Diabetic microvascular disease (MVD) has been associated with increased bone fragility. The objective was to analyse the relationship between MVD and trabecular microstructure – assessed by the trabecular bone score (TBS) – in type 2 diabetic (T2D) patients. A second aim was to know the relationship between vitamin D and MVD.
Patients and methodsCross-sectional study, which included men >50 years and postmenopausal women participating in a population-based cohort, diagnosed with T2D. The presence of nephropathy, neuropathy and/or retinopathy was classified as MVD+. Clinical and laboratory variables, TBS, 25(OH)D and BMD by DXA, were evaluated. Bivariate and multivariate analysis were performed.
ResultsWe evaluated 361 patients (51.1% women), 63.8 (9) years old. Of them, 92 were MVD+ and presented poorer metabolic control, longer duration of T2D, lower TBS [1.235 (0.1) vs. 1.287 (0.1); p = 0.007] and lower levels of 25(OH)D [18.3 (7) vs. 21.6 (8) ng/mL; p = 0.0001). There were no differences between MVD+ and MVD− with regard to BMD or P1NP and β-CTX markers. After adjusting for confounders, including HbA1c and duration of T2D, the TBS value in MVD+ was 1.252 (95% CI 1.230–1.274) vs. 1.281 (95% CI 1.267–1.295) in MVD− (p = 0.034). MVD was associated with a 25(OH)D level <20 ng ml with an adjusted OR of 1.88 (95% CI 1.06–3.31; p = 0.028).
ConclusionsThe MVD+ patients presented a significantly lower TBS, after adjusting for confounders. Furthermore, multivariable analysis showed a significant relationship between a low 25(OH)D level and a prevalent MVD.
La enfermedad microvascular (EMV) diabética ha sido asociada con una fragilidad ósea incrementada. El objetivo fue analizar la relación entre la EMV y la microestructura trabecular – evaluada mediante el índice trabecular óseo (trabecular bone score, TBS) – en pacientes diabéticos tipo 2 (DM2). Adicionalmente, conocer la relación entre la vitamina D y la EMV.
Pacientes y métodosDiseño transversal analítico, que incluyó varones > 50 años y mujeres postmenopáusicas con DM2, participantes en una cohorte poblacional. Se clasificó como EMV+ la presencia de nefropatía, neuropatía y/o retinopatía. Fueron analizadas variables clínicas, de laboratorio, el TBS, la 25-hidroxivitamina D [25(OH)D] y la densidad mineral ósea (DMO). Se realizaron análisis bivariable y multivariable.
ResultadosFueron evaluados 361 pacientes (51,1% mujeres), de 63,8 (9) años. De ellos, 92 tenían EMV, con un peor control metabólico, mayor duración de la DM2, menor TBS (1,235 [0,1] vs. 1,287 [0,1]; p = 0,003) y menores niveles de 25(OH)D (18,3 [7] vs. 21,6 [8] ng/mL; p = 0,0001). No hubo diferencias entre EMV+ y EMV− en la DMO ni en los marcadores P1NP y β-CTX. Tras ajustar por confusores, incluyendo HbA1c y duración de la DM2, el TBS en EMV+ fue 1,252 (IC 95% 1,230–1,274) vs. 1,281 (IC 95% 1,267–1,295) en EMV− (p = 0,034). La EMV se asoció a un nivel de 25(OH)D < 20 ng/mL con una OR ajustada = 1,88 (IC 95% 1,06–3,31; p = 0,028).
ConclusionesLos pacientes con EMV presentaron un TBS significativamente menor, tras ajustar por confusores. El análisis multivariable mostró asimismo una asociación significativa entre un nivel bajo de 25(OH)D y la EMV prevalente.
Diabetes mellitus (DM) is a major public health problem worldwide. It is characterised by high plasma glucose levels and its nature often includes chronic complications that have a significant negative affect on the quality of life, and add a high economic burden on patients and health systems1. Type 1 DM (T1DM) is associated with an autoimmune destruction of the pancreatic cells β leading to an absolute insulin deficiency. Type 2 DM (T2DM), which accounts for more than 90% of DM cases, involves a functional deterioration of the cell β, with a synergistic role being played by insulin resistance and inflammation. The diabetic complications include microvascular diseases (MVD) – retinopathy, nephropathy, and neuropathy – and macrovascular disorders – ischemic heart disease (IHD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD)2.
Both types of diabetes have an increased risk of fracture. T1DM is associated with an increased risk of hip fracture (RR = 8.9; 95% CI 7.1–11.2), vertebral fracture (VF) and proximal humerus. With respect to T2DM, reports show an RR for hip fractures of 2.7 (95% CI 1.7–4.4) and an increased risk of VF in women (OR = 1.9; 95% CI 1.1–3.1) and in men (OR = 4.7; 95% CI 2.1–10.2)1. In patients with T1DM the bone mineral density (BMD) is decreased, while in those with T2DM it is often normal or even increased, compared to non-diabetic subjects of the same age3.
The Trabecular Bone Score (TBS) is a parameter obtained from the bone densitometry image. Two-dimensional densitometry images are transformed into three-dimensional structures using software installed on the densitometer itself, and the score measures the texture of an image that correlates with the 3D determination of the trabecular structure. This dimensionless score provides an indirect estimate of the trabecular architecture, correlating well with the histomorphometric parameters, and several studies have shown that low TBS values are associated with an increased risk of fracture due to fragility, regardless of the BMD4. The TBS presents lower values in diabetic subjects than in non-diabetic subjects4, and it has proved useful in assessing the fracture risk in patients with T2DM4.
Inflammation, oxidative stress, low bone turnover, adipokine alterations, WNT dysregulation, and increased risk of falls related to sarcopenia or vitamin D deficiency are determinant factors of fracture risk in patients with T2DM1. It has also been suggested that MVD may be a key factor in skeletal disorders of the disease1,3, suggesting that these microvascular alterations inside the diabetic bone could cause adverse changes in bone microstructure and, consequently, cause its quality to deteriorate5.
However, other authors have not observed a specific relationship between MVD and bone fragility6, and from that perspective it has been argued that MVD would simply act as a marker of long-term DM or DM with poor metabolic control6.
There is an open debate on this issue7, with several aspects that have not yet been clarified. One of them is the possible relationship between MVD and a decreased TBS, on the assumption that MVD causes a deterioration in the trabecular microstructure which is an analysis to our knowledge that has not yet performed. There is also controversy about the relationship between vitamin D deficiency and MVD8,9.
Based on the above, we have proposed this study with the main objective of comparing the value of TBS in type 2 diabetic patients with and without MVD. A secondary objective has been to determine the association between plasma levels of 25-hydroxyvitamin D (25(OH)D) and MVD.
Patients and methodsParticipants and study designA cross-sectional, analytical observational study nested in a cohort was conducted. The patients belong to a prospective population-based study, the Camargo Cohort, started in 2006 and set up to study bone metabolic diseases in the general population of our geographic area. The cohort consists of males who are 50 years of age and older and postmenopausal females, and their composition and design have been previously published10. At the baseline visit, all patients were provided with a questionnaire on bone metabolism and general diseases, current or past medication use, and risk factors for osteoporosis and fractures. They also underwent a laboratory study (general and specific aimed at assessing bone metabolism), a simple x-ray of the spine, a bone densitometry (DXA) and a calcaneal ultrasonography. After receiving information about the purpose of the cohort study, the subjects were invited to participate, and all participants gave their informed written consent. The Camargo Cohort study was approved by the Cantabria Clinical Research Ethics Committee (Internal Code: 2016.003). The Declaration of Helsinki postulates for human research studies were followed.
The study inclusion criteria were a previous diagnosis of T2DM and an initial assessment that ruled out the presence of diseases or treatments with an effect on bone metabolism, such as primary hyperparathyroidism, hyperthyroidism or the consumption of bisphosphonates, estrogens, raloxifene, strontium ranelate, teriparatide, l-thyroxine, anticonvulsants or glucocorticoids, in the year prior to inclusion.
Clinical variablesWeight (in kg) and abdominal circumference (in cm) were logged. Body mass index (BMI) was measured in kg/m2, with obesity considered as BMI > 30 kg/m2. Glomerular filtration (GFR), expressed in mL/min/1.73 m2, was estimated according to the CKD-EPI formula11. Vertebral fracture (VF) was classified according to the grades of the Genant et al. semiquantitative scale12. The diagnosis of macrovascular disease (IHD, CVD, PAD) was made according to the clinical history.
Microvascular disease (MVD)The definition of MVD was given due to the presence of nephropathy, neuropathy and/or retinopathy in a diabetic patient, classed as nephropathy when albuminuria ≥ 30 mg/g creatinine and/or a sustained reduction in GFR estimated below 60 mL/min/1.73 m2 for at least 3 months; as neuropathy when a pathological monofilament test or the criterion of the American Diabetes Association of “the presence of symptoms and/or signs of peripheral nerve dysfunction in a diabetic person in the absence of another diagnosis”; and as retinopathy when confirmed by the ophthalmologist’s clinical diagnosis after an examination of the fundus2,8.
The sample was classified into subjects without microvascular disease (MVD−) and subjects with microvascular disease (MVD+). Additionally, 4 subgroups were defined: group A (MVD- with macrovascular disease), group B (MVD- without macrovascular disease), group C (MVD+ with macrovascular disease) and group D (MVD + without macrovascular disease).
Laboratory parametersBlood samples were obtained from a vein in the forearm in the early hours of the morning, after a 12-h fast. Concentrations of calcium, phosphorus, albumin, total alkaline phosphatase (tALP), C-reactive protein (CPR), thyrotropin (TSH), total cholesterol, high-density lipoprotein-bound cholesterol (HDLc), triglycerides and glycosylated hemoglobin (HbA1c) were obtained by an automated method (ADVIA® 2400 Chemistry System Autoanalyzer, Siemens, Germany). Procollagen Type 1 N-terminal Propeptide (P1NP) and β-C-terminal telopeptide of type 1 collagen (β-CTX) were analysed as bone turnover markers (BTMs). The concentrations of 25(OH)D, P1NP, β-CTX and intact parathyroid hormone (iPTH) were determined by an automated electrochemiluminescence method (Elecsys® 2010, Roche Diagnostics, GmbH, Mannheim, Germany). The detection limits of iPTH, P1NP and β-CTX were 6 pg/mL, 5 ng/mL and 0.01 ng/mL and the ranges of normality were 15–65 pg/mL, 15−78 ng/mL and 0.069−0.760 ng/mL, respectively. The 25(OH)D variable was analysed quantitatively. Given the variations in vitamin D in relation to sunlight and the seasons13, the plasma level of 25(OH)D was analysed according to the month and season of the year in which the blood was taken. The latitude of our geographical area (Camargo, Cantabria, Spain) is 43.4086° N.
Bone mineral density and TBSThe BMD was assessed by DXA with the QDR-4500 machine by Hologic® in 3 locations; lumbar spine, femoral neck and total hip. In vivo precision was 0.4–1.5% and the results were expressed in gr/cm2. All measurements were performed by the same technician. The TBS was obtained from lumbar spine DXA images (L1-L4) using specific software (TBS iNsight® v2.1, Medimaps, Mérignac, France) installed on the densitometer. A degraded trabecular microstructure was considered as a TBS value < 1.230, partially degraded between 1.230−1.310, and a normal TBS > 1.31014.
Statistical analysisQuantitative variables were expressed as mean (standard deviation, SD) or median [interquartile range], as appropriate. After checking the assumption of normality with the Kolmogorov-Smirnov test, contrast tests were used such as the Student’s t-test, ANOVA, Kruskal-Wallis H or the Median test. The Jonckheere-Terpstra test was used to calculate the p-value of trend. The categorical variables were expressed as a percentage, and the test was used for comparison χ2. Correlation analyses were performed using Pearson’s r or Spearman’s rho.
In order to perform additional analyses, the quantitative variables of age, HbA1c and duration of the diabetes were transformed into dichotomous variables according to the median of the distributions. The strength of an association was expressed with the odds ratio (OR) and its corresponding 95% confidence interval (95% CI). Two multivariate analyses (logistic regression and general linear model) were performed to discover the relationships between MVD and TBS, adjusting for possible confounding variables. A p-value of <0.05 was considered as significant.
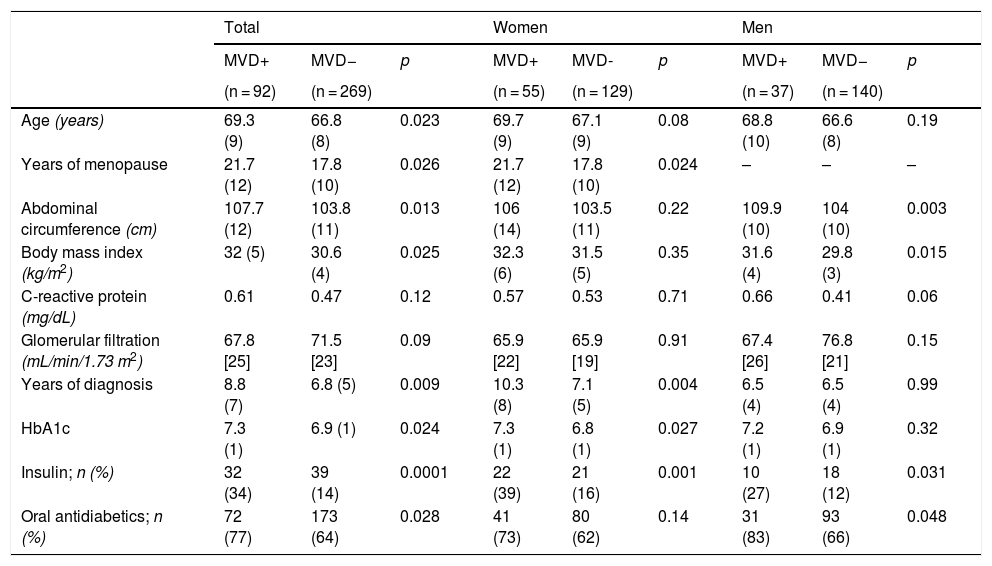
ResultsSample descriptionOf the 388 people with T2DM included in the Camargo Cohort, 15 were ruled out for not meeting the inclusion criteria and 12 due to a lack of data (Fig. 1). Finally, 361 patients were analysed. Of these, 269 (74.5%) were MVD−, distributed between group A (n = 52) and group B (n = 217). The remaining 92 (25.5%) were MVD+, distributed between group C (n = 26) and group D (n = 66). Table 1 summarises the general characteristics of the analysed sample. The study-patients had a mean age of 63.8 (9.7) years, an age range of 47–91 years, an HbA1c of 7%, and an estimated GFR of 72 mL/min/1.73 m2.
Clinical characteristics of the patients of the sample.
| Total | Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MVD+ | MVD− | p | MVD+ | MVD- | p | MVD+ | MVD− | p | |
| (n = 92) | (n = 269) | (n = 55) | (n = 129) | (n = 37) | (n = 140) | ||||
| Age (years) | 69.3 (9) | 66.8 (8) | 0.023 | 69.7 (9) | 67.1 (9) | 0.08 | 68.8 (10) | 66.6 (8) | 0.19 |
| Years of menopause | 21.7 (12) | 17.8 (10) | 0.026 | 21.7 (12) | 17.8 (10) | 0.024 | – | – | – |
| Abdominal circumference (cm) | 107.7 (12) | 103.8 (11) | 0.013 | 106 (14) | 103.5 (11) | 0.22 | 109.9 (10) | 104 (10) | 0.003 |
| Body mass index (kg/m2) | 32 (5) | 30.6 (4) | 0.025 | 32.3 (6) | 31.5 (5) | 0.35 | 31.6 (4) | 29.8 (3) | 0.015 |
| C-reactive protein (mg/dL) | 0.61 | 0.47 | 0.12 | 0.57 | 0.53 | 0.71 | 0.66 | 0.41 | 0.06 |
| Glomerular filtration (mL/min/1.73 m2) | 67.8 [25] | 71.5 [23] | 0.09 | 65.9 [22] | 65.9 [19] | 0.91 | 67.4 [26] | 76.8 [21] | 0.15 |
| Years of diagnosis | 8.8 (7) | 6.8 (5) | 0.009 | 10.3 (8) | 7.1 (5) | 0.004 | 6.5 (4) | 6.5 (4) | 0.99 |
| HbA1c | 7.3 (1) | 6.9 (1) | 0.024 | 7.3 (1) | 6.8 (1) | 0.027 | 7.2 (1) | 6.9 (1) | 0.32 |
| Insulin; n (%) | 32 (34) | 39 (14) | 0.0001 | 22 (39) | 21 (16) | 0.001 | 10 (27) | 18 (12) | 0.031 |
| Oral antidiabetics; n (%) | 72 (77) | 173 (64) | 0.028 | 41 (73) | 80 (62) | 0.14 | 31 (83) | 93 (66) | 0.048 |
MVD+: with microvascular disease; MVD−: without microvascular disease; HbA1c: glycosylated hemoglobin.
Quantitative variables, expressed as mean (SD) or median (interquartile range).
The MVD + patients presented nephropathy (n = 50), neuropathy (n = 15), retinopathy (n = 11), or a combination of all three (n = 16). 78 patients were registered with macrovascular disease: CI (n = 25), CVD (n = 21), PAD (n = 13) or a combination of these (n = 18).
Bivariate analysisIn the 361 diabetic patients, HbA1c had significant inverse correlations with the TBS (r = −0.17; p = 0.002), with the BMD-LS (r = −0.11; p = 0.049), and with P1NP and β-CTX (r = −0.26; p = 0.0001 and r = −0.20; p = 0.0001, respectively). No significant associations were observed between HbA1c and 25(OH)D or BMD levels in the femoral neck or total hip. The years of duration of diabetes and the TBS showed no correlation (r = 0.03; p = 0.47). 25(OH)D levels were negatively correlated with iPTH (r = −0.27; p = 0.0001), and positively correlated with the TBS (r = 0.11; p = 0.03), in a ratio that remained significant after adjusting for sex, age, iPTH levels, BMI and season of the year.
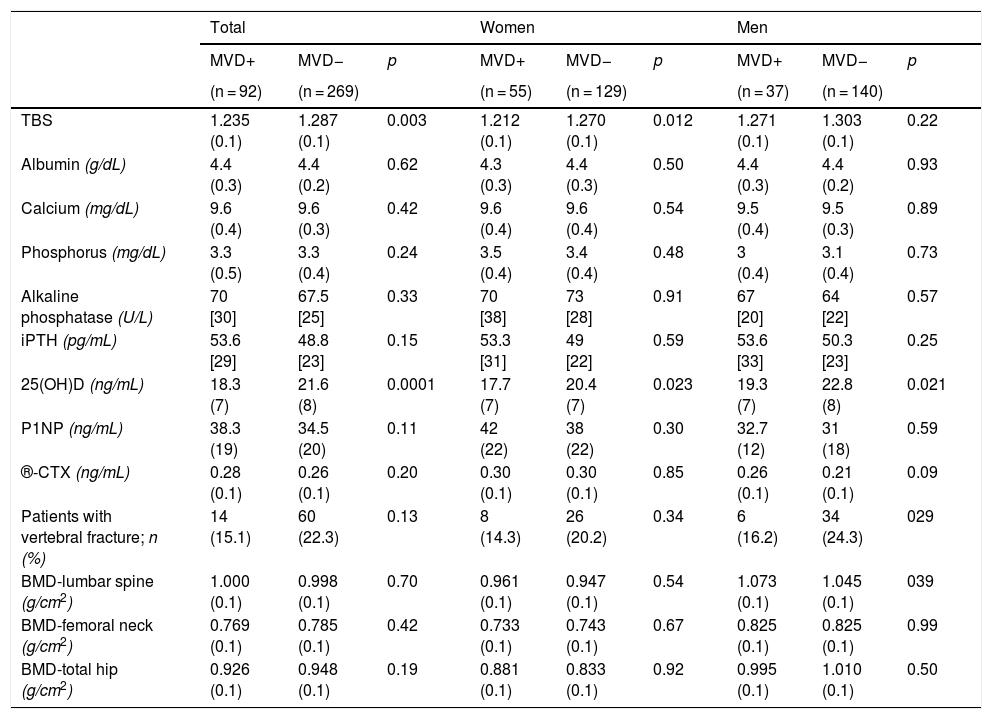
The MVD + patients were older, had poorer metabolic control, increased insulin and oral antidiabetic use, a lower plasma level of 25(OH)D, and a lower TBS. These characteristics were maintained when stratified by sex (Tables 1 and 2). The value of the BMD in the 3 locations and of the analysed BTMs, P1NP and β-CTX, was similar in both groups, and there were no significant differences in the prevalence and severity of VF (Table 2).
Parameters related to bone metabolism in the patients of the sample.
| Total | Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MVD+ | MVD− | p | MVD+ | MVD− | p | MVD+ | MVD− | p | |
| (n = 92) | (n = 269) | (n = 55) | (n = 129) | (n = 37) | (n = 140) | ||||
| TBS | 1.235 (0.1) | 1.287 (0.1) | 0.003 | 1.212 (0.1) | 1.270 (0.1) | 0.012 | 1.271 (0.1) | 1.303 (0.1) | 0.22 |
| Albumin (g/dL) | 4.4 (0.3) | 4.4 (0.2) | 0.62 | 4.3 (0.3) | 4.4 (0.3) | 0.50 | 4.4 (0.3) | 4.4 (0.2) | 0.93 |
| Calcium (mg/dL) | 9.6 (0.4) | 9.6 (0.3) | 0.42 | 9.6 (0.4) | 9.6 (0.4) | 0.54 | 9.5 (0.4) | 9.5 (0.3) | 0.89 |
| Phosphorus (mg/dL) | 3.3 (0.5) | 3.3 (0.4) | 0.24 | 3.5 (0.4) | 3.4 (0.4) | 0.48 | 3 (0.4) | 3.1 (0.4) | 0.73 |
| Alkaline phosphatase (U/L) | 70 [30] | 67.5 [25] | 0.33 | 70 [38] | 73 [28] | 0.91 | 67 [20] | 64 [22] | 0.57 |
| iPTH (pg/mL) | 53.6 [29] | 48.8 [23] | 0.15 | 53.3 [31] | 49 [22] | 0.59 | 53.6 [33] | 50.3 [23] | 0.25 |
| 25(OH)D (ng/mL) | 18.3 (7) | 21.6 (8) | 0.0001 | 17.7 (7) | 20.4 (7) | 0.023 | 19.3 (7) | 22.8 (8) | 0.021 |
| P1NP (ng/mL) | 38.3 (19) | 34.5 (20) | 0.11 | 42 (22) | 38 (22) | 0.30 | 32.7 (12) | 31 (18) | 0.59 |
| ®-CTX (ng/mL) | 0.28 (0.1) | 0.26 (0.1) | 0.20 | 0.30 (0.1) | 0.30 (0.1) | 0.85 | 0.26 (0.1) | 0.21 (0.1) | 0.09 |
| Patients with vertebral fracture; n (%) | 14 (15.1) | 60 (22.3) | 0.13 | 8 (14.3) | 26 (20.2) | 0.34 | 6 (16.2) | 34 (24.3) | 029 |
| BMD-lumbar spine (g/cm2) | 1.000 (0.1) | 0.998 (0.1) | 0.70 | 0.961 (0.1) | 0.947 (0.1) | 0.54 | 1.073 (0.1) | 1.045 (0.1) | 039 |
| BMD-femoral neck (g/cm2) | 0.769 (0.1) | 0.785 (0.1) | 0.42 | 0.733 (0.1) | 0.743 (0.1) | 0.67 | 0.825 (0.1) | 0.825 (0.1) | 0.99 |
| BMD-total hip (g/cm2) | 0.926 (0.1) | 0.948 (0.1) | 0.19 | 0.881 (0.1) | 0.833 (0.1) | 0.92 | 0.995 (0.1) | 1.010 (0.1) | 0.50 |
MVD+: with microvascular disease; MVD−: without microvascular disease; TBS: trabecular bone score; iPTH: intact parathyroid hormone; 25(OH)D: 25-hydroxyvitamin D; P1NP: aminoterminal propeptide of type 1 collagen; β-CTX: C-terminal telopeptide of type 1 collagen; BMD: bone mineral density.
Quantitative variables, expressed as mean (SD) or median (interquartile range).
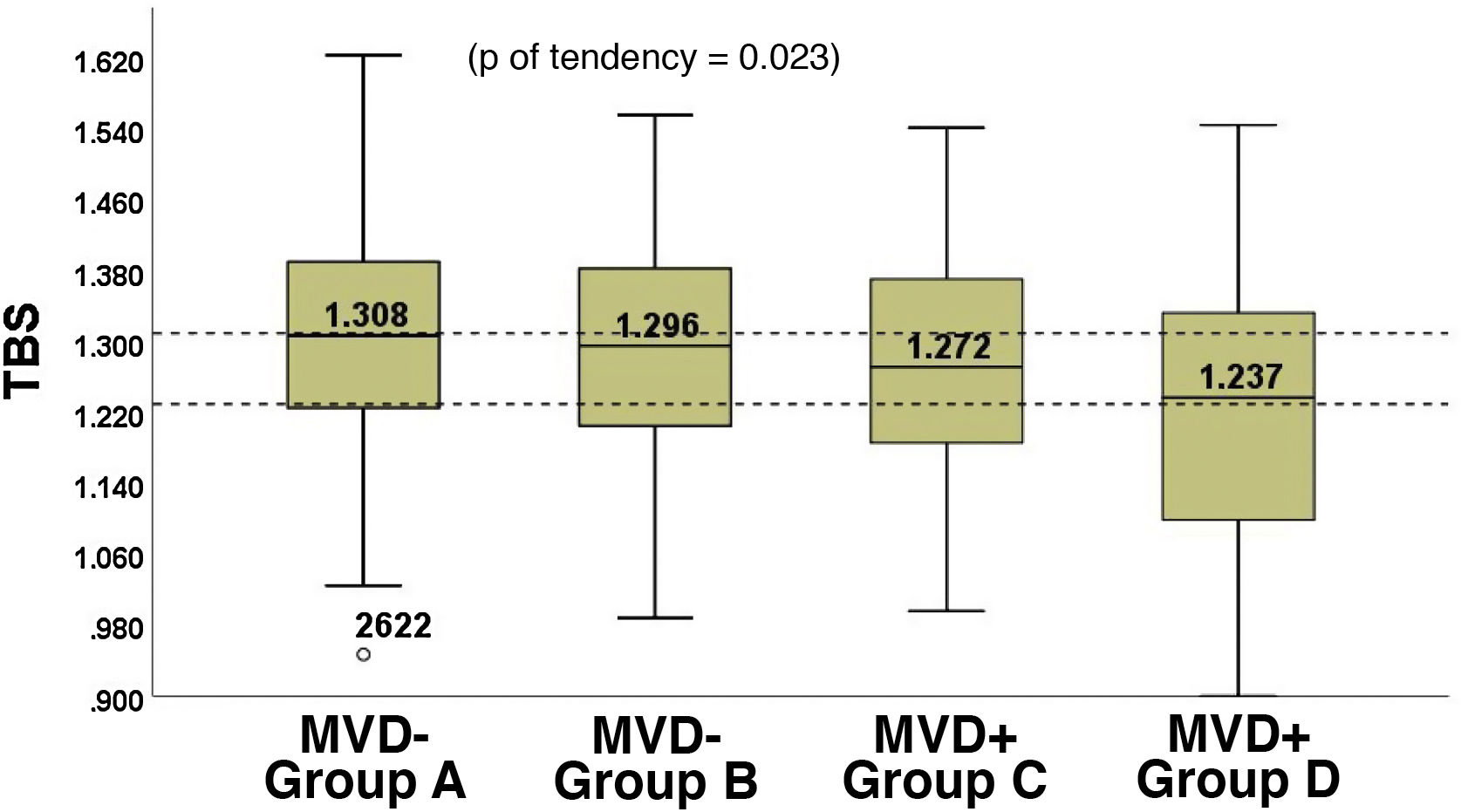
The group with isolated MVD (Group D, 68.2 [9.6] years) presented the lowest TBS, while the group with isolated macrovascular disease (Group A, 69.7 [9.2] years) presented the highest TBS. Between both of them, the TBS values of the group without macrovascular or microvascular disease (Group B, 66.1 [8.6] years) and of the group with both diseases (Group C, 71.8 [9.3] years) were positioned. (Fig. 2). The lowest TBS was observed in patients with retinopathy (TBS = 1.186 [0.2]), followed by nephropathy (TBS = 1.234 [0.2]) and neuropathy (TBS = 1.252 [0.2]), although the differences were not significant (p = 0.59).
46.2% of the MVD + patients and 30.1% of MVD− patients had a TBS < 1.230, while 36.6% of those with MVD+ and 47.2% of those with MVD− had TBS values >1.310 (p = 0.01).
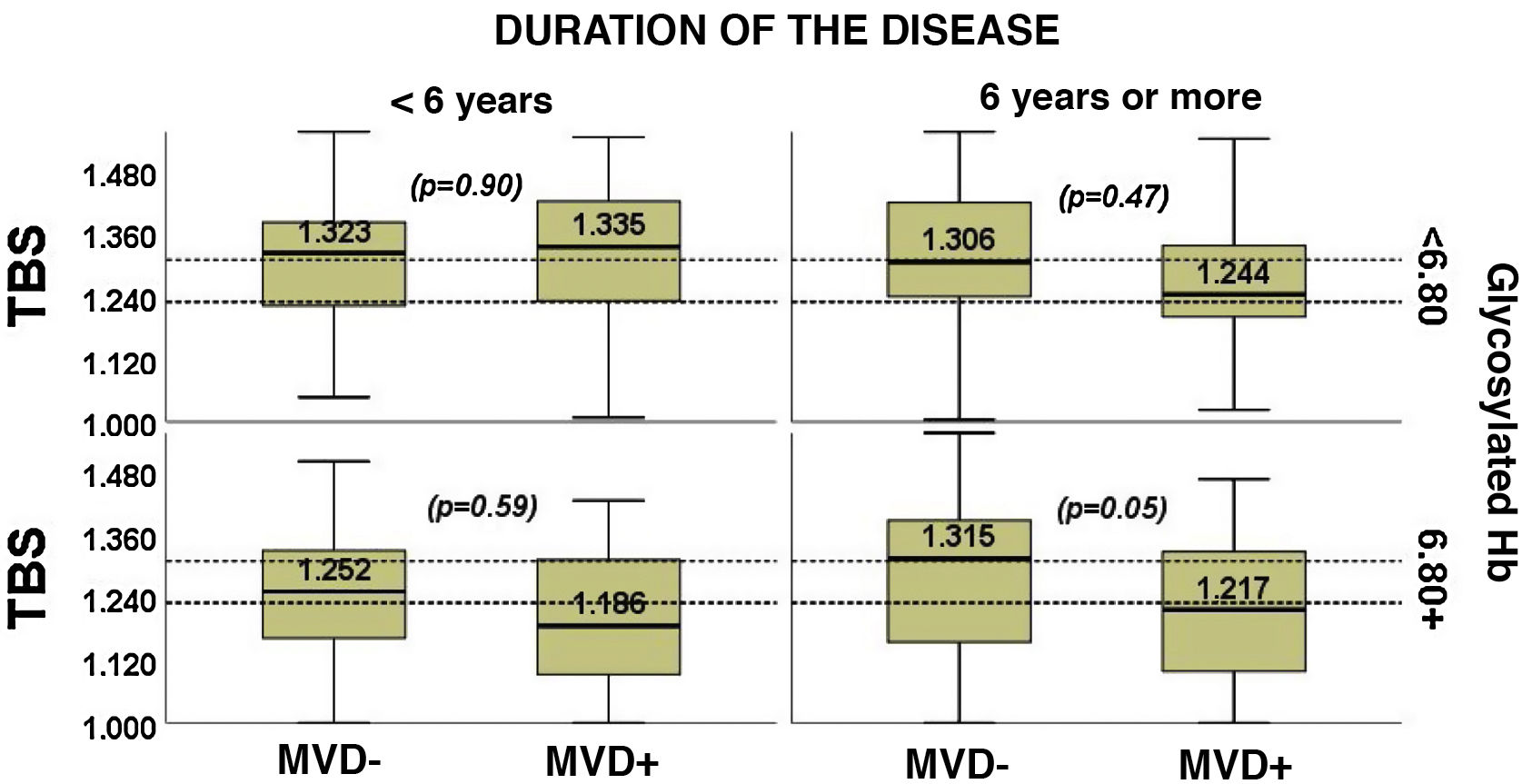
When the patients were classified simultaneously according to the HbA1c value and the duration of disease, it was seen that the MVD+ patients had an elevated HbA1c accompanied by TBS < 1.230, regardless of the duration of the disease (Fig. 3).
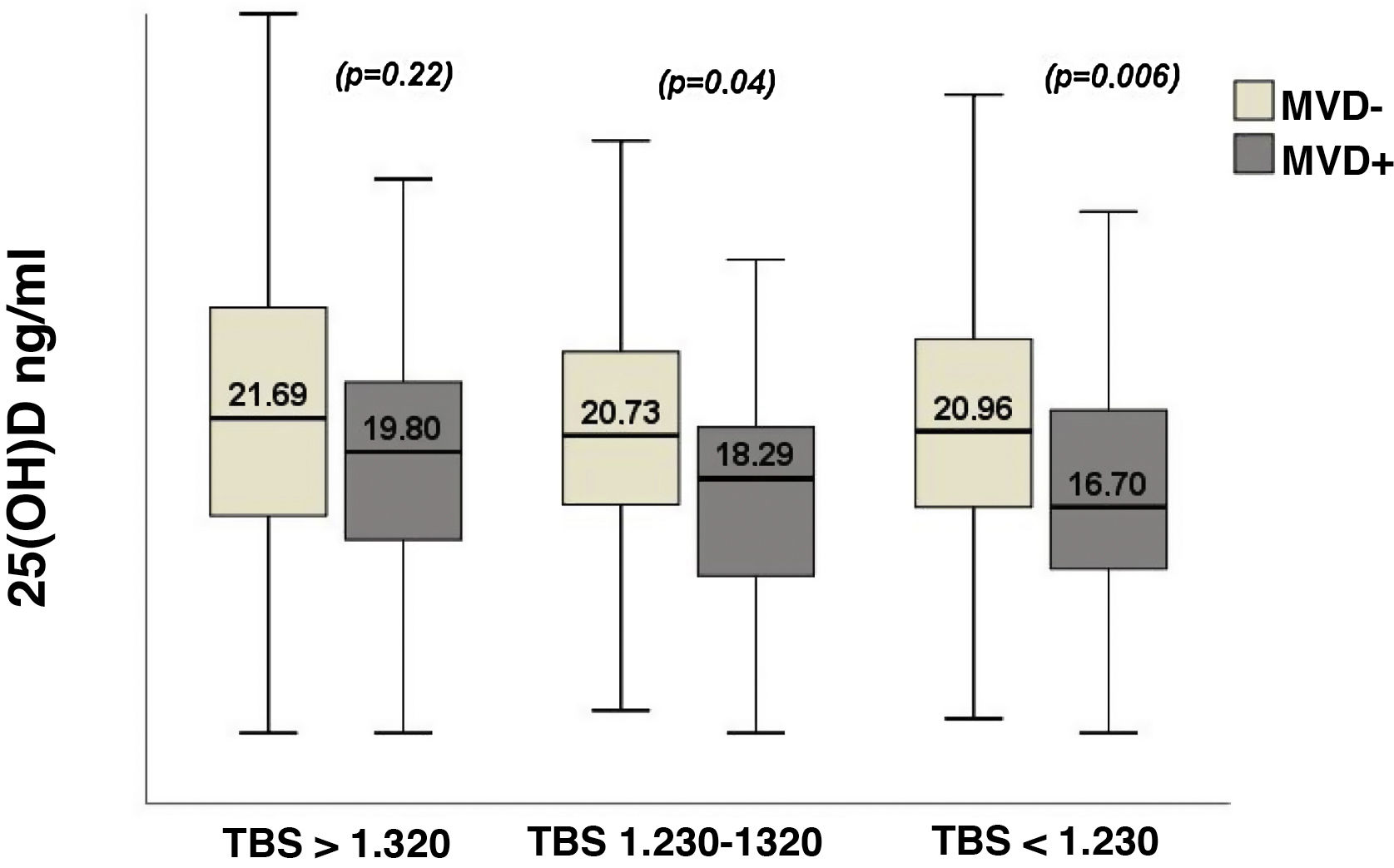
Fig. 4 shows the variations in 25(OH)D levels relative to the TBS. MVD+ patients showed significantly lower values of 25(OH)D in the presence of a TBS corresponding to a degraded or partially degraded trabecular architecture, while in the MVD- patients the values of 25(OH)D did not show any variation in relation with the TBS (Fig. 4). The MVD was associated with a significant risk as it presented a plasma level of 25(OH)D < 20 ng/mL, with an OR = 1.88 (95% CI 1.06–3.31; p = 0.028), after adjusting for sex, age, BMI, season of the year, the GFR, iPTH levels, the TBS, lumbar BMD and the presence of prevalent VF.
Multivariable modelsThe mean value of TBS in patients with MVD+ was 1.252 (95% CI 1.230−1.274), compared with a value of 1.281 (95% CI 1.267−1.295) in those MVD− (p = 0.034), after adjusting for sex, age, BMI, MS, tobacco and alcohol use, insulin treatment, OADs and statins, GFR, 25(OH)D levels, iPTH, P1NP and β-CTX, lumbar BMD, HbA1c, duration of diabetes and the presence of macrovascular disease. Additionally, MVD was associated with an increased risk of developing a TBS value <1.230, with an OR = 2.66 (95% CI 1.18–6.00; p = 0.018), after adjusting for the same confounders as in the general linear model. The ROC curve associated with the logistic regression model showed an area under the curve of 0.898 (95% CI 0.861−0.934); p = 0.0001.
DiscussionMicrovascular disease and TBSIn this study conducted with patients with T2DM, those with MVD+ had a significantly lower TBS than the MVD− patients, after adjusting for the duration of the disease and the degree of metabolic control, etc. In contrast, the BMD showed no significant differences between the two groups. To our knowledge, this is the first study to show a relationship between diabetic MVD and a low TBS.
The different clinical manifestations of MVD were associated with the bone fragility of the DM. Thus, retinopathy was associated with a low BMD15 and with an increased risk of incidental fracture16. In a prospective study of 3654 subjects > 49 years old, Ivers et al.16 observed, after a two-year follow-up, that diabetic retinopathy, a duration of diabetes > 10 years, advanced cataracts and insulin treatment were significantly associated with an increased risk of fracture, and suggested that retinopathy would act as a marker of advanced and severe diabetes. Regarding nephropathy, Vestergaard et al.17 studied 124,655 cases of fracture and 373,962 controls matched according to sex and age, and observed that only nephropathy, and not other complications of DM, increased the risk of fracture in patients with T1DM and T2DM. The authors suggested the possibility of an association of nephropathy with high blood glucose levels, which could weaken bone strength. In relation to neuropathy and the incidence of fracture, proprioceptive impairment has been associated as a trigger to an increased risk of falls. However, other lines of research have recently been proposed, such as the molecular and cellular link between neuropathy and the bone, and the changes in blood microcirculation associated with this complication18.
There is an open debate about if MVD simply develops in parallel with diabetic bone disease due to similar pathophysiological mechanisms (both are markers of long-term disease and poor glycemic control), or if there is a specific causal relationship between the two entities18. There is evidence that changes in the bone structure in the diabetic patient may be partly related to poor perfusion, so that microvascular complications would act as risk factors for bone deterioration in T1DM and T2DM5. In fact, in line with previous studies19, recent studies in patients with MVD have shown a relationship between low transcutaneous oxygen pressure – as a microvascular blood flow measure – and an increase in cortical porosity – assessed by high-resolution peripheral quantitative computed tomography, HR-pQCT – after adjusting for age, sex and BMI20. Based on these observations, it has been suggested that bone fragility could be part of an extended clinical spectrum of MVD21.
A proposed pathophysiological mechanism, which would mediate between microvascular complications and bone fragility4,5, are the advanced glycation end products (AGEs). AGEs are a heterogeneous group of molecules generated by the non-enzymatic reaction of proteins, including type I collagen, that increase in the bone matrix with age and hyperglycemia. Through the nuclear factor κ-β, the AGEs increase oxidative stress and the expression of inflammatory mediators22, as well as the apoptosis of osteoblasts23 and reduce the bone material strength index measured by microindentation20. All of this occurs with adverse effects on the biomechanical properties of cortical and trabecular bone1.
The significant poor metabolic control presented by MVD+ patients in our study would facilitate the accumulation of AGEs, which curiously appear to also accelerate atherosclerotic calcification in the bone microcirculation24.
The need for further studies to examine the values of bone parameters for different values of HbA1c and the different duration of diabetes is ongoing6. Both can influence the risk of fracture, and act as confounder variables that must be considered23. Following this concept, we have analysed both variables and we have not seen any relationship between the duration of diabetes and the TBS. On the contrary, we have observed a significant inverse correlation between HbA1c and the TBS. In the same sense, in people with MVD+, the impact of metabolic control on the TBS has been greater than that of the duration of diabetes: in particular, in MVD+ patients, a high HbA1c was accompanied by a TBS in the range of degraded trabecular structure, regardless of the duration of the disease (Fig. 3). Both the duration of the diabetes and the value of HbA1c were included as adjustment variables in the multivariable models.
The effect of MVD on the bone was not been reflected in the BMD or plasma levels of P1NP and β-CTX. Published studies indicate that the DXA does not adequately characterise increased bone fragility in patients with T2DM and MVD21. Based on our results, the MVD appears to affect another determinant of bone fragility, independent of BMD, such as the trabecular structure. Regarding the BTMs, the data published in the MVD are scarce and inconsistent21. However, in the interpretation of the results it is worth noting the possible interference of the AGEs in the measurement of β-CTX, and the fact that the BTMs reflect the overall activity of the skeleton, rather than a defect in the cellular activity of a particular compartment, the cortical or trabecular bone.
Microvascular disease and plasma level of 25(OH)DAccording to our results, a low level of 25(OH)D was associated with the prevalent MVD, and in turn, the MVD was associated with a significant risk of presenting 25(OH)D <20 ng/mL levels, after adjusting for the confounding variables.
Vitamin D deficiency is currently considered a risk factor for cardiovascular disease, and these patients present an increased risk for DM, coronary heart disease, and stroke, as well as increased cardiovascular mortality25. In a previous study by the Camargo Cohort of 998 men > 50 years of age from the general population, it was observed that vitamin D deficiency was inversely related to abdominal aortic calcification (AAC), evaluated according to the 24-point scale. Specifically, as the plasma level of 25(OH)D descended (>30, 20.1–30, 10.1–20 and ≤10 ng/mL), increased values of AAC (2.7 ± 4.9, 3.1 ± 4.5, 4 ± 0.7 and 4.4 ± 4.1, respectively; p = 0.03)26.
Also, according to the publication, there seems to be an association between vitamin D deficiency and microcirculation disorders, such as cerebral small vessel disease, retinopathy, nephropathy, peripheral neuropathy, or coronary microcirculation disorders27. In the same way, a longitudinal work, based on data from the FIELD study8, showed that low plasma levels of 25(OH)D, at 5 years of follow-up, were predictors of MVD or macrovascular disease, regardless of treatment and duration of diabetes. Based on these observations, the sequence “vitamin D deficiency-microvascular disorders-cardiovascular disease”, with endothelial activation and inflammation as mediating factors, has been put forward27.
Additionally, vitamin D deficiency has traditionally been considered to greatly affect the cortical bone28; however, recent studies on the general population have linked vitamin D deficiency to an altered trabecular structure, after adjusting for confounding variables29,30. Although there is no strong evidence of an association between vitamin D deficiency and TBS in the general population28, in our study on the T2DM population a direct correlation was observed between 25(OH)D levels and TBS values (r = 0.11; p = 0.03), which remained significant after adjusting for age, sex, BMI, iPTH levels and the season of the year.
According to our results, patients with MVD associated at least two adverse circumstances for bone trabecular structure: a poor metabolic control – which facilitates the accumulation of AGEs1,3 – and a low plasma level of 25(OH)D, which based on the recent studies, could contribute to the development of MVD27 and a reduction in the TBS29,30.
The study has some limitations that need to be considered. First, the cross-sectional design makes it possible to detect associations, but not to establish causality. The number of patients in the cohort who met inclusion criteria was relatively low, with the added effect of successive stratification on statistical power and the risk of making a type II error. Dietary intake of dairy products or calcium and/or vitamin D supplements have not been analysed as possible confounding variables. On the other hand, we believe there are two points of interest. To our knowledge, it is the first study to demonstrate a relationship between diabetic MVD and impaired trabecular architecture as measured by the TBS. Finally, the analysed population belongs to a large prospective study, the Camargo Cohort, which has been an added advantage in terms of reliability and data quality.
ConclusionsIn this study, performed on 361 type 2 diabetic patients belonging to the general population, with an acceptable metabolic control and a low prevalence of VF, MVD+ patients presented a significantly lower TBS than the MVD- patients, after adjusting for the confounding variables. There has also been a consistent and significant association between low plasma levels of 25(OH)D and the prevalent MVD.
Conflict of interestThe authors state that they have no conflicts of interest.
FundingThe study is funded in part by the Carlos III Health Institute (PI18/00762), which includes ERDF funds from the EU (Ministry of Economy and Competitiveness, Spain).
Please cite this article as: Maamar el Asri M, Pariente Rodrigo E, Díaz-Salazar de la Flor S, Pini Valdivieso S, Ramos Barrón MC, Olmos Martínez JM, et al. Índice trabecular óseo y niveles de 25-hidroxivitamina D en las complicaciones microvasculares de la diabetes mellitus tipo 2. Med Clin (Barc). 2022;158:308–314.