Elevated blood urea nitrogen to serum albumin (BUN/ALB) ratio had been identified as an independent risk factor related to mortality in community-acquired and hospital-acquired pneumonia. This study aimed to investigate whether this clinical index can predict the clinical outcomes of E. coli bacteraemia.
Material and methodsClinical data were collected from patients with E. coli bacteraemia attended at our hospital between January 2012 and December 2018. The endpoints were mortality within 30 days after the diagnosis of E. coli bacteraemia and intensive care (IC) requirement. Cox regression analysis was performed to evaluate the risk factors.
ResultsA total of 398 patients with E. coli bacteraemia were enrolled in this study and 56 patients died within 30 days after bacteraemia onset. Multivariate Cox regression analysis showed that age greater than 65 years, lymphocyte count<.8×10e9/L, elevated BUN/ALB ratio, increased SOFA score, carbapenem resistance, central venous catheterization before onset of bacteraemia, and infection originating from abdominal cavity were independent risk factors for 30-day mortality (P<.05). The risk factors associated with IC requirement were similar to those for 30-day mortality except central venous catheterization before onset of bacteraemia. The area under the receiver-operating characteristic curve for BUN/ALB ratio predicting 30-day mortality and IC requirement was similar to that for SOFA score, but higher than that for lymphocyte count. The cut-off points of BUN/ALB ratio to predict 30-day mortality and IC requirement were both .3.
ConclusionsBUN/ALB ratio is a simple but independent predictor of 30-day mortality and severity in E. coli bacteraemia. A higher BUN/ALB ratio at the onset of bacteraemia predicts a higher mortality rate and IC requirement.
Se ha identificado la elevación de la proporción de nitrógeno ureico en sangre con respecto a albúmina sérica (NUS/ALB) como un factor de riesgo independiente asociado a la mortalidad de la neumonía adquirida en la comunidad y la neumonía intrahospitalaria. El objetivo de este estudio fue investigar si este índice clínico puede predecir los resultados clínicos de bacteremia por E. coli.
Material y métodosSe recopilaron los datos clínicos de los pacientes con bacteremia por E. coli atendidos en nuestro hospital entre enero de 2012 y diciembre de 2018. Las variables de evaluación fueron la mortalidad a 30 días tras el diagnóstico de bacteremia por E. coli y la necesidad de cuidados intensivos (CI). Se realizó un análisis de regresión de Cox para evaluar los factores de riesgo.
ResultadosSe incluyó en el estudio a un total de 398 pacientes con bacteremia por E. coli, falleciendo 56 pacientes en el plazo de 30 días tras el inicio de la bacteremia. El análisis de regresión de Cox multivariante reflejó que la edad superior a 65 años, el recuento linfocitario <0,8×109/l, la elevación del ratio NUS/ALB, el incremento de la puntuación SOFA, la resistencia al carbapenem, la cateterización venosa central anterior al inicio de la bacteremia y la infección originada por la cavidad abdominal eran factores de riesgo independientes de la mortalidad a 30 días (p<0,05). Los factores de riesgo asociados a la necesidad de CI fueron similares a los de la mortalidad a 30 días, exceptuando la cateterización venosa central anterior al inicio de la bacteremia. El área bajo la curva característica operador-receptor para el ratio NUS/ALB que predice la mortalidad a 30 días, y la necesidad de CI fue similar a la puntuación SOFA, aunque superior a la correspondiente al recuento linfocitario. Los puntos de corte del ratio NUS/ALB para predecir la mortalidad a 30 días y la necesidad de CI se situaron en 0,3.
ConclusionesEl ratio NUS/ALB es un factor predictivo simple, pero independiente de la mortalidad a 30 días y de la gravedad en la bacteremia por E. coli. Un mayor ratio NUS/ALB al inicio de la bacteremia predice una tasa de mortalidad y una necesidad de CI superiores.
Bloodstream infections (BSIs) have increased the incidence of sepsis-related mortality and are associated with prolonged hospitalization.1 In developed countries, nearly 6% and 3% of all intensive care unit (ICU) admissions, and 40% and 49% of hospital mortality are due to BSIs and BSI associated septic shock, respectively.2Escherichia coli (E. coli) is one of the most common causative gram-negative bacterium,3 and the leading cause of BSIs,4 with an incidence ranging from 31.9% to 81.0% among major gram-negative species in 28 European countries.5 In China, the proportion of E. coli related BSIs (EC-BSI) was between 19.8% and 23.0% during 2010–2012.6 Researches from North America and Europe have ranked it among the top seven causes of death,7 resulting in serious problems and economic burden in the field of public health.
Knowledging whether a patient has BSI can guide proper therapy, transferring the patient to ICU, empirically initiating appropriate antibiotic treatment and thinking of differential diagnosis. It's of great importance to develop tools that can predict bacteremia accurately in patients suspected of harboring a moderate to severe BSI. Although many models for predicting bacteraemia in adults have been developed, very few have been prospectively validated and performed well. Moreover, even these are not yet used in clinical practice.8 Reluctance to use these models is probably rooted in the additional work required to enter data and use the models. Hence, it's helpful to find an easy achieved but reliable clinical biomark that can assist physicians to make decisions on the best possible treatment by assigning risk profiles or a clinical index that can predict disease severity and prognosis. Hematological and biochemical biomarkers have historically been employed to facilitate diagnostic process and guide therapy. Although many clinical biomarkers have been investigated, few are currently applied in clinical practice because of the complexity and heterogeneity of bacteremia.9–11 Traditional biomarkers of illness severity, e.g. lactate or leukocyte count may lack the specificity to distinguish these situations. Procalcitonin (PCT) level was associated with severity of illness in patients with severe pneumonia or BSIs. Inability to decrease PCT level by greater than 80% from baseline to day 4 and day 28 was an independent predictor of mortality.12 Nevertheless, the use of PCT as a biomarker for diagnosis of sepsis is limited because PCT level also increases in noninfectious diseases. It cannot be used as a single diagnostic test for sever BSI, as false-negative results could lead to mortality. C-reactive protein (CRP), lipopolysaccharide binding protein (LBP), interleukin (IL)-6, soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), and soluble urokinase plasminogen activator receptor (suPAR), all have a lower sensitivity and specificity as a biomarker for sever BSI compared with PCT.9 Presepsin, soluble cluster of differentiation 14 subtype (sCD14), has recently gained significant attention as a promising prognostic biomarker in bacteremia.13 It is purported to possess superior diagnostic capacity for sever bacteremia compared to PCT. Presepsin was also found to be a valuable tool in prognostication of mortality.14 However, its diagnostic and prognostic capacities in direct comparison to other biomarkers and physiologic parameters in critically ill patients yielded mixed results.15
The Sequential Organ Failure Assessment (SOFA) score is a grading system, which helps in predicting mortality in bacteremic patients by evaluating the severity of a patient's condition based on the degree of organ dysfunction. Increased SOFA score is indicative of severe organ disturbance and poor prognosis.16 However, this scoring system may be affected by subjectivity of clinicians who perform the evaluation. For example, it is often difficult for clinicians to assess the mental status of elderly patients or patients who suffer from dementia, as a result the severity score may vary among clinicians.17
Recently, we found that decreased lymphocyte count and elevated blood urea nitrogen to serum albumin (BUN/ALB) ratio were independent risk factors associated with mortality in patients with hospital-acquired pneumonia (HAP).18 However, it is still unclear whether these clinical indices can predict the mortality or disease severity in patients with EC-BSI. Therefore, we hypothesized that the BUN/ALB ratio increases with a decrease in lymphocyte count in critically ill patients with E. coli bacteremia and correlates with mortality. Thus, in this single-center, retrospective, observational study, we aimed to evaluate the factors that affect 30-day mortality and disease severity in patients with EC-BSI, in order to improve its prognosis in the future.
Material and methodsPatients and data collectionA total of 398 patients with EC-BSI attended at the Third-Affiliated Hospital of Sun Yat-sen University, China, from January 2012 to December 2018 were enrolled in the present study (shown in Fig. 1). The inclusion criterion was one or more E. coli positive blood cultures from patients who had clinical manifestations of BSI, such as fever and shaking chills at the same time. The following situations were excluded: (1) Culture results showing the presence of different bacteria in blood samples drained simultaneously from both sides of the body, eventhough E. coli was isolated from one of the samples; and (2) Blood culture results showing polymicrobial infection.
Clinical information of the patients, including age, gender, community-acquired or nosocomial infection, clinical features, SOFA score at the onset of bacteremia, possible origin of bacteremia, underlying diseases, drug resistance of pathogen and drug sensitivity analyses, medication records, history of invasive surgery, and prognosis, were collected and evaluated. This study conformed to the standards of medical ethics and was approved by the Research Ethics Board of the Third-Affiliated Hospital of Sun Yet-sen University.
Microbiological identificationIdentification of E. coli strains was performed using standard microbiologic methods with an FX200 automatic blood culture instrument (BACTE) in the microbiology laboratory. Antimicrobial susceptibility testing was performed using the micro dilution method (MicroScan system; Baxter Health Care, West Sacramento, CA, USA), and the results were interpreted according to the National Committee for Clinical Laboratory Standards guidelines published in 2012. ESBL production was screened by measuring the minimum inhibitory concentrations of cefotaxime, ceftazidime, and aztreonam.
Relevant definitionsThe date blood sample was collected which yielded E coli was regarded as the date of bacteremia onset. Nosocomial infection was defined as infection that occurred after more than 48h of hospital admission, or infection that occurred within 48h of admission in cases where the patients had been hospitalized in other medical institutes 2 weeks prior to admission.19 30-Day mortality was defined as death within 30 days from the date of bacteremia onset. Survival was defined as the time interval between diagnosis of E. coli bacteremia and death or the last follow-up. Carbapenem-resistant E. coli (CRE) were identified by resistance to imipenem, meropenem, or ertapenem.20 Long-term bedbound patients were defined as patients who were bedbound for more than 14 days and could not recover. Hypoalbuminemia was defined as serum albumin level lower than 30g/L.
Statistical analysisStudents’ t test was used to compare continuous variables, and Pearson's chi-square test or Fisher's exact test was used to compare categorical variables and percentages. The main endpoints of this study were 30-day mortarity and intensive care (IC) requirement. Cox proportional-hazards regression was used to identify variables that predict the given clinical outcomes. Hazard ratios (HRs) and 95% confidence intervals (CIs) were pooled to measure the effects of clinical parameters on prognosis. Variables significantly associated with mortality at the 0.20 level in univariate analysis were considered for multivariate backward analysis. Kaplan–Meier method was adopted to assess the impact of specific parameters on survival, and log-rank test was performed for comparing the differences on survival between groups. Receiver-operating characteristic (ROC) curve analysis was performed to identify the best cutoff value for mortality prediction. The maximum value of Youden index (calculated by the formula: sensitivity+specificity−1) was selected as the optimal threshold value (cutoff point). P values<0.05 were considered statistically significant, and all probabilities were two-tailed. All statistical analyses were performed using SPSS Statistics version 20 (IBM Corp, Armonk, NY, USA).
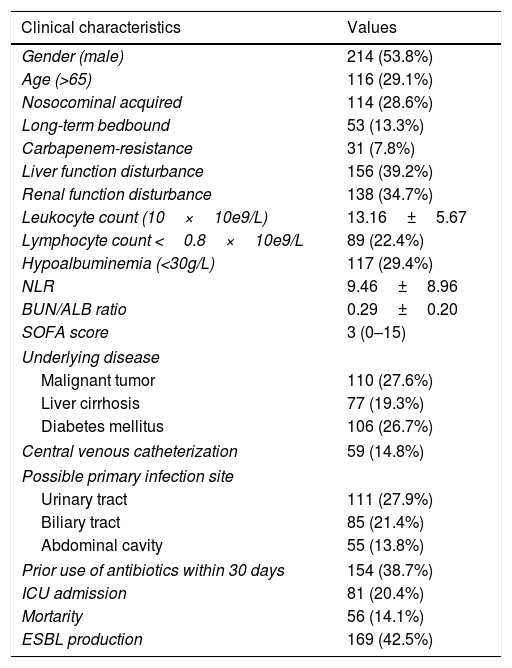
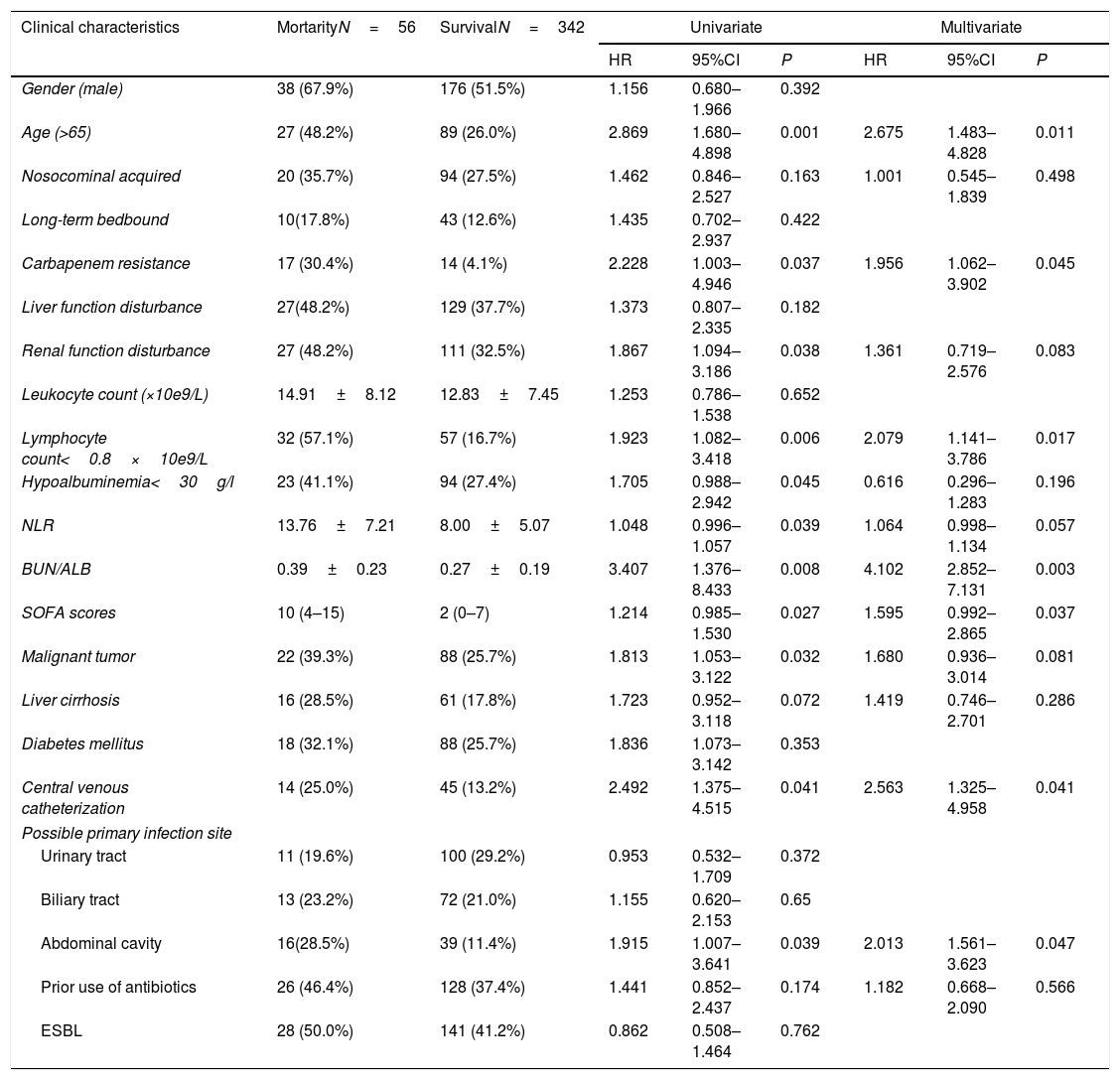
ResultsDemographic data and clinical characteristicsDuring the observational period, a total of 398 cases of E. coli bacteremia, including 169 (42.5%) ESBL-producing cases and 31 (7.8%) carbapenem-resistant cases, were analyzed in our study. The top three underlying disease were malignant tumor (27.6%), diabetes mellitus (26.7%), and liver cirrhosis (19.3%). E. coli bacteremia mainly originated from urinary tract (27.9%), biliary tract (21.4%) and abdominal cavity (13.8%). The mean leukocyte count and neutrophil-to-lymphocyte ratio (NLR) was (13.16±5.67)×10e9/L and (9.46±8.96) respectively, while 22.4% of the patients had lymphocyte count lower than 0.8×10e9/L. Hypoalbuminemia was observed in 29.4% of the patients and the average blood urea nitrogen to serum albumin (BUN/ALB) ratio was (0.29±0.20). A total of 81 (20.4%) patients were admitted to ICU and 56 (14.1%) cases died within 30 days. Moreover, the 30-day mortality did not differ between the patients in the ESBL-producing EC-BSI and non-ESBL-producing EC-BSI groups (16.6% vs 12.2%, P=0.259; Table 1).
Clinical characteristics of patients enrolled in the present study.
| Clinical characteristics | Values |
|---|---|
| Gender (male) | 214 (53.8%) |
| Age (>65) | 116 (29.1%) |
| Nosocominal acquired | 114 (28.6%) |
| Long-term bedbound | 53 (13.3%) |
| Carbapenem-resistance | 31 (7.8%) |
| Liver function disturbance | 156 (39.2%) |
| Renal function disturbance | 138 (34.7%) |
| Leukocyte count (10×10e9/L) | 13.16±5.67 |
| Lymphocyte count <0.8×10e9/L | 89 (22.4%) |
| Hypoalbuminemia (<30g/L) | 117 (29.4%) |
| NLR | 9.46±8.96 |
| BUN/ALB ratio | 0.29±0.20 |
| SOFA score | 3 (0–15) |
| Underlying disease | |
| Malignant tumor | 110 (27.6%) |
| Liver cirrhosis | 77 (19.3%) |
| Diabetes mellitus | 106 (26.7%) |
| Central venous catheterization | 59 (14.8%) |
| Possible primary infection site | |
| Urinary tract | 111 (27.9%) |
| Biliary tract | 85 (21.4%) |
| Abdominal cavity | 55 (13.8%) |
| Prior use of antibiotics within 30 days | 154 (38.7%) |
| ICU admission | 81 (20.4%) |
| Mortarity | 56 (14.1%) |
| ESBL production | 169 (42.5%) |
Notes: Data were presented by numbers (percentage), median (discontinuous) or mean±standard deviation (x±s) (continuous).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; BUN/ALB ratio, blood urea nitrogen to serum albumin ratio; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit; ESBL, extended-spectrum beta-lactamase.
The results of univariate Cox regression analysis revealed that the following clinical factors were significantly different between the survival group and the 30-day mortality group: age greater than 65 years, carbapenem resistance, renal function disturbance, lymphocyte count less than 0.8×10e9/L, increased NLR level, hypoalbuminemia, elevated BUN/ALB ratio, elevated SOFA score at the onset of bacteremia, complicated with malignant tumor, central venous catheterization before onset of bacteremia, and infection originating from abdominal cavity. Multivariate Cox regression analysis showed that age greater than 65 years (HR=2.675, 95% CI:1.483–4.828, P=0.011), lymphocyte count less than 0.8×10e9/L (HR=2.079, 95% CI:1.141–3.786, P=0.017), elevated BUN/ALB ratio (HR=4.102, 95% CI:2.852–7.131, P=0.003), elevated SOFA score at the onset of bacteremia (HR=1.595, 95% CI: 0.992–2.865, P=0.037), carbapenem resistance (HR=1.956, 95% CI:1.062–3.902, P=0.045), central venous catheterization before onset of bacteremia (HR=2.563, 95% CI:1.325–4.958, P=0.041), and infection originating from abdominal cavity (HR=2.013, 95% CI:1.561–3.623, P=0.047) were independent risk factors associated with 30-day mortality (Table 2).
Univariate and multivariate cox regression analyses of risk factors related to 30-day mortality caused by E. coli bacteremia.
| Clinical characteristics | MortarityN=56 | SurvivalN=342 | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |||
| Gender (male) | 38 (67.9%) | 176 (51.5%) | 1.156 | 0.680–1.966 | 0.392 | |||
| Age (>65) | 27 (48.2%) | 89 (26.0%) | 2.869 | 1.680–4.898 | 0.001 | 2.675 | 1.483–4.828 | 0.011 |
| Nosocominal acquired | 20 (35.7%) | 94 (27.5%) | 1.462 | 0.846–2.527 | 0.163 | 1.001 | 0.545–1.839 | 0.498 |
| Long-term bedbound | 10(17.8%) | 43 (12.6%) | 1.435 | 0.702–2.937 | 0.422 | |||
| Carbapenem resistance | 17 (30.4%) | 14 (4.1%) | 2.228 | 1.003–4.946 | 0.037 | 1.956 | 1.062–3.902 | 0.045 |
| Liver function disturbance | 27(48.2%) | 129 (37.7%) | 1.373 | 0.807–2.335 | 0.182 | |||
| Renal function disturbance | 27 (48.2%) | 111 (32.5%) | 1.867 | 1.094–3.186 | 0.038 | 1.361 | 0.719–2.576 | 0.083 |
| Leukocyte count (×10e9/L) | 14.91±8.12 | 12.83±7.45 | 1.253 | 0.786–1.538 | 0.652 | |||
| Lymphocyte count<0.8×10e9/L | 32 (57.1%) | 57 (16.7%) | 1.923 | 1.082–3.418 | 0.006 | 2.079 | 1.141–3.786 | 0.017 |
| Hypoalbuminemia<30g/l | 23 (41.1%) | 94 (27.4%) | 1.705 | 0.988–2.942 | 0.045 | 0.616 | 0.296–1.283 | 0.196 |
| NLR | 13.76±7.21 | 8.00±5.07 | 1.048 | 0.996–1.057 | 0.039 | 1.064 | 0.998–1.134 | 0.057 |
| BUN/ALB | 0.39±0.23 | 0.27±0.19 | 3.407 | 1.376–8.433 | 0.008 | 4.102 | 2.852–7.131 | 0.003 |
| SOFA scores | 10 (4–15) | 2 (0–7) | 1.214 | 0.985–1.530 | 0.027 | 1.595 | 0.992–2.865 | 0.037 |
| Malignant tumor | 22 (39.3%) | 88 (25.7%) | 1.813 | 1.053–3.122 | 0.032 | 1.680 | 0.936–3.014 | 0.081 |
| Liver cirrhosis | 16 (28.5%) | 61 (17.8%) | 1.723 | 0.952–3.118 | 0.072 | 1.419 | 0.746–2.701 | 0.286 |
| Diabetes mellitus | 18 (32.1%) | 88 (25.7%) | 1.836 | 1.073–3.142 | 0.353 | |||
| Central venous catheterization | 14 (25.0%) | 45 (13.2%) | 2.492 | 1.375–4.515 | 0.041 | 2.563 | 1.325–4.958 | 0.041 |
| Possible primary infection site | ||||||||
| Urinary tract | 11 (19.6%) | 100 (29.2%) | 0.953 | 0.532–1.709 | 0.372 | |||
| Biliary tract | 13 (23.2%) | 72 (21.0%) | 1.155 | 0.620–2.153 | 0.65 | |||
| Abdominal cavity | 16(28.5%) | 39 (11.4%) | 1.915 | 1.007–3.641 | 0.039 | 2.013 | 1.561–3.623 | 0.047 |
| Prior use of antibiotics | 26 (46.4%) | 128 (37.4%) | 1.441 | 0.852–2.437 | 0.174 | 1.182 | 0.668–2.090 | 0.566 |
| ESBL | 28 (50.0%) | 141 (41.2%) | 0.862 | 0.508–1.464 | 0.762 | |||
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; BUN/ALB ratio, blood urea nitrogen to serum albumin ratio; ESBL, extended-spectrum beta-lactamase; SOFA, Sequential Organ Failure Assessment.
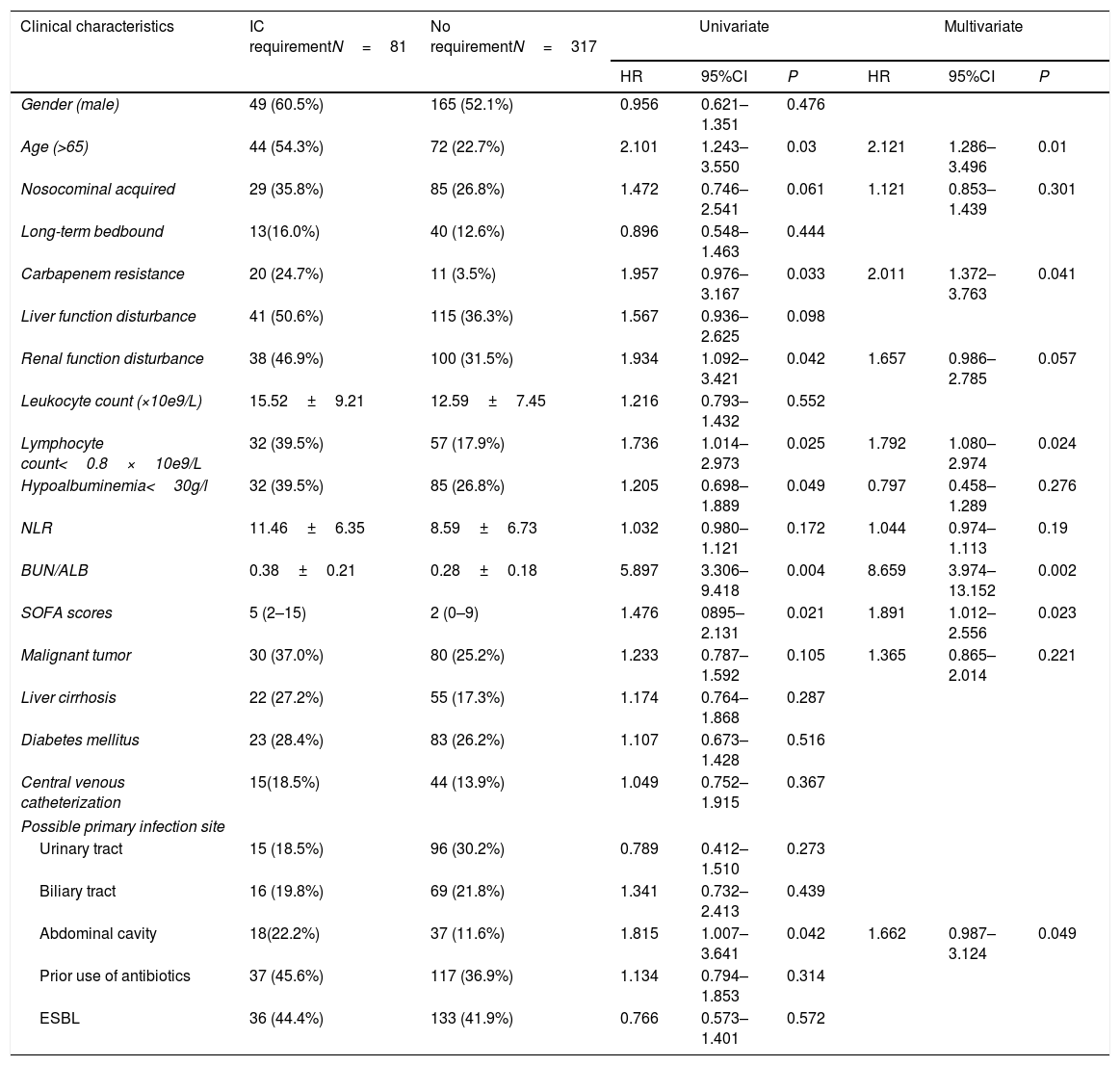
To evaluate the risk factors associated with disease severity, the characteristics of patients who received IC and those who did not receive IC were compared (Table 3). The results showed that patients who required IC were more likely of advanced age, with renal function disturbance and hypoalbuminemia. Multivariate analysis revealed that independent factors associated with IC requirement included: age greater than 65 years (HR=2.121, 95% CI:1.286–3.496, P=0.01), lymphocyte count less than 0.8*10e9/L (HR=1.792, 95% CI:1.080–2.974, P=0.024), elevated BUN/ALB ratio (HR=8.659, 95% CI:3.974–13.152, P=0.002), elevated SOFA score at the onset of bacteremia (HR=1.891, 95% CI:1.012–2.556, P=0.023), carbapenem resistance (HR=2.011, 95% CI:1.372–3.763, P=0.041), and infection originating from abdominal cavity (HR=1 .662, 95% CI:0.987–3.124, P=0.049).
Univariate and multivariate cox regression analyses of risk factors related to intensive case requirement caused by E. coli bacteremia.
| Clinical characteristics | IC requirementN=81 | No requirementN=317 | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |||
| Gender (male) | 49 (60.5%) | 165 (52.1%) | 0.956 | 0.621–1.351 | 0.476 | |||
| Age (>65) | 44 (54.3%) | 72 (22.7%) | 2.101 | 1.243–3.550 | 0.03 | 2.121 | 1.286–3.496 | 0.01 |
| Nosocominal acquired | 29 (35.8%) | 85 (26.8%) | 1.472 | 0.746–2.541 | 0.061 | 1.121 | 0.853–1.439 | 0.301 |
| Long-term bedbound | 13(16.0%) | 40 (12.6%) | 0.896 | 0.548–1.463 | 0.444 | |||
| Carbapenem resistance | 20 (24.7%) | 11 (3.5%) | 1.957 | 0.976–3.167 | 0.033 | 2.011 | 1.372–3.763 | 0.041 |
| Liver function disturbance | 41 (50.6%) | 115 (36.3%) | 1.567 | 0.936–2.625 | 0.098 | |||
| Renal function disturbance | 38 (46.9%) | 100 (31.5%) | 1.934 | 1.092–3.421 | 0.042 | 1.657 | 0.986–2.785 | 0.057 |
| Leukocyte count (×10e9/L) | 15.52±9.21 | 12.59±7.45 | 1.216 | 0.793–1.432 | 0.552 | |||
| Lymphocyte count<0.8×10e9/L | 32 (39.5%) | 57 (17.9%) | 1.736 | 1.014–2.973 | 0.025 | 1.792 | 1.080–2.974 | 0.024 |
| Hypoalbuminemia<30g/l | 32 (39.5%) | 85 (26.8%) | 1.205 | 0.698–1.889 | 0.049 | 0.797 | 0.458–1.289 | 0.276 |
| NLR | 11.46±6.35 | 8.59±6.73 | 1.032 | 0.980–1.121 | 0.172 | 1.044 | 0.974–1.113 | 0.19 |
| BUN/ALB | 0.38±0.21 | 0.28±0.18 | 5.897 | 3.306–9.418 | 0.004 | 8.659 | 3.974–13.152 | 0.002 |
| SOFA scores | 5 (2–15) | 2 (0–9) | 1.476 | 0895–2.131 | 0.021 | 1.891 | 1.012–2.556 | 0.023 |
| Malignant tumor | 30 (37.0%) | 80 (25.2%) | 1.233 | 0.787–1.592 | 0.105 | 1.365 | 0.865–2.014 | 0.221 |
| Liver cirrhosis | 22 (27.2%) | 55 (17.3%) | 1.174 | 0.764–1.868 | 0.287 | |||
| Diabetes mellitus | 23 (28.4%) | 83 (26.2%) | 1.107 | 0.673–1.428 | 0.516 | |||
| Central venous catheterization | 15(18.5%) | 44 (13.9%) | 1.049 | 0.752–1.915 | 0.367 | |||
| Possible primary infection site | ||||||||
| Urinary tract | 15 (18.5%) | 96 (30.2%) | 0.789 | 0.412–1.510 | 0.273 | |||
| Biliary tract | 16 (19.8%) | 69 (21.8%) | 1.341 | 0.732–2.413 | 0.439 | |||
| Abdominal cavity | 18(22.2%) | 37 (11.6%) | 1.815 | 1.007–3.641 | 0.042 | 1.662 | 0.987–3.124 | 0.049 |
| Prior use of antibiotics | 37 (45.6%) | 117 (36.9%) | 1.134 | 0.794–1.853 | 0.314 | |||
| ESBL | 36 (44.4%) | 133 (41.9%) | 0.766 | 0.573–1.401 | 0.572 | |||
Abbreviations: IC, intensive care; NLR, neutrophil-to-lymphocyte ratio; BUN/ALB ratio, blood urea nitrogen to serum albumin ratio; ESBL, extended-spectrum beta-lactamase; SOFA, Sequential Organ Failure Assessment.
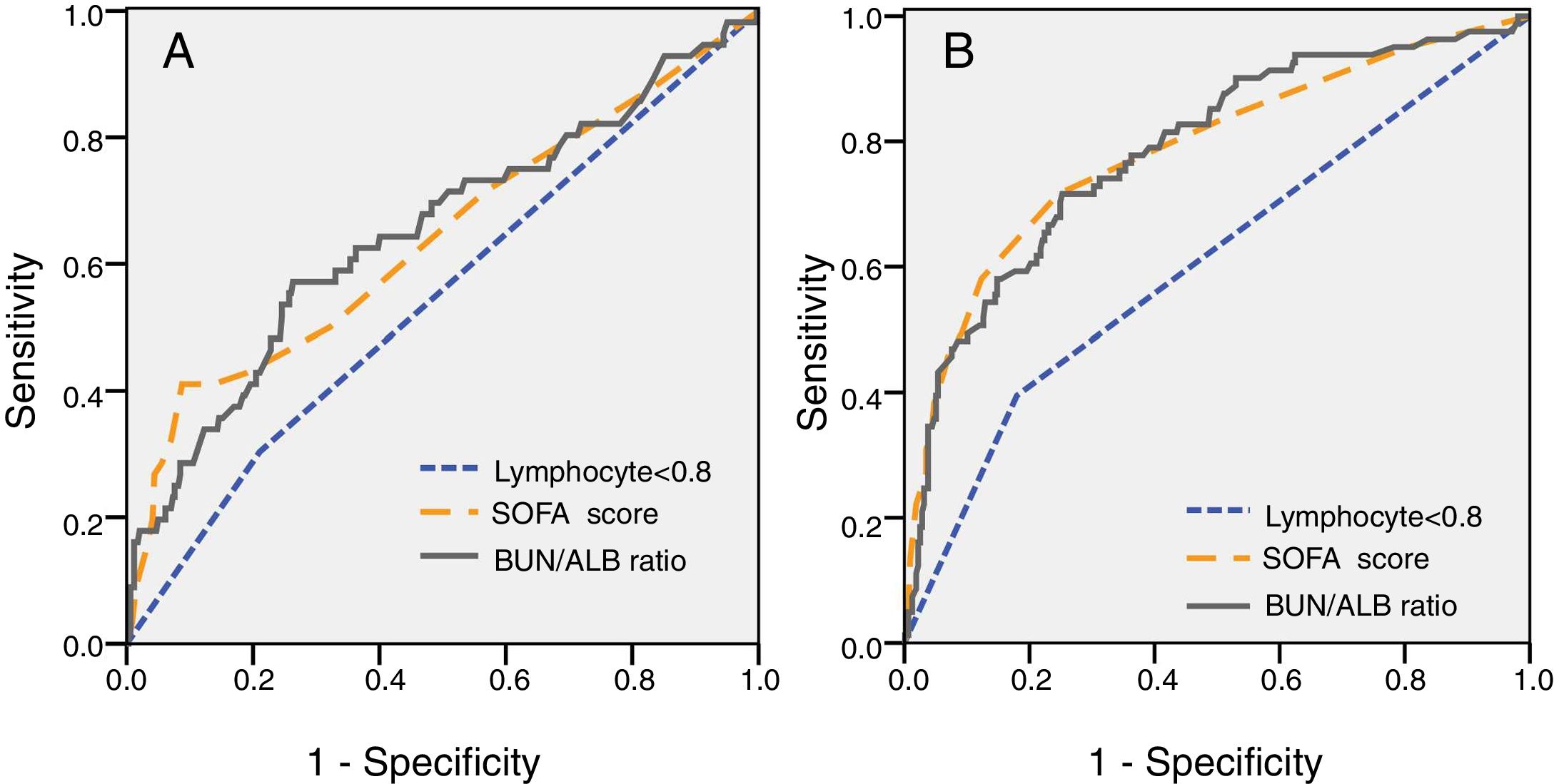
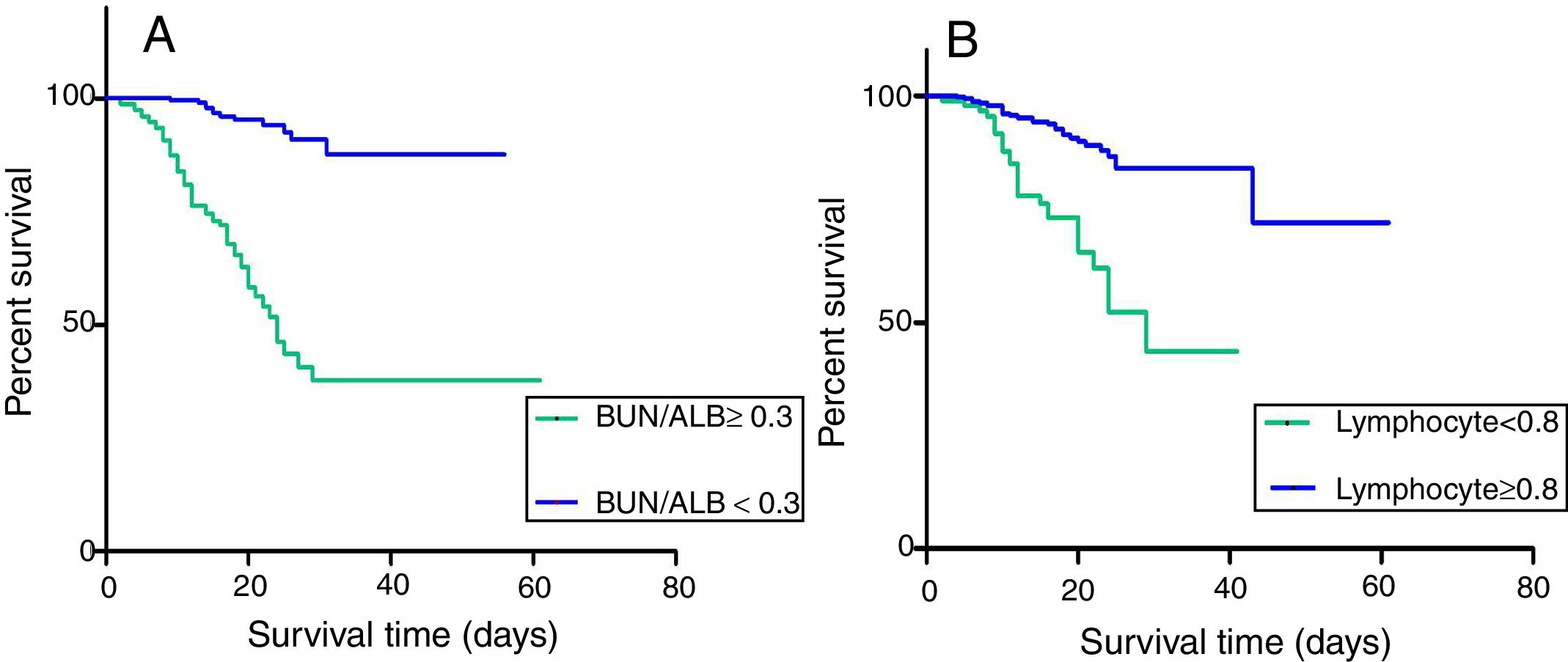
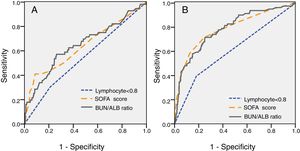
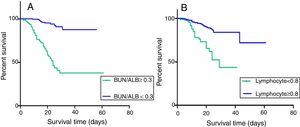
For further understanding the prediction of 30-day mortality by SOFA score, lymphocyte count and BUN/ALB ratio, ROC curves were plotted. The area under the ROC curve (AUC) was 0.712 (95% CI:0.591–0.805, P=0.003) for BUN/ALB ratio, 0.705 (95% CI:0.601–0.786, P=0.005) for SOFA score, and 0.547 (95% CI:0.463–0.630, P=0.264) for lymphocyte count respectively. The results indicated that the BUN/ALB ratio was a better predictor of 30-day mortality as compared to lymphocyte count (Fig. 2A). The optimal cutoff value of the BUN/ALB ratio for predicting mortality was 0.3, with 67.5% sensitivity and 65.1% specificity. As the cutoff point was 0.3, patients with BUN/ALB ratio lower than 0.3 were significantly associated with better survival as compared to patients with BUN/ALB ratio greater than 0.3, according to the result of Kaplan–Meier survival curves (P<0.001). Moreover, patients with lymphocyte count less than 0.8×10e9/L were significantly associated with poor survival as compared to those with higher lymphocyte count (P=0.024) (Fig. 3).
(A) Analysis of receiver-operating characteristic curve to predict 30-day mortality of EC-BSI (A) and IC requirement of EC-BSI (B). (A) The AUC was 0.712 for BUN/ALB ratio (P=0.003), 0.705 for SOFA score (P=0.005), and 0.547 for lymphocyte count (P=0.264) respectively. The cutoff point of BUN/ALB ratio to predict 30-day mortality was 0.3. (B) The AUC was 0.811 for BUN/ALB ratio (P=0.001), 0.813 for SOFA score (P=0.001), and 0.618 for lymphocyte count (P=0.023) respectively. The cutoff point of BUN/ALB ratio to predict IC requirement was 0.3. Abbreviations: EC-BSI, Escherichia coli bloodstream infections; AUC, area under the curve; BUN/ALB, blood urea nitrogen to serum albumin ratio; SOFA, Sequential Organ Failure Assessment; IC, intensive care;.
(A) Kaplan–Meier survival curves for 30-day survival in EC-BSI patients with different BUN/ALB ratio. BUN/ALB ratio≥0.3 was significantly associated with a worse 30-day survival (P<0.001). (B) Kaplan–Meier survival curves for 30-day survival in EC-BSI patients with different levels of lymphocyte count. Lymphocyte count<0.8×10e9/L was significantly associated with a worse 30-day survival (P=0.024). Abbreviations: BUN/ALB, blood urea nitrogen to serum albumin ratio; EC-BSI, Escherichia coli bloodstream infections.
ROC curve analysis was also performed to evaluate the predictive value of different clinical indices on disease severity. The ROC curve for predicting IC requirement is shown in Fig. 2B. The AUC was 0.811 (95% CI 0.744–0.857, P=0.001) for BUN/ALB ratio, 0.813 (95% CI 0.751–0.863, P=0.001) for SOFA score, and 0.618 (95% CI 0.546–0.691, P=0.023) for lymphocyte count respectively. The optimal cutoff value of the BUN/ALB ratio for predicting IC requirement was 0.3 as well, with 71.9% sensitivity and 73.7% specificity.
DiscussionThe growing incidence of E. coli bacteremia has become one of the most significant epidemiological changes in recent decades. Hence, early and accurate identification of patients with high mortality risk is critical for appropriate management. In the present study, we investigated the possible independent risk factors of disease severity and 30-day mortality in EC-BSI by analyzing the clinical characteristics of patients with this BSI.
Our results showed that the 30-day mortality rate was 14.1%, similar to the mortality rates reported by other studies from 10.3% to 33.3%.21–23 The results of multivariate Cox regression analysis in our study showed that age greater than 65 years, carbapenem resistance, central venous catheterization before onset of bacteremia, infection originating from abdominal cavity, elevated SOFA score at the onset of bacteremia, lymphocyte count less than 0.8×10e9/L, and increased BUN/ALB ratio were independent risk factors associated with mortality within 30-days. The first four factors were also reported in previous similar researches.16,24 The same risk factors were also related to IC requirement, except central venous catheterization before onset of bacteremia. Old age usually has a negative impact on prognosis and may increase disease severity and risk of mortality owing to deterioration of organ function with advanced age. Central venous catheterization has been considered as an independent risk factor for BSIs with multidrug-resistant Enterobacteriaceae.25 Carbapenem antibiotics are one of the most effective drug classes for treating Enterobacteriaceae BSIs. For carbapenem resistant E. coli bacteremia, the selection of antimicrobials for effective therapy was fewer. Studies have shown that mortality rate is significantly higher for carbapenem-resistant Enterobacteriaceae (CRE) infections than for carbapenem-sensitive Enterobacteriaceae infections.26 In the present study, it was observed that abdominal cavity was the third common primary infection site, and BSI originating from abdominal cavity was found to be an independent risk factor for 30-day mortality. It is well known that E. coli is an opportunistic pathogen that can cause infections following mucosal disruption associated with invasive procedures.35 The high proportion of infections originating from abdominal cavity in this study might be attributed to the large number of patients with severe liver cirrhosis and peritonitis and patients who underwent hepatobiliary-related surgeries in our hospital. Owing to the poor health status of patients with these underlying conditions, bacterial infection is one of the most common causes of death in these patients,27 this explains the observation that BSI originating from abdominal cavity was an independent risk factor for ICU admission and mortality in the present study. The SOFA score is commonly used by clinicians to assess patients’ general condition. Several studies have demonstrated the correlation between elevated SOFA score and poor clinical outcomes in patients with BSIs.16,28 In the present study, patients admitted to the ICU or patients who died within 30 days after onset of bacteremia showed higher SOFA scores, which is in accordance with the previous studies.
Studies have shown that hypoalbuminemia indicates the severity of inflammation and can serve as an additional independent risk parameter for mortality and prognosis. An acute decrease in serum albumin level immediately after infection predicts poor prognosis.29 Blood urea nitrogen (BUN) is an important parameter reflecting the complex interrelation among nutritional status, protein metabolism and renal condition of the patient. Multiple studies have indicated the association between BUN level and mortality in various situations.30,31 A combination of the two parameters, i.e., the BUN/ALB ratio has been reported to be a simple but an independent predictor of mortality and severity of community-acquired pneumonia.17 In a previous study, we had found that elevated BUN/ALB ratio was associated with higher 30-day mortality in HAP.18 Compared to SOFA score, the BUN/ALB ratio is simpler and easier to calculate, thus is more convenient for clinical use. Moreover, this ratio is not affected by individual subjectivity from different clinicians. Our results showed that an elevated BUN/ALB ratio (with a cutoff point 0.3) was significantly associated with poor survival in patients with EC-BSI and IC requirement. Patients with severe condition often suffered from hypoperfusion of kidney and hypoalbuminemia, resulting in increased BUN level and an elevated BUN/ALB ratio. This will help clinicians better understand the meaning of high BUN/ALB ratio and evaluate patients’ prognosis.
Lymphocytes are important immune cells that play a role in body's defense against pathogens, reflecting the immune condition of a patient. In serious infection, as inflammatory disease progresses, lymphocyte count generally decreases and results in impaired immunity.19 In general, blood neutrophil count increases with the progress of inflammatory disease. The NLR is an indicator of systemic inflammation based on complete blood count values. Numerous studies have shown that a high NLR can be an independent predictor of poor prognosis in various clinical situations, including malignancies, cardiovascular diseases, acute respiratory distress syndrome, and fibrotic liver diseases.11,32,33 A recent meta analysis showed that a higher NLR was also associated with poor prognosis in patients with sever bacteremia.34 Nevertheless, in certain conditions like cachexia, the neutrophil count does not increase, resulting in “false negative” condition when evaluating disease progression, and the association between NLR and clinical prognosis remains controversial.35 In the present study, we compared NLR levels between patients survived or died within 30 days. The results indicated that there was no significant difference between the two groups, while NLR level was not associated with the 30-day mortality. This was not consised with the resultes reported in the previous researches. The possible reason was that a considerable portion of the patients included in our study were complicated with liver cirrosis, hematological disease or receiving chemotherapy, which suffered from neutropenia and resulted in decreased but not increased NLR levels. Comparison between subgroups excluding patients with these complications in the future work is necessary. Moreover, it had been reported previously that decreased lymphocytes were associated with mortality in sever bacteremia or pneumonia.15 Blood lymphocyte count less than 0.8×10e9/L was an independent risk factor for 30-day mortality in HAP.36 Our results revealed that blood lymphocyte count less than 0.8×10e9/L increased the 30-day mortality and ICU admission rate in EC-BSI. However, according to the result of ROC curve analysis, BUN/ALB ratio was a better predictor of 30-day mortality and disease severity in EC-BSI as compared to lymphocyte count.
Our results showed that ESBL was not an independent risk factor for 30-day mortality in EC-BSI, and there was no difference on 30-day mortality between patients in the ESBL-producing EC-BSI and non-ESBL-producing EC-BSI groups (16.6% vs 12.2%, P=0.259). This result was similar to a previous study,37 but contrasted with other studies which demonstrated significantly higher mortality in patients infected with ESBL-producing E. coli than in those infected with non-ESBL-producing E. coli.38,39 It may be attributed to that patients were administered appropriate empiric and definitive antibiotics therapy between two groups.
There were certain limitations to this investigation. First, as a retrospective study, coding practices can vary over time because of policy and other systemic changes, owing to the retrospective study design, which may result in bias.40 Second, the study was carried out in a single center, and the results may not be applicable in other settings. Third, the sample size was relative small, and some comparison analysis between subgroups was limited. Therefore, further study with multicenter and large sample size to analyze the relationship between BUN/ALB ratio and mortality or disease severity is necessary.
ConclusionThe present study investigated various clinical parameters to identify risk factors for 30-day mortality and IC requirement in EC-BSI, which will enable clinicians to identify individuals who are at high risk of mortality. Our results indicated that the BUN/ALB ratio at the onset of bacteremia is a simple but reliable predictor of mortality and disease severity in EC-BSI. A high BUN/ALB ratio indicates high risk of mortality or IC requirement in patients with EC-BSI.
Conflict of interestThe authors declare no conflicts of interest.