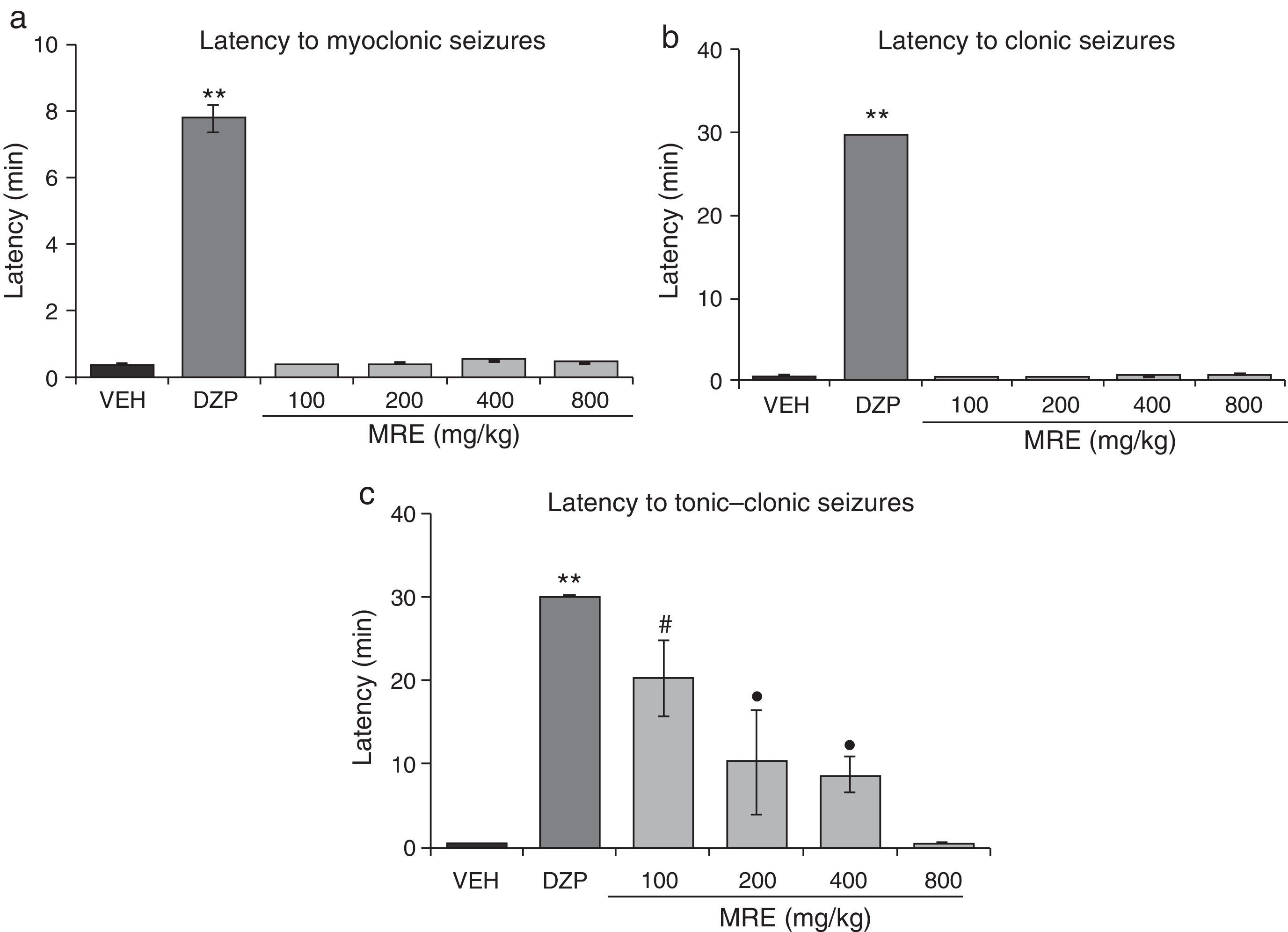
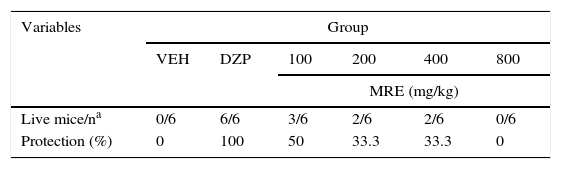
The statistical analysis demonstrated significant differences (F5.30=298.64; P<.001, Fig. 2a) in latency to both myoclonic seizures and clonic seizures (F5.30=20147.09; P<.001; Fig. 2b). The post hoc test demonstrated that only DZP showed increased latency when comparing all the groups. We also found significant differences in latency to the onset of tonic–clonic seizures (F5.30=12.41; P<.001). The post hoc test showed that the groups treated with MRE (100–400mg/kg but not 800mg/kg) displayed greater latency to tonic–clonic seizures than those receiving the vehicle, although still with less efficacy than DZP (Fig. 2c). A similar outcome was found for the variable of protection against PTZ lethality (Table 3).
Model of PTZ-induced seizures. MRE did not modify latency to either myoclonic or clonic seizures. However, it increased latency to tonic–clonic seizures in a manner inversely proportional to the dose used. DZP produces anticonvulsant effects. (a) **P<.001 vs all groups. (b) **P<.001 vs all groups. (c) **P<.001 vs all groups; #P<.001 vs VEH, MRE 400, and MRE 800; ●P<.001 vs VEH and MRE 800. DZP: diazepam group; MRE: methanolic root extract group; VEH: vehicle group.
Protective effects of MRE against PTZ lethality.
| Variables | Group | |||||
|---|---|---|---|---|---|---|
| VEH | DZP | 100 | 200 | 400 | 800 | |
| MRE (mg/kg) | ||||||
| Live mice/na | 0/6 | 6/6 | 3/6 | 2/6 | 2/6 | 0/6 |
| Protection (%) | 0 | 100 | 50 | 33.3 | 33.3 | 0 |
DZP, diazepam; MRE, methanolic root extract; VEH, vehicle.
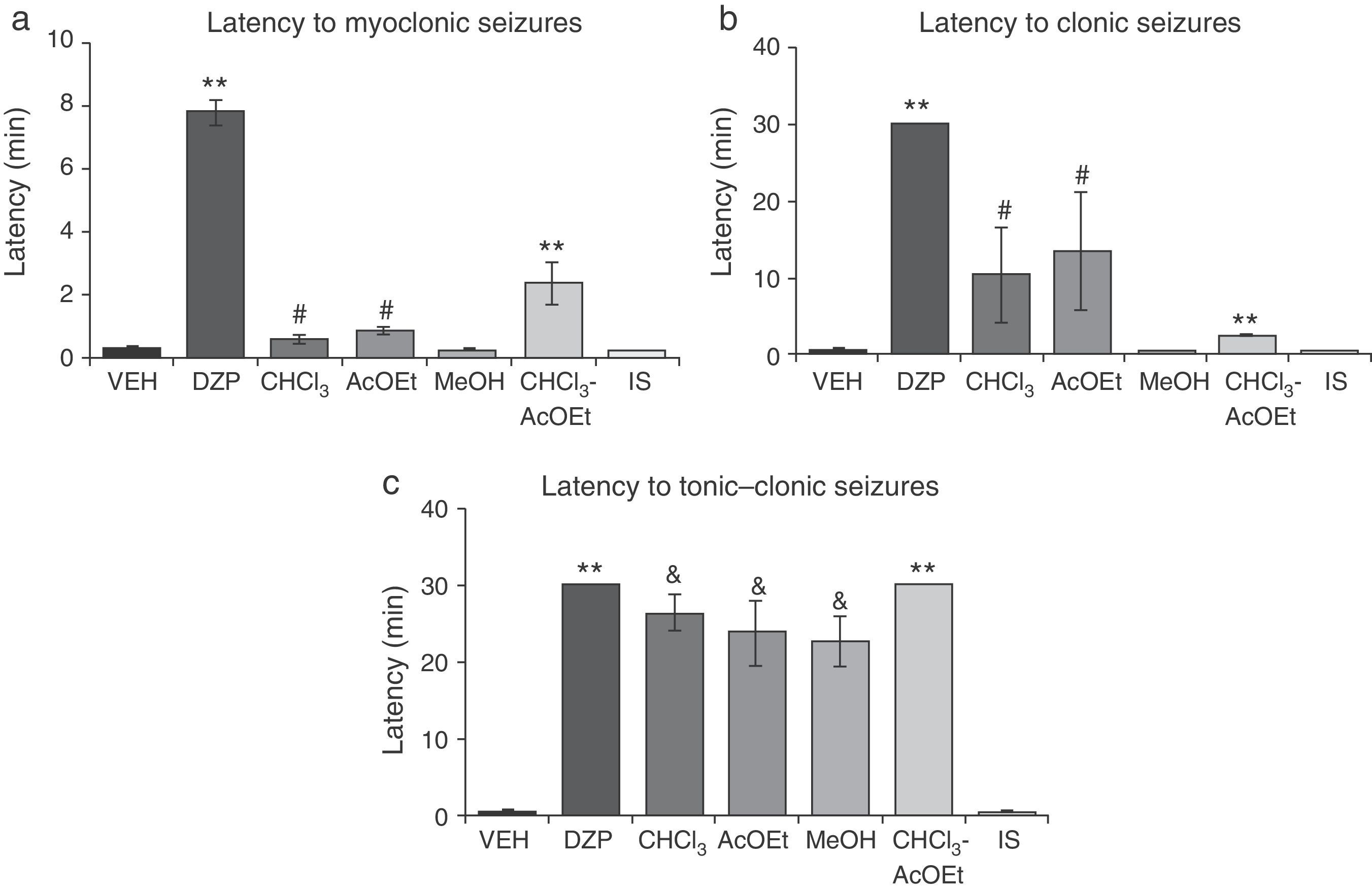
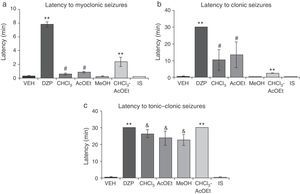
Latency to myoclonic seizures (F6.35=80.66; P<.001) and clonic seizures (F6.35=8.71; P<.001) varied significantly depending on the treatment. The post hoc test showed that the CHCl3 and AcOEt fractions significantly increased latency periods compared to VEH, MeOH, and IS fractions; DZP and CHCl3–AcOEt fractions resulted in greater latencies with respect to all groups (Fig. 3a and b). Latency to tonic–clonic seizures also displayed significant differences (F6.35=38.42; P<.001). According to the post hoc test, CHCl3, AcOEt, and MeOH showed greater latency than VEH and IS, while the DZP and CHCl3–AcOEt fractions displayed the longest latency periods (Fig. 3c).
Model of PTZ-induced seizures. CHCl3, AcOEt, and CHCl3–AcOEt fractions and DZP show anticonvulsant effects. (a) **P<.001 vs all groups; #P<.001 vs VEH, MeOH, and IS. (b) **P<.001 vs all groups; #P<.001 vs VEH, MeOH, and IS. (c) **P<.001 vs all groups; &P<.001, compared to VEH and IS. AcOEt: ethyl acetate group; CHCl3: chloroform group; CHCl3–AcOEt: chloroform–ethyl acetate group; DZP: diazepam group; MeOH: methanol group; IS: inorganic salt group; VEH: vehicle group.
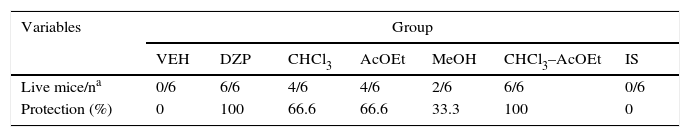
MSE fractions displayed less protective effects against PTZ lethality than DZP. However, the CHCl3–AcOEt fraction demonstrated total protection, similar to that produced by MSE and DZP. The IS fraction provided no protection (Table 4).
Protective effects of MSE fractions against PTZ lethality.
| Variables | Group | ||||||
|---|---|---|---|---|---|---|---|
| VEH | DZP | CHCl3 | AcOEt | MeOH | CHCl3–AcOEt | IS | |
| Live mice/na | 0/6 | 6/6 | 4/6 | 4/6 | 2/6 | 6/6 | 0/6 |
| Protection (%) | 0 | 100 | 66.6 | 66.6 | 33.3 | 100 | 0 |
AcOEt, ethyl acetate; CHCl3, chloroform; CHCl3–AcOEt, chloroform–ethyl acetate; DZP, diazepam; MSE, methanolic stem extract; MeOH, methanol; IS, inorganic salt; VEH, vehicle.
This study explores the anticonvulsant activity of K. pinnata MRE and MSE in BALB/c mice with PTZ-induced seizures. Preclinical studies have demonstrated that clinically effective anticonvulsants (valproate, DZP, and carbamazepine) reduce the severity of PTZ-induced seizures.7,18 The MRE extract increased latency to tonic–clonic seizures; this increase was inversely proportional to the dose. The same was true for protection against PTZ lethality, such that the most effective dose was 100mg/kg. However, that dose was less effective than DZP. The MSE extract, however, boosted latency to myoclonic and clonic seizures in a dose-dependent manner with respect to the vehicle, although it was still less effective than DZP. On the other hand, all the evaluated doses increased latency to tonic–clonic seizures and were 100% protective against PTZ lethality with the same efficacy as DZP. In accordance with the preliminary phytochemical analysis, MRE and MSE effects on tonic–clonic seizures can be traced to the presence of sterols in both extracts. Sterols such as β-sitosterol and stigmasterol have been identified in K. pinnata leaf extract.19,20 These compounds contain a base structure of cyclopentanoperhydrophenanthrene, similar to neuroactive steroids such as progesterone and allopregnanolone, which possess anticonvulsant activity through the activation of a GABAA receptor.21,22
On the other hand, effects produced by MSE on latency to myoclonic and clonic seizures might be due to the presence of such terpenes as α-amyrin and β-amyrin, which have already been identified in K. pinnata.1 These terpenes have demonstrated effects on the central nervous system, including anticonvulsant effects on PTZ models23 and anxiolytic effects in the elevated plus-maze in a manner similar to that of DZP, which could indicate that the mechanism of action is shared.24 Additionally, β-amyrin has been shown to increase the release of cerebral noradrenalin in mice,25 linking the interaction of the noradrenergic system to the anticonvulsant effect of β-amyrin.
We also observed that anticonvulsant activity was preserved in the CHCl3 and AcOEt fractions of MSE, although with less efficiency than the complete extract or DZP, probably because of a decrease in the sterol and terpene concentrations, which were also identified in these fractions of the stem extract. On combining the CHCl3 and AcOEt fractions, the anticonvulsant efficacy is recovered and protection against PTZ lethality reaches the same level as DZP.
One limitation of the present study was that we failed to determine the precise sterols or terpenes responsible for the anticonvulsant effect of the plant and the link between them, which is an area that could be studied in the future.
ConclusionK. pinnata root and stem extracts exert anticonvulsant effects in PTZ-induced seizure models. In addition, stem extracts provide total protection against PTZ lethality with the same efficacy as DZP. The metabolites responsible for the stem extract activity are found in the CHCl3 and AcOEt fractions, which suggests this activity is due to sterols and terpenes. MRE activity, on the other hand, may be attributable to the presence of sterols.
Conflicts of interestThe authors have no conflicts of interest to declare.
The lead author received financial support from CONACyT (Reg. 229628) for his doctorate studies in neuroethology.
Please cite this article as: Mora-Pérez A, Hernández-Medel MR. Actividad anticonvulsivante del extracto metanólico de tallo y raíz de Kalanchoe pinnata Lam. en ratones: Comparación con diazepam. Neurología. 2016;31:161–168.