To describe the process of cultural adaptation and validation of the Boston Carpal Tunnel Questionnaire (BCTQ) measuring symptom intensity, functional status and quality of life in carpal tunnel syndrome patients and to report the psychometric properties of this version.
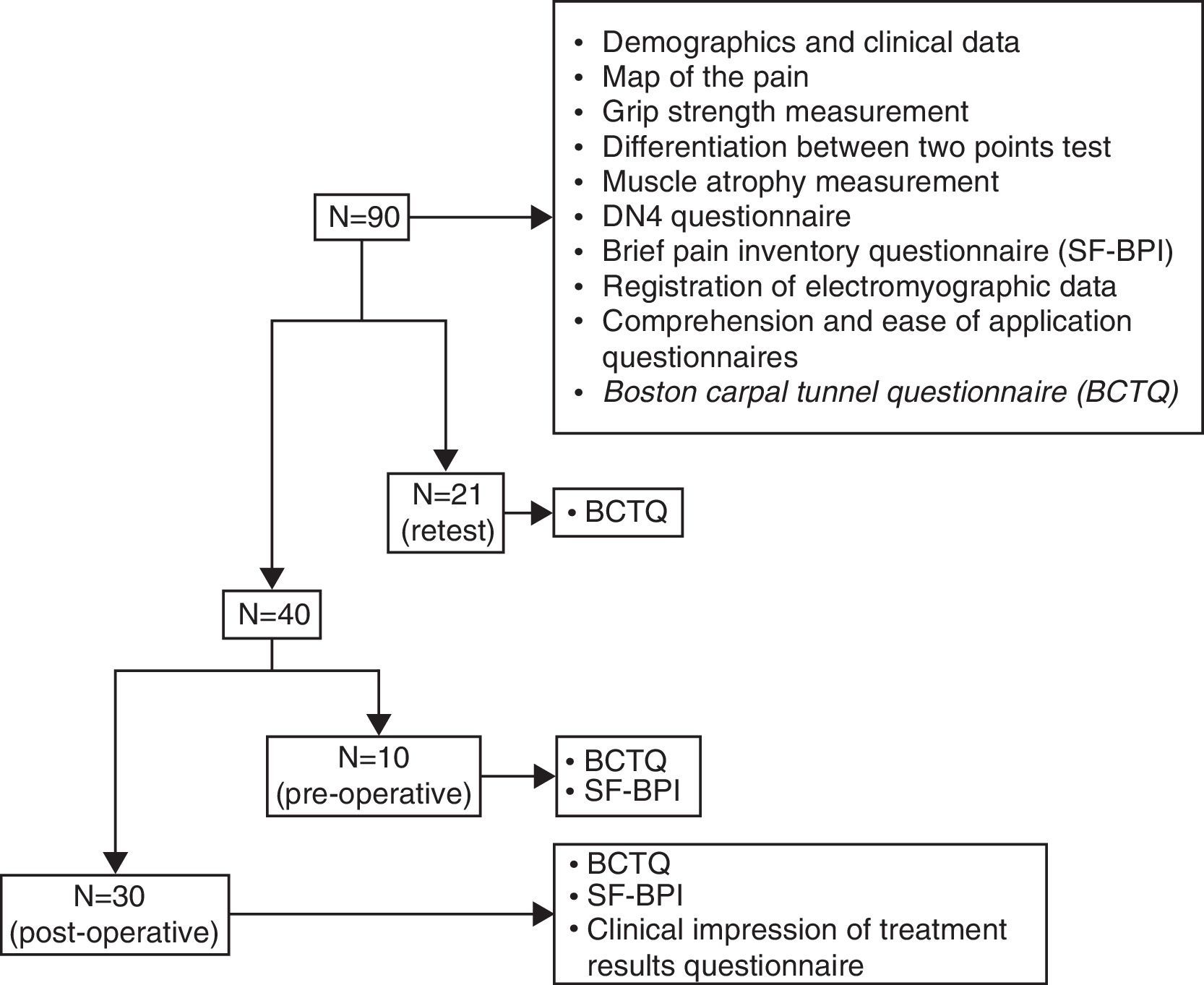
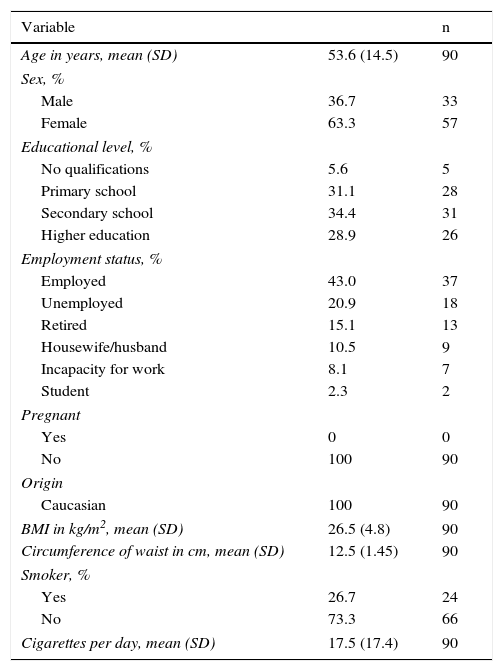
Material and methodsA 3 expert panel supervised the adaptation process. After translation, review and back-translation of the original instrument, a new Spanish version was obtained, which was administered to 2 patient samples: a pilot sample of 20 patients for assessing comprehension, and a 90 patient sample for assessing structural validity (factor analysis and reliability), construct validity and sensitivity to change. A re-test measurement was carried out in 21 patients. Follow-up was accomplished in 40 patients.
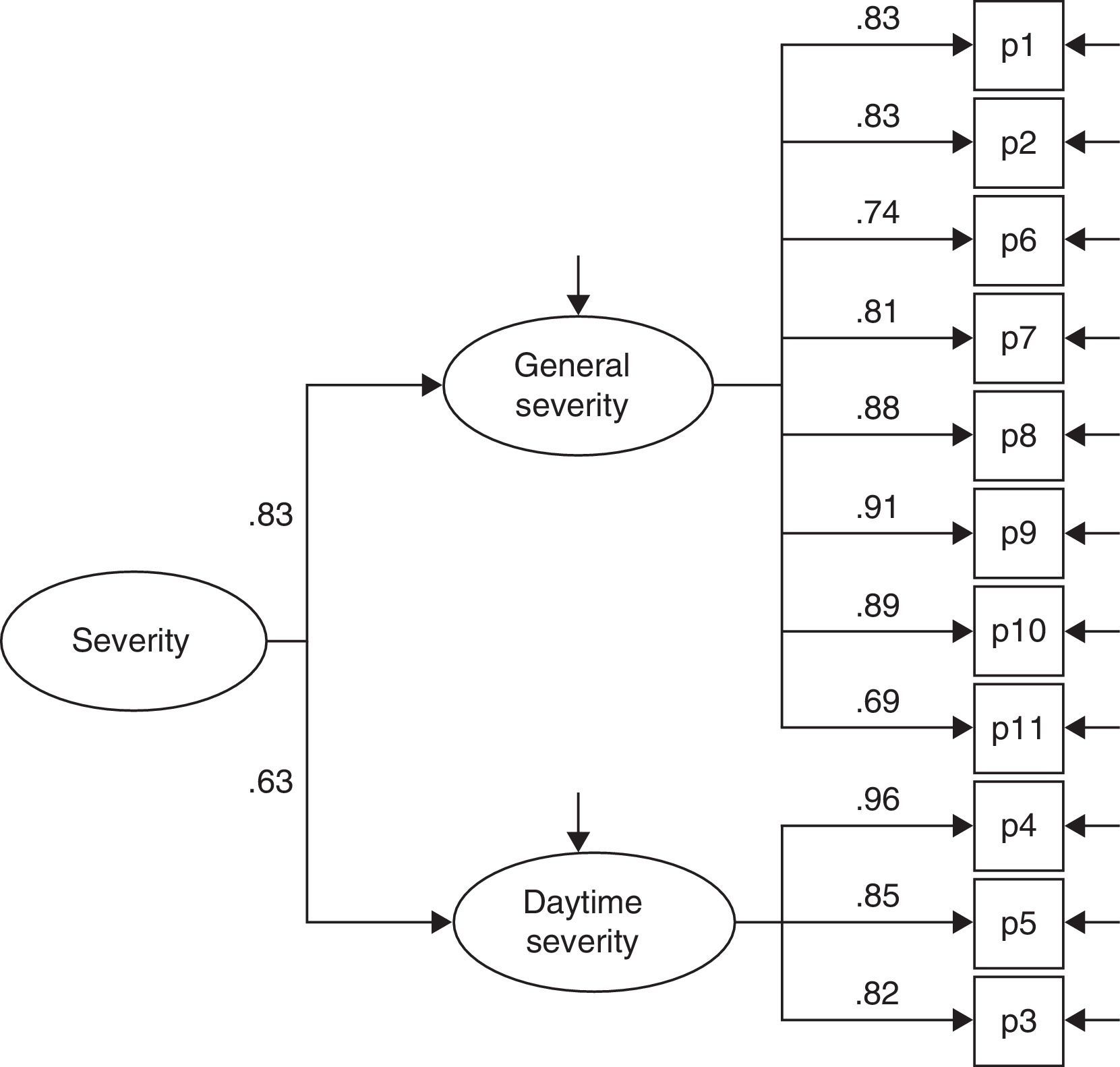
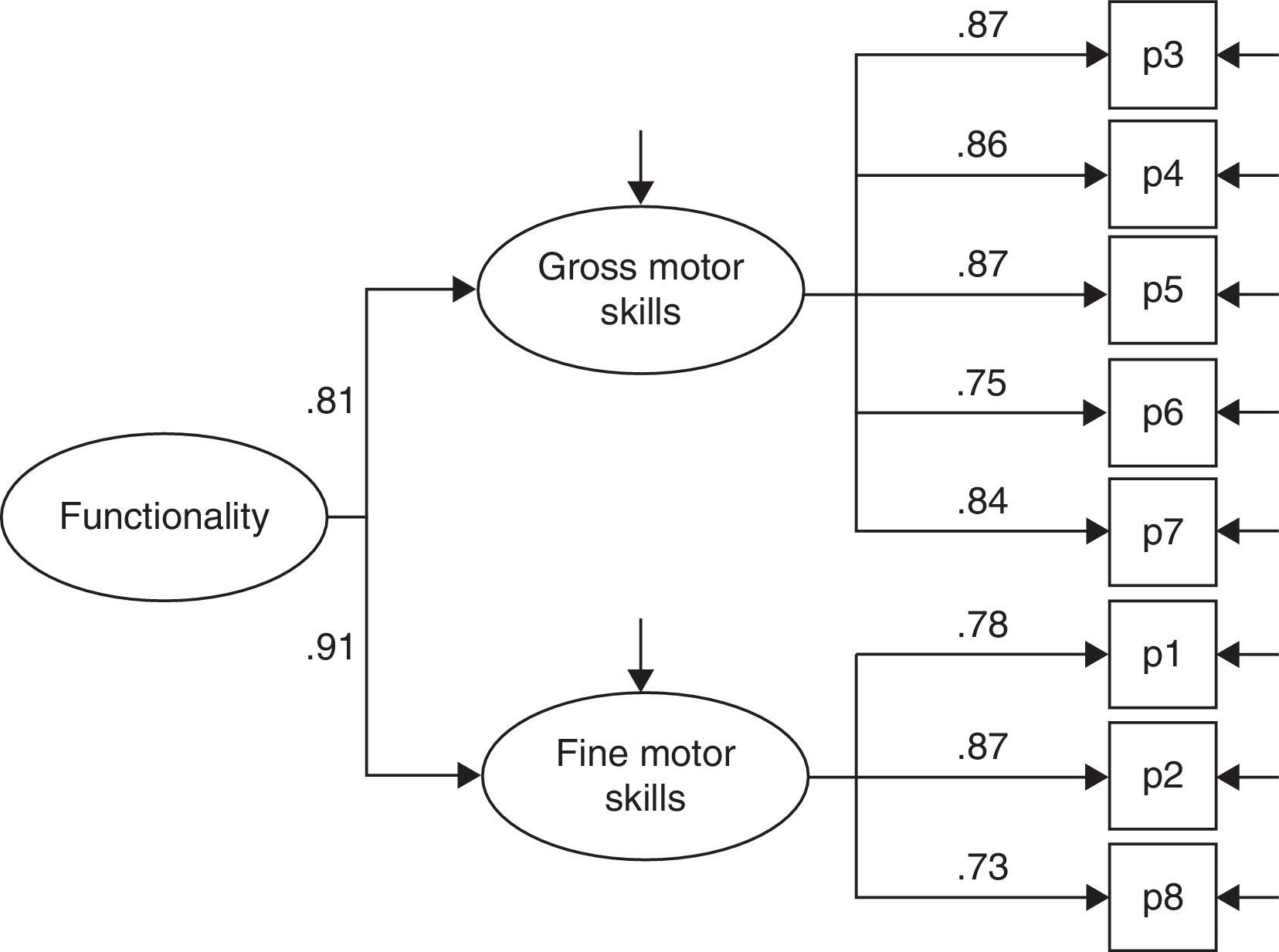
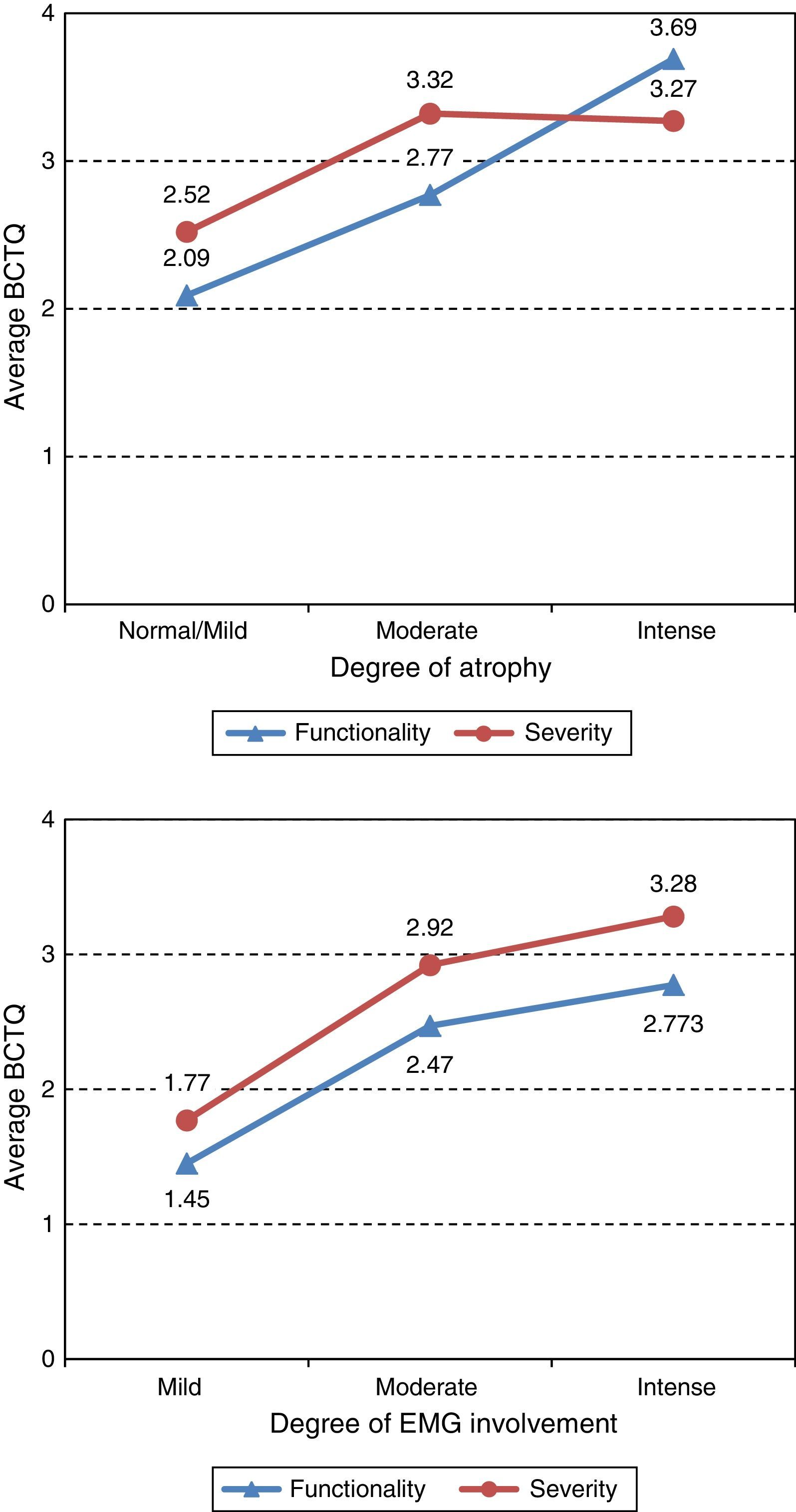
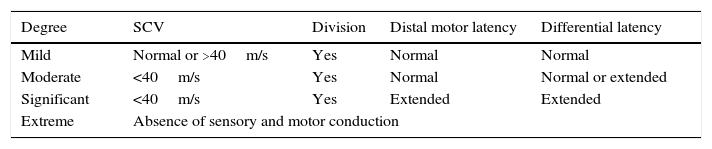
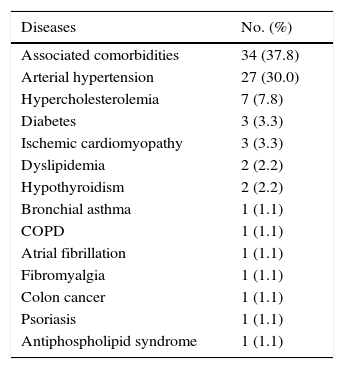
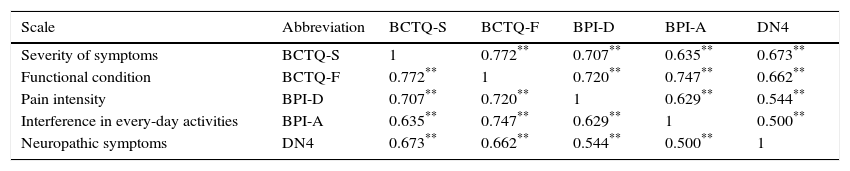
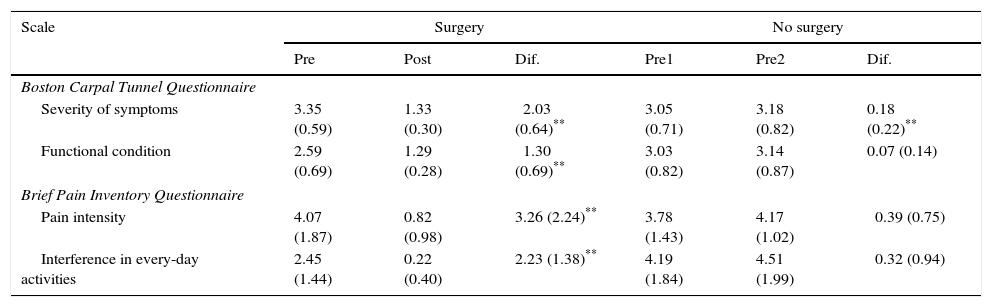
ResultsThe questionnaire was well accepted by all participants. Celling effect was observed for 3 items. Reliability was very good, internal consistency: αS=0.91 and αF=0.87; test⿿retest stability: rS=0.939 and rF=0.986. Both subscales fitted to a general dimension. Subscales correlated with dynamometer measurements (rS=0.77 and rF=0.75) and showed to be related to abnormal 2-point discrimination, muscle atrophy and electromyography deterioration level. Scores properly correlated with other validated instruments: Douleur Neuropatique 4 questions and Brief Pain Inventory. BCTQ demonstrated to be sensitive to clinical changes, with large effect sizes (dS=⿿3.3 and dF=⿿1.9).
ConclusionsThe Spanish version of the BCTQ shows good psychometric properties warranting its use in clinical settings.
Describir el proceso de adaptación cultural y validación al español del cuestionario Boston Carpal Tunnel Questionnaire (BCTQ) de intensidad de los síntomas, capacidad funcional y calidad de vida en pacientes con síndrome del túnel carpiano, e informar de sus propiedades psicométricas.
Material y métodosUn panel de 3 expertos supervisó el proceso de adaptación. Tras la traducción, revisión y retrotraducción del instrumento se obtuvo un cuestionario en español que fue administrado a 2 muestras de pacientes: una muestra piloto de 20 pacientes para valorar la comprensibilidad y una de 90 pacientes para comprobar la validez estructural (análisis factorial y fiabilidad), la validez de constructo y la sensibilidad al cambio. Se realizó medición retest a 21 pacientes. Se realizó seguimiento a 40 pacientes.
ResultadosEl cuestionario fue bien entendido por todos los participantes. Tres ítems presentaron efecto suelo. La fiabilidad fue muy buena, consistencia interna: αS=0,91 y αF=0,87; estabilidad temporal test-retest: rS=0,939 y rF=0,986. Se comprobó que ambas subescalas del cuestionario se ajustaban a una dimensión general. Las subescalas correlacionaron con las medidas del dinamómetro (rS=0,77 y rF=0,75) y mostraron relación con la discriminación anormal entre 2 puntos, la atrofia muscular y el nivel de afectación según electromiografía. Las puntuaciones correlacionaron adecuadamente con cuestionarios ya validados: Douleur Neuropatique 4 questions y Cuestionario Breve de Dolor. El BCTQ demostró ser sensible a los cambio clínicos, con tamaños del efecto grandes (dS=⿿3,3 y dF=⿿1,9).
ConclusionesLa versión en castellano del BCTQ ha demostrado tener buenas propiedades psicométricas, lo que garantiza su uso en el ámbito clínico.