SARS-CoV-2 has been and is a major global Public Health challenge. Since the beginning of the pandemic, different comorbidities have been postulated and associated with spectra of increased severity and mortality. The objectives of this research are: 1) to analyse the factors associated with SARS-CoV-2 infection (COVID-19) in a health area in northern Spain; 2) to understand the possible role of influenza vaccination and pneumococcal vaccination in the development of COVID-19.
Materials and methodA test-negative case-control study was conducted. Variables related to personal and vaccination history were considered. Although the epidemiological definition of the case varied over time, the reference definition was that corresponding to 31/01/2020 in Spain. A bivariate and multivariate analysis was performed.
ResultsThe sample included 188 patients, of which 63 were cases and 125 controls. The results show that obesity increases the risk 2.4-fold of suffering this infection (IC 95% 1,301–4,521) and ARA-2 increases it 2.2-fold (95% CI 1,256–6,982). On the other hand, anti-pneumococcal vaccination of 13 serotypes showed results close to statistical significance (OR = 0.4; 95% CI 0.170–1,006).
ConclusionObesity and the use of ARA-2 increases the risk of COVID-19. Scientific knowledge about factors associated with COVID-19 should be expanded. The authors consider that the present research raises the need further investigate the role of vaccines in this infection and their possible heterologous properties.
El SARS-CoV-2 ha supuesto un importante reto de salud pública. Desde el inicio de la pandemia, se han postulado diferentes comorbilidades que se han asociado con espectros de mayor gravedad y mortalidad. Los objetivos de la presente investigación son: 1) analizar los factores asociados a la infección por SARS-CoV-2 (COVID-19) en un área sanitaria del norte de España; 2) conocer el posible papel de la vacunación antigripal y antineumocócica en el desarrollo de la COVID-19.
Materiales y métodosSe ha realizado un estudio de casos y controles de test negativo. Se tuvieron en cuenta variables relacionadas con los antecedentes personales y vacunales. A pesar de que la definición epidemiológica de caso fue variando a lo largo del tiempo, se tuvo como referencia la correspondiente al 31 de enero del 2020 en España. Se efectuó un análisis bivariante y multivariante.
ResultadosLa muestra incluyó a 188 pacientes, de los cuales 63 fueron casos y 125 controles. Los resultados muestran que la obesidad aumenta 2,4 veces el riesgo de padecer esta infección (IC 95% 1.301 a 4.521) y los antagonistas de los receptores de la angiotensina II (ARA-2) lo aumentan 2,2 veces (IC 95% 1.256 a 6.982). Por otro lado, la vacunación antineumocócica conjugada de 13 serotipos mostró resultados cercanos a la significación estadística (OR = 0,4; IC 95% 0,170 a 1.006).
ConclusionesLa obesidad y el uso de fármacos ARA-2 aumentan el riesgo de la COVID-19. El conocimiento científico sobre los factores asociados a la COVID-19 debe seguir ampliándose. La presente investigación plantea la necesidad de profundizar en el papel de las vacunas sobre esta infección y sus posibles propiedades heterólogas.
Most of the pandemics described in recent decades have originated from viruses of animal origin.1 Coronaviruses represent a family of viruses with a great capacity for recombining genetic material between different coronavirus genomes. Severe acute respiratory syndrome (SARS) was described in Asia in 2003 and Middle East respiratory syndrome (MERS-CoV) in 2012, both originating from previously unknown coronavirus variants.2 In January 2020, the World Health Organisation (WHO) declared an international public health emergency3 following the emergence in China, a month earlier, of a new coronavirus variant called SARS-CoV-2, followed by the declaration of a pandemic situation on 11th March after more than 118,000 cases were reported globally.4
SARS-CoV-2 has posed a major global public health challenge while testing the capacity of the health system. Clinically, SARS-CoV-2 (COVID-19) infection leads to a wide range of clinical manifestations, from asymptomatic or subclinical cases, mild symptoms compatible with the common cold or flu-like illness, to its most severe manifestation in the form of severe acute respiratory syndrome with multi-organ involvement and, ultimately, death.5
As with other infectious diseases, clinical variability is largely explained by the intrinsic characteristics of the host.6 Thus, since the beginning of the pandemic, different comorbidities or risk factors have been postulated as being associated with higher severity and mortality spectra. In general, it appears that this virus affects, as a priority, ageing, multi-pathological and possibly also polypharmacy-exposed populations.7 In this regard, with the data available to date, there is some consensus on the role of age, obesity, dyslipidaemia, cardiovascular disease, diabetes mellitus, arterial hypertension (HT) and chronic pulmonary, hepatic or neurological disease, among others, as risk factors.8,9 However, since the beginning of the pandemic, the effect of some antihypertensive treatments is under discussion due to the penetration of the virus into the cell through the angiotensin-converting enzyme 2 (ACE-2) receptor present mainly in the kidney, lungs and heart.10–12
The biggest limitation to the global approach to SARS-CoV-2 is the low natural immunity of the population, as well as the absence of preventive tools linked to vaccination. Therefore, a major scientific race for an effective vaccine is currently underway, while at the same time exploring whether any of the vaccines available for the prevention of other diseases,13 such as the BCG vaccine against severe forms of tuberculosis, could have heterologous properties in the prevention of SARS-CoV-2.14
Therefore, and considering all of the above, the objectives of this research are: 1) to analyse the factors associated with SARS-CoV-2 infection in a healthcare area in northern Spain; 2) to know the possible role of influenza and pneumococcal vaccination in the development of SARS-CoV-2 infection.
Material and methodsScope of studyThe study has been carried out in a regional hospital in the north of Spain with a reference population of 61,267 inhabitants that shows a progressive decreasing trend in recent years (loss of approximately 750–1000 inhabitants/year). It is an aging, multi-pathological and polypharmacy-exposed population with more than 26% of people over 65 years of age. The hospital is equipped with more than 120 beds and has a Mother and Child Unit and a Psychiatric Unit.15,16
Type of studyA test-negative case-control study was conducted.
Case definitionPatients who from 28 February 2020 (start date of epidemiological surveillance for SARS-CoV-2 in the health area where the research is conducted) until 08 May 2020 met the case definition of new SARS-CoV-2 coronavirus infection and were positive after polymerase chain reaction (PCR) testing for this virus.
It is important to note that the definition of a case of infection by the new SARS-CoV-2 coronavirus was modified in accordance with the updates published by the Epidemiological Surveillance Service of the Health Department of the Autonomous Community of reference, in line with the information published by the Centre for the Coordination of Health Alerts and Emergencies of the Ministry of Health, Consumer Affairs and Social Welfare, with the initial definition corresponding to 31 January 2020. This definition has been the following:
Cases meeting at least one epidemiological criterion and the following clinical criteria shall be investigated for 2019-nCoV infection.
A. Epidemiological criteriaA.1 Any person with a history of travel to Hubei province, China, in the 14 days prior to the onset of symptoms or A.2 Any person who, in the 14 days prior to the onset of symptoms, has been in close contact with a probable or confirmed case.
Close contact is defined as:
- -
Anyone who has provided care to a probable or confirmed case*: health care workers who have not used appropriate protective measures, family members or friends, as well as persons who have had other similar types of physical contact.
- -
Any person who was in the same location as a probable or confirmed case* at a distance <2 m (e.g., cohabitants, visitors).
- -
Close contact on an aircraft is defined as passengers within a two-seat radius of a probable or confirmed case* and crew who have had contact with such cases.
*At this stage and following the recommendations of the WHO and the European Centre for Disease Prevention and Control (ECDC), until further epidemiological information is available, contact will be considered with probable or confirmed cases within 14 days before and 14 days after the onset of symptoms of such cases.
B. Clinical criteriaAny person with clinical symptoms consistent with acute respiratory infection, of any severity, presenting with fever and any of the following symptoms: dyspnoea, cough, or malaise.
Control selectionTwo controls were selected for each case. Patients eligible were those who from 28 February 2020 until 08 May 2020 met the case definition of infection with the new SARS-CoV-2 coronavirus and, after PCR testing for this virus, were found to be negative.
In order to minimise possible selection bias, negative test controls were matched to each case, based on the variables "sex" (male/female), "age" (age in years) and "severity" (hospital admission/domicile).
Selection and definition of variablesA literature search was carried out in the PubMed database to identify articles written in English published in the last three months related to the coronavirus. The variables considered were mainly those included in the meta-analysis published by Yang et al.17 In addition, others were added which, although not widely researched, were considered clinically relevant. Finally, the selected variables were:
- -
Age (years)
- -
Gender (male/female)
- -
Copies of viral ribonucleic acid (RNA) at diagnosis (copies/1000 cells)
- -
Severity (domicile/admission)
- -
Final outcome (death/discharge)
- -
Diabetes (yes/no)
- -
Diabetes type (1/2)
- -
Diabetes treatment (oral antidiabetics/insulin)
- -
Obesity (yes/no)
- -
Dyslipidemia (yes/no)
- -
High blood pressure (yes/no)
- -
Treatment of arterial hypertension (angiotensin converting enzyme inhibitors [ACEIs]/angiotensin II receptor blockers [ARBs])
- -
Metabolic syndrome18 (yes/no)
- -
Chronic liver disease (yes/no)
- -
Chronic kidney disease (yes/no)
- -
Immunodeficiency/immunosuppression (yes/no)
- -
Cardiovascular disease (yes/no)
- -
Chronic lung disease (yes/no)
- -
Neurological or neuromuscular disease (yes/no)
- -
Chronic disease 1 (yes/no)
- -
Chronic disease 2 (number of chronic diseases)
- -
Polypathology19 (yes/no)
- -
Polypharmacy20 (yes/no)
- -
Hospitalization in the previous three months (yes/no)
- -
Hospital stay (number of days)
- -
Complications (intensive care unit [ICU]/death/other complications)
- -
2019/20 flu vaccine (yes/no)
- -
13-v pneumococcal conjugate vaccine (yes/no)
- -
23-v pneumococcal polysaccharide vaccine (yes/no)
- -
Sequential regimen of 13-v pneumococcal conjugate vaccine + 23-v polysaccharide (yes/no)
- -
2019/20 flu vaccine + 13-v pneumococcal conjugate vaccine (yes/no)
- -
2019/20 flu vaccine + 23-v pneumococcal polysaccharide vaccine (yes/no)
- -
2019/20 flu vaccine + 13-v pneumococcal conjugate vaccine + 23-v pneumococcal polysaccharide vaccine (yes/no)
A descriptive statistical analysis was performed for each variable (univariate analysis), calculating absolute and relative frequencies for qualitative variables, and means and standard deviations (SD), as measures of central tendency and dispersion, for quantitative variables. Secondary variables were created from the initial variables, mainly to organize them into ranges ("age" and "viral RNA copies at the time of diagnosis") or to combine them ("metabolic syndrome", "flu vaccine 2019/20", "13-v pneumococcal conjugate vaccine" and "23-v pneumococcal polysaccharide vaccine"). A bivariate analysis was performed to assess the association between the selected variables. The X2 test was used for the qualitative dichotomous variables. Students’t test was used for the quantitative variables. On the other hand, Pearson's correlation coefficient was calculated to measure the statistical relationship between the continuous variables "number of stay days", "number of viral RNA copies" and "number of chronic diseases".
The analysis was carried out with the Statistical Package for the Social Sciences version 23.0 and EPIDAT version 3.1.
Ethical aspectsThis research was approved by the Clinical Research Ethics Committee of the Autonomous Community (reference 2020.260).
ResultsSample overviewThe sample consisted of 188 patients, of which 63 were cases and 125 controls. Of them, 52.1% were women and the mean age was 64.66 years (SD ± 19.97). No statistically significant differences were observed between age (95% CI −5.75 to 6.44), sex (95% CI 0.53–1.80) and patient location (95% CI 0.48–1.74) between cases and controls.
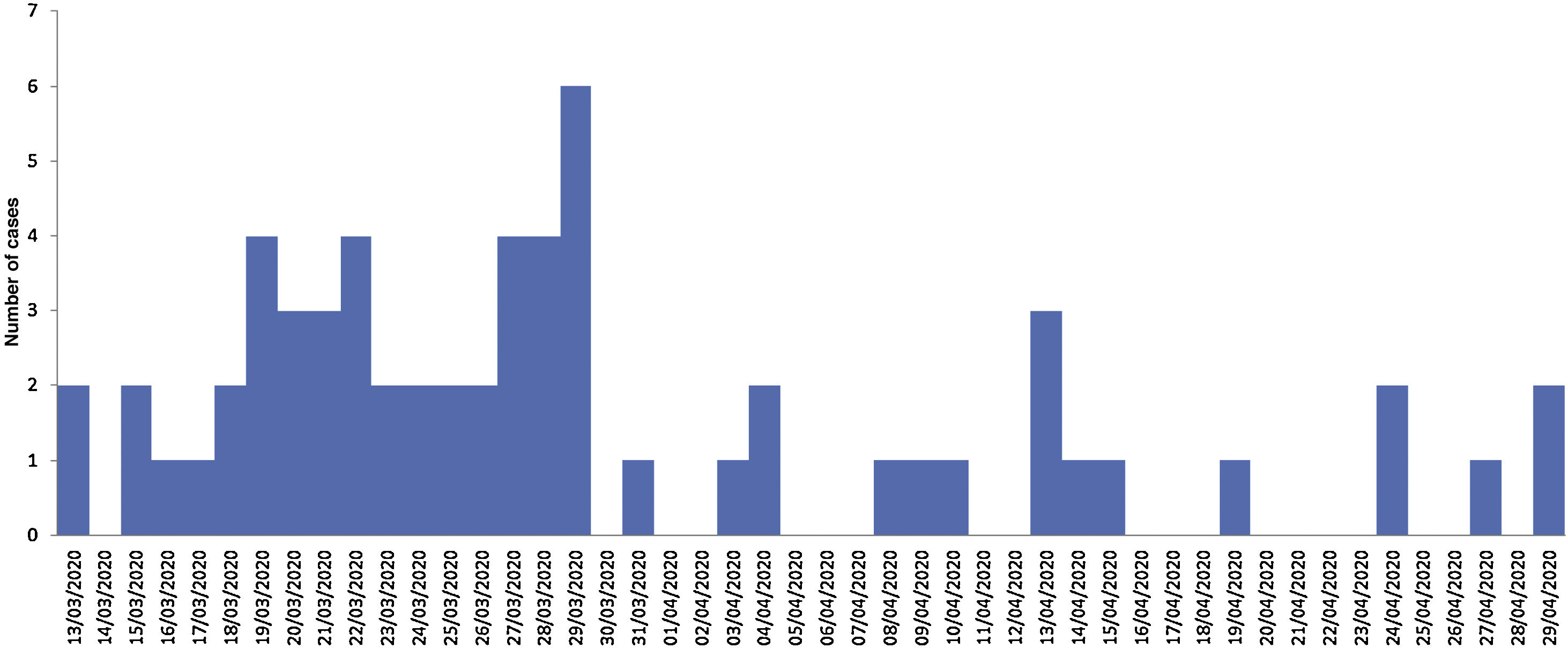
Description of casesOf the 63 registered cases, 47.6% were men. The most common age group was ≥65 years (55.6%), followed by 15–49 years (23.8%), 50–64 years (17.5%) and 0–14 years (3.2%). The mean age was 64.89 years (SD ± 20.13). Fig. 1 shows the epidemic curve according to the date of diagnosis. Regarding the need for hospital admission, 42 patients (66.7%) required hospitalization. The mean hospital stay in this group was 13.05 days (SD ± 9326 days). Regarding the number of viral RNA copies at the time of diagnosis, 69.8% was greater than 50,000 copies/mL. Finally, the overall case fatality rate was 14.28%, while the case fatality rate for inpatients was 21.42%.
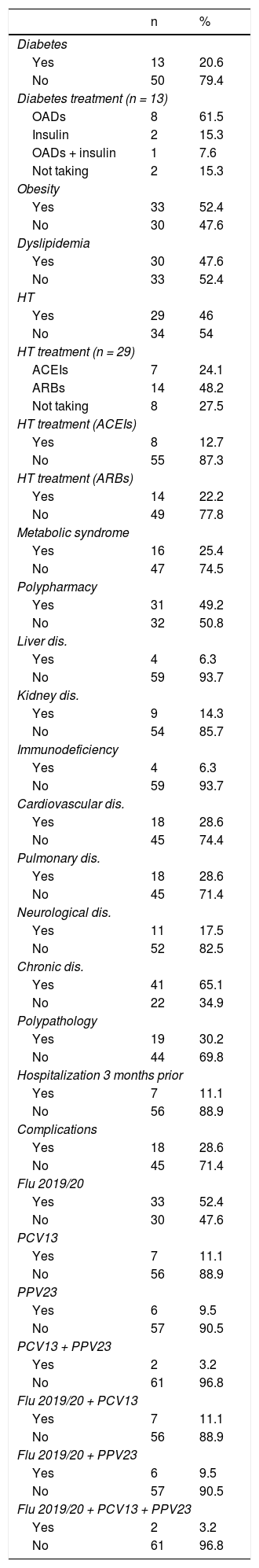
Table 1 shows the distribution of the main personal history of patients with COVID-19, as well as the vaccination history (against influenza and pneumococcus) in those aged ≥65 or chronic patients. Overall, obesity was found to be the most common history finding (52.4%) followed by dyslipidaemia (47.6%). 49.2% met the definition of "polypharmacy" and, overall, 52.4% had some chronic disease.
Distribution of the main personal and vaccination history of patients with SARS-CoV-2 infection.
| n | % | |
|---|---|---|
| Diabetes | ||
| Yes | 13 | 20.6 |
| No | 50 | 79.4 |
| Diabetes treatment (n = 13) | ||
| OADs | 8 | 61.5 |
| Insulin | 2 | 15.3 |
| OADs + insulin | 1 | 7.6 |
| Not taking | 2 | 15.3 |
| Obesity | ||
| Yes | 33 | 52.4 |
| No | 30 | 47.6 |
| Dyslipidemia | ||
| Yes | 30 | 47.6 |
| No | 33 | 52.4 |
| HT | ||
| Yes | 29 | 46 |
| No | 34 | 54 |
| HT treatment (n = 29) | ||
| ACEIs | 7 | 24.1 |
| ARBs | 14 | 48.2 |
| Not taking | 8 | 27.5 |
| HT treatment (ACEIs) | ||
| Yes | 8 | 12.7 |
| No | 55 | 87.3 |
| HT treatment (ARBs) | ||
| Yes | 14 | 22.2 |
| No | 49 | 77.8 |
| Metabolic syndrome | ||
| Yes | 16 | 25.4 |
| No | 47 | 74.5 |
| Polypharmacy | ||
| Yes | 31 | 49.2 |
| No | 32 | 50.8 |
| Liver dis. | ||
| Yes | 4 | 6.3 |
| No | 59 | 93.7 |
| Kidney dis. | ||
| Yes | 9 | 14.3 |
| No | 54 | 85.7 |
| Immunodeficiency | ||
| Yes | 4 | 6.3 |
| No | 59 | 93.7 |
| Cardiovascular dis. | ||
| Yes | 18 | 28.6 |
| No | 45 | 74.4 |
| Pulmonary dis. | ||
| Yes | 18 | 28.6 |
| No | 45 | 71.4 |
| Neurological dis. | ||
| Yes | 11 | 17.5 |
| No | 52 | 82.5 |
| Chronic dis. | ||
| Yes | 41 | 65.1 |
| No | 22 | 34.9 |
| Polypathology | ||
| Yes | 19 | 30.2 |
| No | 44 | 69.8 |
| Hospitalization 3 months prior | ||
| Yes | 7 | 11.1 |
| No | 56 | 88.9 |
| Complications | ||
| Yes | 18 | 28.6 |
| No | 45 | 71.4 |
| Flu 2019/20 | ||
| Yes | 33 | 52.4 |
| No | 30 | 47.6 |
| PCV13 | ||
| Yes | 7 | 11.1 |
| No | 56 | 88.9 |
| PPV23 | ||
| Yes | 6 | 9.5 |
| No | 57 | 90.5 |
| PCV13 + PPV23 | ||
| Yes | 2 | 3.2 |
| No | 61 | 96.8 |
| Flu 2019/20 + PCV13 | ||
| Yes | 7 | 11.1 |
| No | 56 | 88.9 |
| Flu 2019/20 + PPV23 | ||
| Yes | 6 | 9.5 |
| No | 57 | 90.5 |
| Flu 2019/20 + PCV13 + PPV23 | ||
| Yes | 2 | 3.2 |
| No | 61 | 96.8 |
OADs: oral antidiabetics; ARBs: angiotensin II receptor antagonists; HT: arterial hypertension; ACEIs: angiotensin converting enzyme inhibitors; PCV13: 13-valent pneumococcal conjugate vaccine; PPV23: 23-valent pneumococcal polysaccharide vaccine.
No statistically significant differences were observed between the days of hospital stay and the presence of any personal and vaccination history.
Regarding the link between continuous variables, a positive relationship was found between the number of chronic diseases and the days of hospital stay (r = 0.251; 95% CI −0.05 to 0.51), however, this relationship was not observed between the number of chronic diseases and the number of viral RNA copies at the time of diagnosis (r = 0.128; 95% CI −0.12 to 0.36) or between the number of viral RNA copies and the days of hospital stay (r = 0.032; 95% CI −0.27 to 0.33).
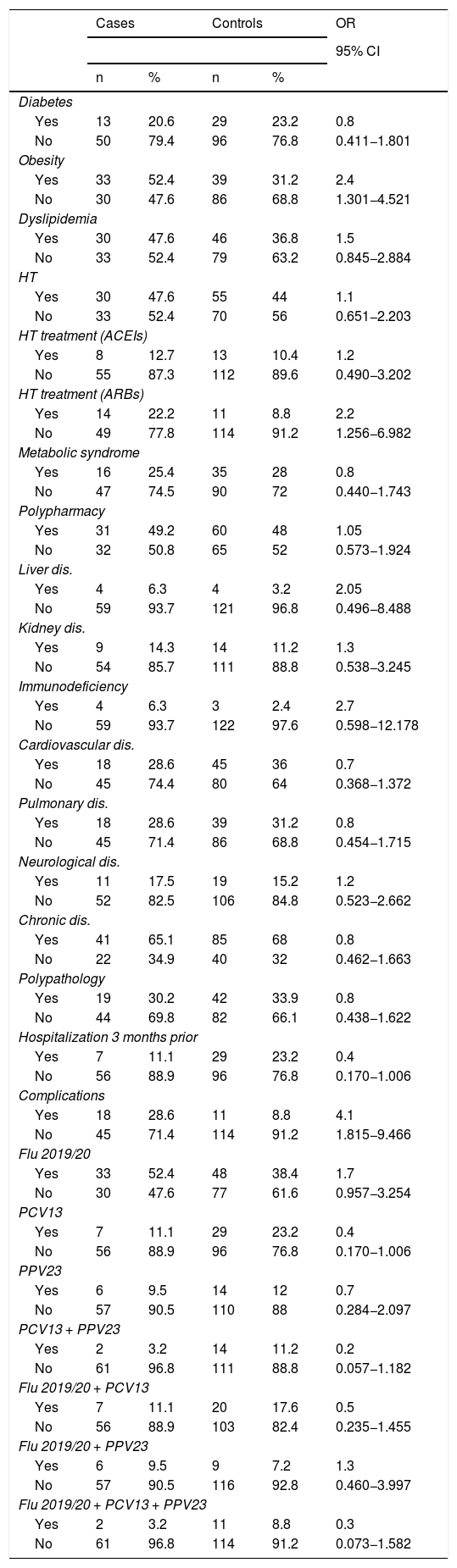
Personal and vaccination historyThe main risk factors related to coronavirus infection were obesity and active treatment with antihypertensive ARB drugs. Thus, the bivariate analysis showed that being obese increases the risk of suffering from this infection 2.4 times (95% CI 1301–4521) and ARBs increases it 2.2 times (95% CI 1256–6982). At the same time, it was found that both obesity and polypharmacy were identified as risk factors for clinical complications such as referral to ICU and death, with an odds ratio (OR) = 3.1 (95% CI 1.4–7.2) for obesity and an OR = 2.3 (95% CI 1004–5249) for polypharmacy. Table 2 shows the rest of the results.
Factors associated with SARS-CoV-2 infection.
| Cases | Controls | OR | |||
|---|---|---|---|---|---|
| 95% CI | |||||
| n | % | n | % | ||
| Diabetes | |||||
| Yes | 13 | 20.6 | 29 | 23.2 | 0.8 |
| No | 50 | 79.4 | 96 | 76.8 | 0.411−1.801 |
| Obesity | |||||
| Yes | 33 | 52.4 | 39 | 31.2 | 2.4 |
| No | 30 | 47.6 | 86 | 68.8 | 1.301−4.521 |
| Dyslipidemia | |||||
| Yes | 30 | 47.6 | 46 | 36.8 | 1.5 |
| No | 33 | 52.4 | 79 | 63.2 | 0.845−2.884 |
| HT | |||||
| Yes | 30 | 47.6 | 55 | 44 | 1.1 |
| No | 33 | 52.4 | 70 | 56 | 0.651−2.203 |
| HT treatment (ACEIs) | |||||
| Yes | 8 | 12.7 | 13 | 10.4 | 1.2 |
| No | 55 | 87.3 | 112 | 89.6 | 0.490−3.202 |
| HT treatment (ARBs) | |||||
| Yes | 14 | 22.2 | 11 | 8.8 | 2.2 |
| No | 49 | 77.8 | 114 | 91.2 | 1.256−6.982 |
| Metabolic syndrome | |||||
| Yes | 16 | 25.4 | 35 | 28 | 0.8 |
| No | 47 | 74.5 | 90 | 72 | 0.440−1.743 |
| Polypharmacy | |||||
| Yes | 31 | 49.2 | 60 | 48 | 1.05 |
| No | 32 | 50.8 | 65 | 52 | 0.573−1.924 |
| Liver dis. | |||||
| Yes | 4 | 6.3 | 4 | 3.2 | 2.05 |
| No | 59 | 93.7 | 121 | 96.8 | 0.496−8.488 |
| Kidney dis. | |||||
| Yes | 9 | 14.3 | 14 | 11.2 | 1.3 |
| No | 54 | 85.7 | 111 | 88.8 | 0.538−3.245 |
| Immunodeficiency | |||||
| Yes | 4 | 6.3 | 3 | 2.4 | 2.7 |
| No | 59 | 93.7 | 122 | 97.6 | 0.598−12.178 |
| Cardiovascular dis. | |||||
| Yes | 18 | 28.6 | 45 | 36 | 0.7 |
| No | 45 | 74.4 | 80 | 64 | 0.368−1.372 |
| Pulmonary dis. | |||||
| Yes | 18 | 28.6 | 39 | 31.2 | 0.8 |
| No | 45 | 71.4 | 86 | 68.8 | 0.454−1.715 |
| Neurological dis. | |||||
| Yes | 11 | 17.5 | 19 | 15.2 | 1.2 |
| No | 52 | 82.5 | 106 | 84.8 | 0.523−2.662 |
| Chronic dis. | |||||
| Yes | 41 | 65.1 | 85 | 68 | 0.8 |
| No | 22 | 34.9 | 40 | 32 | 0.462−1.663 |
| Polypathology | |||||
| Yes | 19 | 30.2 | 42 | 33.9 | 0.8 |
| No | 44 | 69.8 | 82 | 66.1 | 0.438−1.622 |
| Hospitalization 3 months prior | |||||
| Yes | 7 | 11.1 | 29 | 23.2 | 0.4 |
| No | 56 | 88.9 | 96 | 76.8 | 0.170−1.006 |
| Complications | |||||
| Yes | 18 | 28.6 | 11 | 8.8 | 4.1 |
| No | 45 | 71.4 | 114 | 91.2 | 1.815−9.466 |
| Flu 2019/20 | |||||
| Yes | 33 | 52.4 | 48 | 38.4 | 1.7 |
| No | 30 | 47.6 | 77 | 61.6 | 0.957−3.254 |
| PCV13 | |||||
| Yes | 7 | 11.1 | 29 | 23.2 | 0.4 |
| No | 56 | 88.9 | 96 | 76.8 | 0.170−1.006 |
| PPV23 | |||||
| Yes | 6 | 9.5 | 14 | 12 | 0.7 |
| No | 57 | 90.5 | 110 | 88 | 0.284−2.097 |
| PCV13 + PPV23 | |||||
| Yes | 2 | 3.2 | 14 | 11.2 | 0.2 |
| No | 61 | 96.8 | 111 | 88.8 | 0.057−1.182 |
| Flu 2019/20 + PCV13 | |||||
| Yes | 7 | 11.1 | 20 | 17.6 | 0.5 |
| No | 56 | 88.9 | 103 | 82.4 | 0.235−1.455 |
| Flu 2019/20 + PPV23 | |||||
| Yes | 6 | 9.5 | 9 | 7.2 | 1.3 |
| No | 57 | 90.5 | 116 | 92.8 | 0.460−3.997 |
| Flu 2019/20 + PCV13 + PPV23 | |||||
| Yes | 2 | 3.2 | 11 | 8.8 | 0.3 |
| No | 61 | 96.8 | 114 | 91.2 | 0.073−1.582 |
ARBs: angiotensin II receptor antagonists; HT: arterial hypertension; ACEIs: angiotensin converting enzyme inhibitors; PCV13: 13-valent pneumococcal conjugate vaccine; PPV23: 23-valent pneumococcal polysaccharide vaccine.
Regarding the vaccination history, the 13-valent pneumococcal conjugate vaccination showed results close to statistical significance with an OR = 0.4; (95% CI 0.170–1.006) (Table 2).
DiscussionSARS-CoV-2 infection in the present study population has been shown to have a higher incidence in older age groups, as reported in other similar21,22 published research on other types of coronaviruses such as SARS and MERS.23–26 In addition, in most cases, this situation is linked to the increase in the number of chronic diseases and, therefore, to polypharmacy, which, despite not having been found as a risk factor for COVID-19 as it is with other types of infections,27 the results point to an association between polypharmacy and complications, including death. These findings are consistent with those of other authors who link polypharmacy with increased mortality in people over 65,28 although it could also be explained by the high prevalence of chronic diseases in the study sample (52.4%) and of polypharmacy (49.2%), the latter much higher than that described in national studies where it is not exceeded 30% for the year 2017.29
Obesity has been, in recent months, a variable that numerous authors have explored in their COVID-19 related research, and it seems logical to think that it may have a relevant role in the development of this infection. Obese patients are known to have impaired lung function, decreased thoraco-pulmonary compliance and, as a consequence, increased work of breathing Likewise, a decrease in maximum inspiratory pressure is observed. As a consequence of both phenomena, there is an increase in respiratory work and greater muscle fatigue. In addition, an impaired respiratory centre response to hypercapnia has been reported in patients with obesity.30,31 On the other hand, obesity is associated with a low degree of chronic inflammation, as well as an increased risk of thrombosis32 that can be increased by SARS-CoV-2, and with a worse immune response and poor prognosis of respiratory infections with a higher risk of hospitalization and death, as has been shown in the case of influenza virus infection.33–35 In the case of the present study, and as indicated by the results of authors such as Simonnet et al.,36 Richardson et al.37 or Caussy et al.38 in different parts of the world, bivariate analysis indicates that obesity is an important risk factor for the development of SARS-CoV-2 infection (OR = 2.4; 95% CI 1.30–4.52), as well as producing a significant increase in the risk of complications (OR = 4.1; 95% CI 1.81–9.46).
The effect of antihypertensive drugs on the development of COVID-19, as well as its complications, has also been subject to extensive debate since the beginning of the pandemic. Theories have been postulated supporting a detrimental effect and others in the opposite direction advocating a beneficial effect. The hypothetical detrimental effect of these drugs supported by the present research is based on the fact that chronic treatment with ARBs would produce an overexpression of angiotensin-2 converting enzyme (ACE-2), an enzyme used by the virus for endocytosis. This situation would favour the entry of the virus into lung cells, aggravating the infection.39,40 On the contrary, the hypothetical beneficial effect is postulated through the fact that ARBs, by binding to the type 1 angiotensin II (AT1) receptor, would avoid the profibrotic and pro-inflammatory effects that would lead to the stimulation of said receptor, and the overexpression of ACE-2 would degrade angiotensin II into peptides with anti-inflammatory and anti-fibrotic properties.12,41
Contrary to what might be expected, having been hospitalized for any cause in the last three months was identified as a possible protective factor for SARS-CoV-2 (OR = 0.4; 95% CI 0.170–1.006). Although, so far, no publications have been found that assess this specific situation in the context of the current pandemic, it is known that SARS-CoV-2 has a higher impact, like other infectious diseases, on people belonging to vulnerable groups and groups with high social interaction,42–44 so these results could be interpreted as the need for home recovery after such hospitalisation and, therefore, less exposure to meetings or social activities that involve contact with other people. Translated with www.DeepL.com/Translator (free version).
The study of the possible effect of influenza and pneumococcal vaccination on SARS-CoV-2 infection brings a novelty to this research since, so far, except for some specific reference on the possible inverse relationship between influenza vaccination coverage and COVID-19 mortality in Italy,45 no field study on the subject seems to exist in the literature. Knowing that no benefit or harm is expected to be found from these vaccines on the development of COVID-19, it is surprising to note that the pneumococcal 13-valent conjugate vaccine is proposed as a possible protective factor for the development of COVID-19 (OR = 0.4; 95% CI 0.170–1.006). In recent years, the heterologous or non-specific effects of some live attenuated vaccines, beyond the target micro-organisms of each of them, have been the subject of much research.46 This research has focused mainly on BCG and measles vaccines.47,48
It must be said that the findings of the present study may be a chance finding that should in no way suggest a true heterologous effect of this vaccine on SARS-CoV-2 infection, but that, at this stage, it raises the need for further, larger studies to specifically explore the role of this preventive tool. Therefore, the authors do not know whether these results could be related to recently published data on co-infection of SARS-CoV-2 with other viruses and bacteria where, in a Chinese study, 94.2% of patients were co-infected with one or more pathogens, with Streptococcus pneumoniae49 being the most frequently identified pathogen. Based on these results and also considering a scenario in which the influenza virus and SARS-CoV-2 may co-circulate in the coming autumn-winter in the northern hemisphere, some independent authors as well as the WHO are in favour of generalised influenza vaccination and intensification of pneumococcal vaccination in particularly vulnerable groups such as those institutionalised in social-health centres. The aim of these interventions is to be able to combat and minimise the potential overburden on the health care system as these vaccines protect against infections that are a major contributor to respiratory mortality in the elderly.50,51
The present study is not without limitations. On the one hand, although the epidemiological design (case-control study with negative test design) involves certain methodological biases, it is currently the most widely used design in observational studies on the effectiveness of vaccines,52–54 which is the main reason for its choice. Furthermore, this is a local study whose sample may not be representative of the general population. As a future prospect, it seems necessary to further study the risk and protective factors for the development of this infection and, until effective and safe vaccines against SARS-CoV-2 itself are available, the role that other known vaccines can play against it.
ConclusionsTreatment with antihypertensive ARB drugs and obesity are identified as risk factors, while hospitalization in the previous three months for any cause and possibly 13-valent pneumococcal conjugate vaccination are postulated as protective factors.
FundingThis article has not received any type of funding.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Fernández-Prada M, García-González P, García-Morán A, Ruiz-Álvarez I, Ramas-Diez C, Calvo-Rodríguez C. Antecedentes personales y vacunales como factores asociados a la infección por SARS-CoV-2. Med Clin (Barc). 2021;157:226–233.