Autonomic nervous system (ANS) dysfunction is frequently seen in people living with multiple sclerosis (MS). Heart rate variability (HRV) is an easy and objective index for evaluating ANS functioning, and it has been previously used to explore the association between ANS and the experience of symptom burden in other chronic diseases. Given ANS functioning can be influenced by physical and psychological factors, this study investigated whether emotional distress and/or the presence of ANS dysfunction is associated with symptom severity in people living with MS.
MethodsParticipants with MS and healthy controls (HC) with no history of cardiac conditions were recruited to self-collect HR data sampled from a chest strap HR monitor (PolarH10). Short-term HR signal was collected for five minutes, and time and frequency HRV analyses were performed and compared between groups. HRV values were then compared to self-reported distress (Kessler Psychological Distress Scale) and MS participants’ self-reported measures of symptom burden (SymptoMScreen).
ResultsA total of n = 23 adults with MS (51 ± 12 years, 65 % female, median Patient Determined Disease Steps [PDDS]: 3.0) and n = 23 HCs (43 ± 18 years, 40 % female) completed the study procedures. All participants were able to complete the chest strap placement and HR data capture independently. Participants with MS, compared to the HC participants, had a significantly lower parasympathetic activation as shown by lower values of the root mean square of successive differences between normal heartbeats (RMSSD: 21.86 ± 9.84 vs. 43.13 ± 20.98 ms, p = 0.002) and of high-frequency (HF) power band (HF-HRV: 32.69 ± 12.01 vs. 42.39 ± 7.96 nu, p = 0.016), indicating an overall lower HRV in the MS group. Among individuals with MS, HF-HRV was significantly correlated with the severity of self-reported MS symptoms (r = -0.548, p = 0.010). Participants with MS also reported higher levels of distress compared to HC participants (18.32 ± 6.05 vs. 15.00 ± 4.61, p = 0.050), and HRV correlated with the severity of distress in MS participants (r = -0.569, p = 0.007). A significant mediation effect was also observed, with emotional distress fully mediating the association between HRV and symptom burden.
ConclusionsThese findings suggest the potential for ANS dysfunction, as measured by HRV (i.e., lower value of HF power), to be utilized as an objective marker of symptom burden in people living with MS. Moreover, it is apparent that the relationship between HRV and symptom burden is mediated by emotional distress.
Multiple sclerosis (MS) is a chronic and progressive neurological disorder that affects the central nervous system, resulting in a diverse range of symptoms and clinical presentations (Brownlee et al., 2017). Symptom burden in MS encompasses the severity of a range of symptoms including cognitive, motor, and sensory dysfunctions, as well as pain and fatigue (Kister et al., 2013) and it is a significant predictor of the quality of life of individuals with MS (Feigin et al., 2021). MS symptom burden is influenced by various factors beyond undergoing disease processes and can fluctuate from day to day, both between and within individuals (Veldhuijzen van Zanten et al., 2021).
Although our understanding of the role of the autonomic nervous system (ANS) in MS is somewhat limited (Kale et al., 2009), some initial reports suggest the presence of ANS dysfunction at the population level at various stages of the disease course (Gerasimova-Meigal et al., 2021; Pintér et al., 2015). ANS dysfunction has been primarily linked to the ongoing processes of demyelination and inflammation characteristic of the pathophysiology of MS (Brezinova et al., 2004; Racosta et al., 2015). Given the crucial role of the ANS system in regulating involuntary physiologic processes to maintain homeostasis, its dysfunction may intensify the burden of the symptoms experienced (Koutsouraki et al., 2023).
Heart rate variability (HRV) is an easily measurable and objective index for evaluating ANS function. HRV is a measure of the variation in time between successive heartbeats, arising from dynamic fluctuations in the ANS (Rajendra Acharya et al., 2006), and it serves as an index that may be utilized to evaluate the combined activity of the sympathetic and parasympathetic branches of the ANS on heart rate (Singh et al., 2018). A high value of HRV is generally associated with better health and well-being, including a greater adaptability to external stress; whereas a low HRV suggests the opposite (Appelhans & Luecken, 2006). A reduced resting-state HRV indicates a monotonously regular heart rate and is associated with compromised regulatory and homeostatic functions of the ANS, which in turn, affect the body's capacity to regulate emotional responses to internal and external stressful events (Kim et al., 2018). Several physical and psychological factors, such as emotional distress, sleep quality, certain medical conditions, and lifestyle, can influence HRV (Fatisson et al., 2016). Previous studies have shown that emotional distress can directly affect the sympathetic and parasympathetic nervous systems functioning in chronic disease (Kim et al., 2018; Krbot Skorić et al., 2019).
In the context of MS, previous research has shown that individuals with MS display reduced HRV compared to healthy controls (Damla et al., 2018; Studer et al., 2017), with significant impairment in both parasympathetic and sympathetic ANS (Videira et al., 2016). However, despite these findings, there were mixed findings about the correlations between HRV and clinical outcomes, including the number of relapses, or Expanded Disability Status Scale (EDSS) (Kurtzke, 1983) scores, or MRI findings related to lesion locations (Gökaslan et al., 2020; Reynders et al., 2019).
Emotional distress may be a contributor to symptom burden in MS (e.g., Morree et al., 2013; Ziegler, 2004). In support of this notion, previous research has shown that disease activity (i.e., occurrence of relapse) as well as symptoms including fatigue, cognitive impairments, and mobility issues, are likely to worsen during periods of increased stress, such as during major life changes or other stressful events (Gold & Heesen, 2007; Mohr et al., 2004; Vissicchio et al., 2019; Wood & Bhatnagar, 2015). Importantly, the relationship between stress and ANS dysfunction can be bidirectional. For instance, stress can cause or intensify ANS dysfunction (Chrousos, 2009); while a compromised ANS may yield an inability to adequately respond to stressors in the environment (Chu et al., 2023).
There is limited evidence on the association between measures of emotional distress, HRV, and symptom burden in people living with MS. Identifying whether emotional distress and/or the presence of ANS dysfunction, as indicated by HRV recordings, is associated with higher MS symptoms burden could provide insight towards improvement in treatment and patient care. Therefore, this study compared HRV recordings between individuals with MS and healthy controls (HC), and tested the relation of HRV to self-reported distress. We also tested HRV recordings in correspondence to self-reported distress and symptom burden among the participants with MS.
Material and methodsParticipantsParticipants with MS were recruited from NYU Langone MS Comprehensive Care Center during routine outpatient visits and telephone outreach. HCs were recruited by advertisement in the local community.
Eligibility criteria for all participants included: (1) 18 to 80 years of age; (2) no history of cardiac conditions (e.g., heart attack, coronary artery disease, and arrhythmias); (3) no presence of implanted cardiac devices (e.g., pacemaker, defibrillators); and (4) capacity to provide informed consent. Participants in the MS group were required to have a confirmed diagnosis of MS (any subtype) by their treating neurologist at the NYU MS Comprehensive Care Center. Participants in the HC group were required to report overall good health and no current or recent history of diagnosis or treatment for a medical condition.
All eligible participants provided written consent and were scheduled for a single, one-hour-long visit to our outpatient clinic. This study was approved by the New York University School of Medicine Institutional Review Board.
Heart rate recording equipment and proceduresHeart rate (HR) signals were recorded using a commercially available device, the Polar H10 HR sensor (Polar, Finland). The telemetry system Polar H10 HR sensor is a non-invasive monitor extensively used in research studies (Hinde et al., 2021; Speer et al., 2020; Umair et al., 2021) with robust reliability for mobile HR measurement (Gilgen-Ammann et al., 2019; Huang et al., 2021; Pereira et al., 2020; Plews et al., 2017). The chest strap is equipped with interference-preventing electrodes to ensure the HR signal is sampled accurately, and is paired with the App Elite HRV (sampling rate of 130 Hz and accuracy in detecting R-R intervals of 2 ms).
Participants were instructed on how to use the device, and before initiating data acquisition, they were asked to remove all electronic devices in contact with their body, including smartphones, smartwatches, and smart rings, to minimize electrical interference. The study staff confirmed the correct placement of the device before initiating any recording.
Short-term HRV recordings were obtained under the following experimental conditions: seated position and spontaneous breathing. The recording was taken in a quiet and bright room and during the recordings, participants were asked to sit quietly in an upright position with their feet flat on the floor.
Three short-term, 5-minute HR recordings were collected for each participant, one immediately after the other.
HR signal processing and HRV outcomesSignal processing to obtain HRV parameters was performed with the Kubios HRV Premium software (Biosignal Analytics and Medical Imaging Group, Kuopio, Finland).
Technical (e.g., misaligned beats) and physiological (e.g., ectopic beats) artifacts in the R-R interval field were detected via manual inspection as well as a threshold-based beat correction algorithm (median filtering, medium threshold of 0.25 s Aranda et al., 2017; Tarvainen et al., 2014). If the amount of artifact-free data was <4 min, the identified artifacts were replaced with interpolated values using a cubic spline function.
From time-domain analysis, the following HRV parameters were calculated: Mean R-R, or the mean of R-R intervals; the root mean square of successive differences between successive R-R intervals (RMSSD), which reflects the beat-to-beat variance in HR and estimates the vagally mediated changes reflected in HRV; and the standard deviation of NN intervals (SDNN).
Power spectral analysis (frequency-domain) of the HRV was performed with the non-parametric method of fast Fourier transform (FFT). The following HRV power parameters in normal units (nu) were obtained: (1) low-frequency power (LF Power, 0.04–0.15 Hz); (2) high-frequency power (HF Power, 0.15–0.4 Hz); and (3) LF/HF ratio. The normalized HF and LF were used to directly compare frequency-domain measurements among participants (Berntson et al., 1997; Shaffer & Ginsberg, 2017). It is important to note that HF Power corresponds to parasympathetic activity, while LF Power is the result of interplay from both the parasympathetic and sympathetic systems, influenced primarily by vagally mediated baroreflex activity (Pham et al., 2021; Rajendra Acharya et al., 2006). The dominance of sympathetic efferent activity in LF oscillations remains controversial, particularly in resting conditions, where LF Power primarily reflects baroreflex activity rather than sympathetic activity (Pham et al., 2021; Rajendra Acharya et al., 2006). The LF/HF ratio serves as an index of sympathovagal balance, indicative of the balance between sympathetic and parasympathetic activities (Valenza et al., 2018).
Demographic and clinical descriptorsParticipants provided demographic and clinical information, and completed self-report questionnaires via REDCap, a computerized survey delivery and database system. The clinical data were then confirmed with their medical records.
MS disease severity was measured by the Patient-derived MS Severity Score, P-MSSS (Kister & Kantarci, 2020), which is based on self-reported disability (Patient Determined Disease Steps; PDDS Learmonth et al., 2013) and disease duration in years. In addition, the use of disease-modifying therapies (DMT), if any, were recorded.
To control for the potential factors of weight and activity to confound HRV measures, we calculated participants’ body mass index (BMI) and administered the Godin Leisure Time Questionnaire (Godin & Shephard, 1985) (GLT) which characterized participants’ weekly exercise habits.
Self-report measures: emotional distress and MS symptom burdenAll participants completed the Kessler Psychological Distress Scale-10 (K10) (Andrews & Slade, 2001). The K10 is a 10-item questionnaire in which participants rate anxiety and depressive symptoms over the 4 weeks prior to enrollment. Scores range from 10 (minimal-to-no distress) to 50 (extreme distress).
For the MS participants, symptom burden was measured with the SymptoMScreen (Green et al., 2017), a brief inventory in which participants rate their experience across 12 symptom domains, including mobility, hand dexterity, body pain, sensory and bladder function, fatigue, vision, dizziness, mood, and cognition. Participant ratings are recorded on a 7-point Likert scale (0–6), and the total score, ranging from 0 to 72, corresponds to an overall symptom burden of disease.
Statistical analysisAnalyses were performed using IBM SPSS Statistics (Version 25.0. Armonk, NY).
To examine group differences in demographics, we conducted independent samples t-tests and chi-square tests for the categorical variables of sex distribution.
Given that age can be a potential confounder in MS-related HRV changes (McDougall & McLeod, 2003) and the evident difference in age between the MS and HC group, we included age as a covariate in the following analyses. Analyses of covariance (ANCOVA) were conducted to test for differences in time (Mean RR, RMSSD, SDNN) and frequency-domain HRV (LF Power, HF Power, and LF/HF) measures between the MS and HC groups.
In the MS group, bivariate correlation was performed to determine linear relationships between K10, SymptoMScreen, and time and frequency HRV parameters (LF Power, HF Power, and LF/HF ratio) using Pearson's correlation coefficient.
We performed a mediation model to explore the specific interplays between HRV and K10 that may contribute to MS symptom burden. The mediation model was constructed using a nonparametric bootstrapping mediation method by the Hayes’ PROCESS macro in SPSS (Coutts & Hayes, 2023). This model included SymptoMScreen as outcome variable, HRV measures as a predictor and K10 as a mediator after controlling for age. In the model, results were derived from 5000 bootstrapped samples; standardized beta parameter estimates, standard errors, and bias-corrected 95% confidence intervals (95% CI) determined the significance of direct and indirect (mediating) associations. The statistical significance level was set at 0.05.
ResultsDemographic and clinical characteristicsA total of n = 46 participants (n = 23 with MS and n = 23 HCs) were enrolled in the study, with n = 21 MS and n = 19 HC participants providing usable HR data for analyses reported below.
Demographic and clinical characteristics from the resulting n = 21 individuals with MS and n = 19 HC individuals are shown in Table 1. The majority of the patients with MS (n = 15/21, 71%) were on a DMT: n = 7 ocrelizumab, n = 2 natalizumab, n = 3 rituximab, n = 2 glatiramer acetate, and n = 1 fingolimod. Interestingly, despite including some MS participants who exhibited higher levels of disability, there were no group differences in self-reported physical activity (GLT Questionnaire) or BMI. However, as the MS participants were significantly older in age (51.90 ± 12.05) than the HC participants (39.05 ± 19.43 years, p = 0.02), respectively, age was included as a covariate for analyses given its potential relation to HRV values (Garis et al., 2022).
Participant's demographic and clinical features.
As above, from the initial n = 46 participants enrolled, HR recordings were not sufficient for HRV analyses in n = 4 participants from the HC group and n = 2 from the MS group due to noisy HR signals, possibly due to incorrect sensor placement.
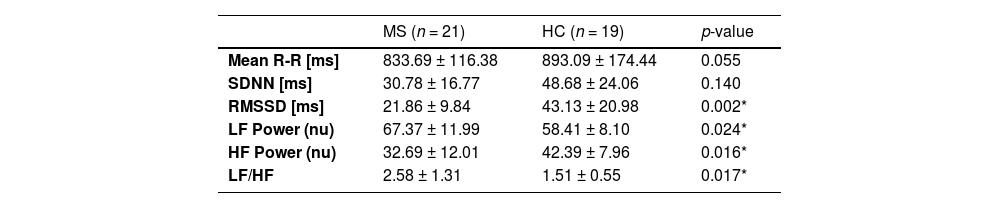
Comparison of HRV recordings between groupsGroup differences in time and frequency HRV measures are reported in Table 2.
ANCOVA comparison of MS vs. HC in the time domain and frequency domain HRV parameters.
| MS (n = 21) | HC (n = 19) | p-value | |
|---|---|---|---|
| Mean R-R [ms] | 833.69 ± 116.38 | 893.09 ± 174.44 | 0.055 |
| SDNN [ms] | 30.78 ± 16.77 | 48.68 ± 24.06 | 0.140 |
| RMSSD [ms] | 21.86 ± 9.84 | 43.13 ± 20.98 | 0.002* |
| LF Power (nu) | 67.37 ± 11.99 | 58.41 ± 8.10 | 0.024* |
| HF Power (nu) | 32.69 ± 12.01 | 42.39 ± 7.96 | 0.016* |
| LF/HF | 2.58 ± 1.31 | 1.51 ± 0.55 | 0.017* |
As we hypothesized, participants with MS had an overall significantly lower HRV in both time and frequency domain parameters. Participants with MS showed a decrease in the time domain HRV measure of RMSSD (21.86 ± 9.84) compared to HCs (43.13 ± 20.98, F[1,37]=11.28, p = 0.002, ƞ2=0.234).
Similarly, participants with MS had a lower value in the frequency domain HRV measure of HF Power when compared to the HC group (F[1,37]=6.44, p = 0.016, ƞ2=0.300). The presence of an autonomic imbalance in those with MS was also reflected in a significantly higher LF/HF ratio (F[1,37]=6.23, p = 0.017, ƞ2=0.170).
Demographic and clinical descriptors as predictors of HRV recordingsAfter controlling for age, hierarchical regression analysis revealed that clinical and demographic descriptors such as disease severity (P-MSSS) and disease duration, as well as BMI and level of physical activity did not account for altered HF Power and LF Power (Table 3).
Hierarchical multiple regression analyses for variables predicting HF Power and LF Power.
BMI= Body Max Index; GLT= Godin Leisure Time Questionnaire (level of physical activity); P-MSSS= Patient-derived MS Severity Score (disease severity). * p<0.05.
The MS group reported higher distress (K10: 18.32 ± 6.05) compared to the HC group (15.00 ± 4.61, p = 0.050, t1,38=2.037, d = 0.617). Results from Pearson's bivariate correlation analyses between time and frequency HRV measures vs. distress (K10) and MS symptom burden (SymptoMScreen) are reported in Table 4. As expected, higher symptom burden was strongly correlated with higher distress (SymptoMScreen vs. K10, r = 0.791, p < 0.001). In turn, higher distress was correlated with reduced resting-state HRV, particularly in the HF power spectrum measurements (r=−0.621, p < 0.001). We also found that higher symptom burden was negatively associated with the HRV measure of HF Power (r=−0.548, p < 0.001), meaning that lower HRV was associated with higher symptom burden experience.
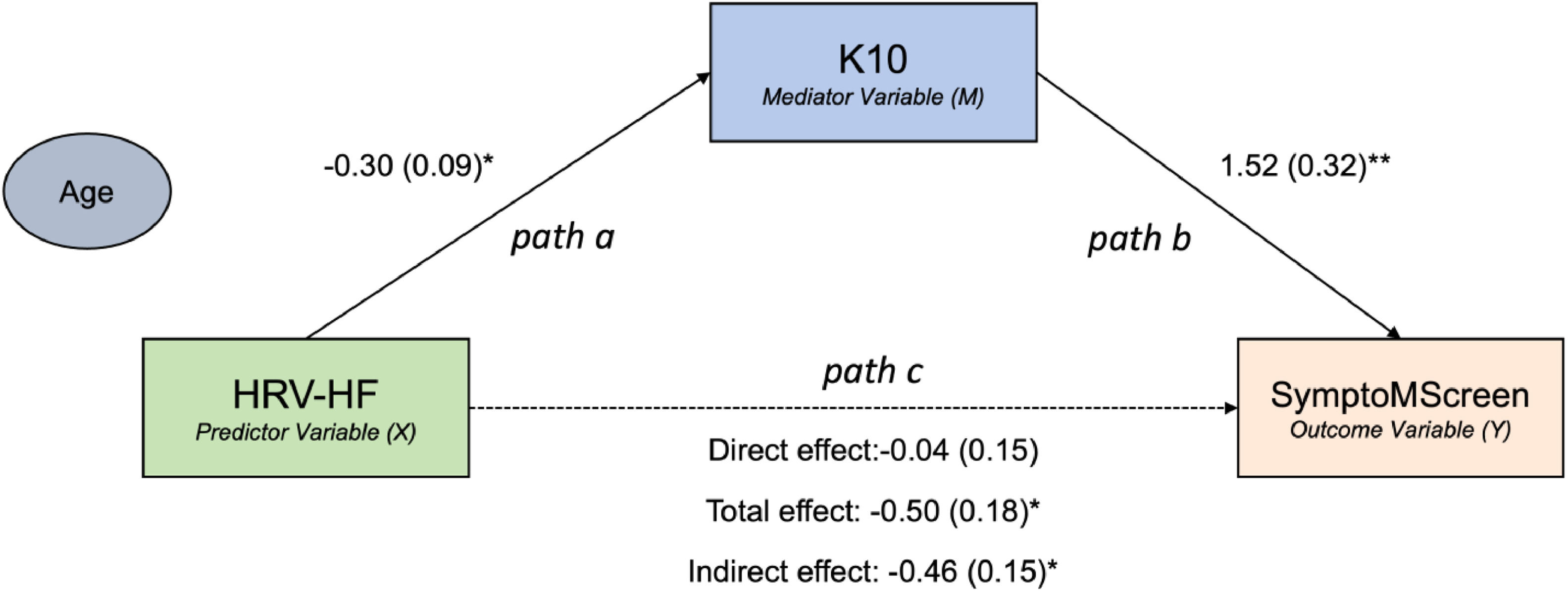
Distress mediates the relationship between HF power HRV and MS symptom burdenWe performed a mediation analysis to understand the directional relationship between HRV, distress, and MS symptom burden. We tested the hypothesis that distress is a mediator in the relationship between HRV and symptom burden severity. There was a significant negative total effect between the HRV measure of HF Power and SymptoMScreen (β = −0.5, 95 % CI [−0.87, −0.12], p = 0.0124). The effect of HF Power on K10 (path a in Fig. 1, β = −0.30, p = 0.003) as well as the effect of K10 on SymptoMScreen (path b in Fig. 1, β = 1.52, p = 0.0002) were both significant. Notably, K10 was found to mediate the relationship between HF Power and SymptoMScreen (β = −0.46, 95% CI [−0.79, −0.15], p < 0.05). Given the direct effect of HF Power on SymptomMScreen was not significant (β = −0.04, 95% CI [−0.36, −0.28], p = 0.79), we concluded that K10 fully mediated this relationship.
To summarize, the mediation analysis showed that lower HF Power indirectly influenced higher SymptoMScreen scores via K10 mediation.
Then, we repeated the mediation analysis using LF Power and LF/HF ratio as measures of HRV and the mediation models were not significant (p > 0.05).
DiscussionOur findings support the notion that individuals with MS tend to have lower HRV compared to healthy individuals, which is consistent with previous findings (Damla et al., 2018; Gerasimova-Meigal et al., 2021; Studer et al., 2017). Particularly, we found that the participants with MS had significantly lower HF Power and RMSSD values, and a significantly higher LF/HF ratio compared to healthy controls, indicating reduced parasympathetic activity, and altered sympathovagal balance, which may cause a decreased resiliency to stress.
Moreover, within patients, we found that reduced resting-state HRV was associated with higher emotional distress. These findings are also consistent with previous research demonstrating a negative association between physiological measures of stress (e.g., allostatic load index Waliszewska-Prosół et al., 2022) and vagal-mediated HRV indicators, such as RMSSD and HF-HRV (Thayer et al., 2010). Low resting vagal tone has been associated with decreased emotional reactivity, attentional and inhibitory control, and impaired emotional regulation, ultimately rendering individuals more susceptible to stress (Browning et al., 2017). Furthermore, people with MS may potentially have damage to the pathways responsible for controlling vagal outflow (Gold & Heesen, 2007; Pintér et al., 2015), which could be additional factors leading to a decrease in vagal tone, and consequently, make individuals more susceptible to distress.
Our study provides novel evidence in support of the interconnection between HRV, distress, and symptom burden in people with MS. The results from the mediation analysis are in support of our hypothesis that ANS dysfunction, particularly in the parasympathetic branch, affects how patients experience symptom burden through emotional distress. Similarly, based on prior research in various neurological and non-neurological conditions, it is known that distress can play an important role in the relationship between parasympathetic activation and symptom severity (Morree et al., 2013; Sharif et al., 2018; Ziegler, 2004). Specifically, our findings suggest that patients with low HF power measurements (lower vagal activation) and elevated distress levels may experience an increased burden of MS symptoms. This supports the notion that distress can influence changes in ANS functioning (Kim et al., 2018; Morree et al., 2013; Ziegler, 2004). In turn, this could exacerbate inflammation in individuals with MS (Garis et al., 2022; Grippo & Scotti, 2013; Liu et al., 2017; Williams et al., 2019), ultimately contributing to the experience of symptom burden. In conclusion, it is unlikely that ANS function directly impacts symptom burden, but it is likely that ANS function is dependent on the level of distress experienced by the individual. It is important to include parasympathetic HRV indicators in the assessment of conditions such as MS while also taking into account other factors like emotional distress (Garis et al., 2022).
The rise of telemedicine has increased the demand for remote monitoring technologies capable of collecting physiological data remotely and on a larger scale through self-assessment (Block et al., 2022; Hilty et al., 2022). For consistency, especially in remote monitoring applications, HR recordings should be done under controlled conditions in a specific body position. Our study confirms the feasibility of using commercially available and portable HR monitoring devices for self-administration, opening up the possibility of adopting HRV as a metric for large-scale at-home self-measurement (Stone et al., 2021). However, we found that in some cases the recorded HR data were not usable, emphasizing the need for developing optimized methods for proper sensor placement to ensure a full data capture even in a remote fashion.
This study was not without its limitations, as first and foremost, the sample size was relatively small, and follow-up studies are likely necessary to support generalizability of the results. Second, the HRV recordings were taken at a single time-point and could not capture changes in HRV over time, which may be more informative. To address these limitations, future studies should include a larger sample of individuals with MS and examine the effects of HRV over time. Larger studies would also be needed to assess whether symptomatic medications and DMTs have an impact on HRV, especially those that have known effects on HR, such as S1P modulators (Constantinescu et al., 2022; Findling et al., 2020).
ConclusionsThese findings suggest that parasympathetic autonomic dysfunction (lower value of HF power) occurs more frequently in people with MS who experience greater symptom burden, and that emotional distress is an important underlying factor mediating this relationship. Moreover, HRV may be a useful marker for monitoring emotional distress in individuals with MS and concomitantly serve as a potential target for interventions. Future studies should focus on examining the effects of HRV-based interventions targeting MS symptoms management by reducing emotional distress, and the use of HRV as a clinical management tool to guide daily functioning and minimizing symptom burden in patients with chronic neurologic conditions.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.