Primary Care is the first point of contact for most patients after the onset of symptoms of inflammatory bowel disease (IBD). Establishing an initial diagnostic process based on compatible symptoms and agreed criteria and referral pathways, depending on the degree of suspicion and the patient's situation, can reduce diagnostic delays. Once the patient is referred to the Digestive specialist and the diagnosis of IBD is established, a treatment and follow-up plan is structured. The management of the patient must be shared with the participation of the family practitioners in the diagnosis and treatment of concomitant or intercurrent pathologies, the recognition of flare-ups or complications (of IBD or treatments), education tasks or adherence control.
With the purpose of developing a comprehensive guide on the management of IBD aimed at Primary Care doctors, we have developed this positioning document collaboratively between the Spanish Society of Primary Care Physicians (SEMERGEN) and the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU).
Atención Primaria es el primer punto de contacto de la mayoría de los pacientes tras el inicio de los síntomas de una enfermedad inflamatoria intestinal (EII). Establecer un proceso diagnóstico inicial ante síntomas compatibles y unos criterios y vías de derivación pactadas, en función del grado de sospecha y de la situación del paciente, pueden disminuir los retrasos diagnósticos. Una vez derivado el paciente al especialista de Digestivo y establecido el diagnóstico de EII se estructura un plan de tratamiento y seguimiento. El manejo del paciente debe ser compartido con la participación del médico de familia en el diagnóstico y tratamiento de las patologías concomitantes o intercurrentes, el reconocimiento de los brotes o de las complicaciones (de la EII o de los tratamientos), las tareas de educación del paciente o el control de la adherencia.
Con el objetivo de realizar una guía integral sobre el manejo de la EII dirigida a médicos de Atención Primaria se ha elaborado este documento de posicionamiento colaborativo entre la Sociedad Española de Médicos de Atención Primaria (SEMERGEN) y el Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU).
Ulcerative colitis (UC) and Crohn's disease (CD) are immune-mediated inflammatory diseases of unknown origin that develop in genetically predisposed individuals and in which the role of the microbiota is increasingly relevant. Multiple studies have described a progressive increase in incidence, with geographical differences, which has been linked to westernisation, industrialisation and lifestyle changes.1 The incidence of inflammatory bowel disease (IBD) in Spain in 2017 was 16.2 cases per 100,000 population, with an increasing prevalence of around 0.5% of the population.2
After the onset of symptoms, primary care (PC) is the first point of contact for most patients, and general practitioners (GPs) are responsible for initiating the diagnostic process and deciding on referral to a gastroenterology specialist to complete the investigations and confirm the diagnosis. It should be noted that, despite the increase in the incidence of IBD, the estimated number of patients with IBD per GP (calculating the number of GPs obtained from the Ministry of Health website and the prevalence of IBD) during their professional life will be between five and eight.3 If we compare it with other gastrointestinal disorders with similar initial symptoms which are much more prevalent, such as irritable bowel syndrome, coeliac disease, lactose intolerance or dyspepsia, IBD remains a rare disease in PC.
Since there are no pathognomonic signs/symptoms or analytical determinations for IBD, we need to develop and validate algorithms or screening tools in patients with symptoms compatible or suggestive of IBD, incorporating faecal calprotectin (FC) into routine clinical practice.4 There is a need to establish agreed criteria and referral pathways (normal or priority) based on the degree of suspicion and the patient's situation to avoid or reduce diagnostic delays.
Once the patient has been referred to a gastroenterology specialist and the IBD diagnosis has been made, a treatment and follow-up plan is structured. Over the past 20 years, multidisciplinary IBD units have been set up in most hospitals and an IBD Unit Certification (IUC) programme5 has been established to try to standardise and improve the standard of care. The units, which include nurses, surgeons and radiologists specialised in IBD, have not taken primary care into account and the coordination and communication between primary and specialised care is suboptimal, as reflected in two studies carried out in our setting.6,7 In the management of IBD there is a tendency towards "hospital-centred care", in which many of the concomitant and intercurrent disorders and comorbidities, which should be treated in PC, end up being treated in IBD units or referred to other hospital departments.8 Referral from primary care does not transfer all patient care to the specialist3; rather, shared care should be provided by defining the care aspects of each speciality to achieve better outcomes.9
GPs have a comprehensive view of the patient and should manage concomitant or intercurrent disorders, detect symptoms suggestive of extraintestinal manifestations related to IBD, be conversant with the treatments used in patients with IBD and the possible side effects, and recognise flare-ups of activity and the treatment thereof in particular cases. They also play a fundamental role in patient education tasks, particularly at the onset of the disease, in monitoring adherence to treatment or in managing psychological disorders, among other problems associated with IBD. However, in a study on the degree of knowledge and comfort in the management of patients with IBD by GPs, the result was suboptimal: 37% felt uncomfortable in general with these patients, a figure that increased to 71% and 91% with the management of immunomodulators or biologics respectively; in addition, 70% would like to have support tools.10 In the review carried out to make these recommendations, no diagnostic guidelines and action plans for IBD designed specifically for PC were found, unlike other diseases, such as asthma or diabetes.3
A survey of 222 primary care physicians and 157 IBD specialists on the management of patients with IBD was recently published.7 Only 34% and 44% respectively described the relationship between the two groups as good; the average level of satisfaction of each speciality with respect to the other was 5 out of 10, and the perception of support and assistance between groups did not reach 5. Additionally, although IBD specialists believed that the importance of PC in diagnosis was 9 out of 10, only 30% of GPs had protocols or consensus on diagnosis, referral and treatment. The relationship between PC and Gastroenterology/IBD units is heterogeneous owing to structural, organisational and budgetary inequalities in our setting, as well as to the lack of protocols and performance standards. There are different degrees of integration, probably related to personal or hospital/health centre initiatives. Coordination and communication between the GP and the IBD specialist should be improved through two-way information links and pathways, training programmes, adapted protocols and systems for accessing IBD units. The way to establish this relationship needs to be agreed on between the different teams and adapted to each circumstance.
With the aim of producing a comprehensive guide on the management of IBD aimed at primary care physicians, this positioning statement has been prepared collaboratively by the Sociedad Española de Médicos de Atención Primaria (SEMERGEN) [Spanish Society of Primary Care Physicians] and the Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) [Spanish Working Group on Crohn's Disease and Ulcerative Colitis].
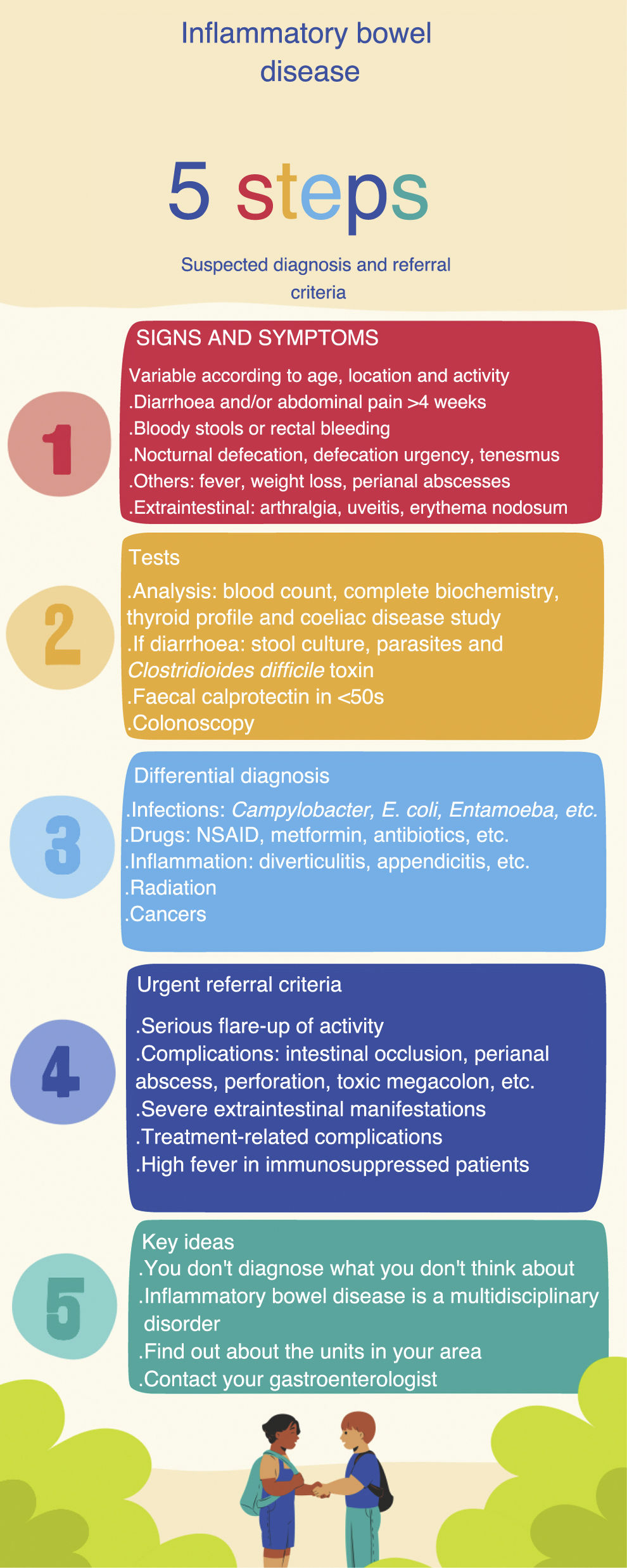
Block 1. Diagnosis and referral of IBD patientsHow can we improve early diagnosis?The diagnostic period comprises three intervals: from the onset of symptoms to the first medical visit in PC; from the first visit to referral to the gastroenterologist; and from referral to the diagnosis of IBD.11,12 The time to diagnosis may be delayed due to the delay in the patient's request for a primary care consultation, prolonged response times due to waiting lists, and difficulty in making a differential diagnosis with other conditions, such as functional disorders or gastrointestinal infectious diseases. The mean time to diagnosis in our setting was 3.5 months (5.6 in CD and 2.7 in UC).2 These data are similar to those from an Italian study from 2017, with a diagnostic delay of 3 months (7 months for CD and 2 months for UC), but with the observation that in up to 14% of patients the diagnosis time was over 24 months.13 A recent systematic review on the time to diagnosis confirmed that the delay was longer in CD than in UC, sometimes estimated at one to two years, possibly because in UC the location is limited to the colon and patients who experience rectal bleeding are more likely to seek medical attention.12
Reducing the time of diagnosis of IBD will allow us to diagnose the patient in earlier stages of the disease, with less inflammation and a better response to treatment.14 It is difficult to determine what an acceptable diagnostic time is, as it will depend on each patient and the clinical situation. The time to diagnosis is highly dependent on the age of the patient, the type of IBD, the extent, the disease activity, and the presenting symptoms. Lack of recognition of symptoms by the physician and the attribution of those symptoms to more common processes, such as infectious diseases or irritable bowel syndrome, can delay the start of tests and referral to a specialist. Response times are defined as the time patients wait to be seen by a specialist or to have additional tests performed. The interval from referral by the primary care physician to the gastroenterologist is generally quantified in months, and is of special relevance, since it has been shown to be the interval with the greatest influence on the time of diagnosis.11 When a primary care physician suspects IBD, this should reduce response times thanks to early and priority referral to a specialist and the performing of diagnostic tests by the gastroenterologist, also on a priority basis. To achieve this, it is necessary to have formats such as infographics, short guidelines, checklists or apps available in the PC clinic where the suspicious signs and symptoms, the tests to be performed and criteria for early referral are listed schematically.3 The systematisation of these tools can help both primary care physicians and specialists to improve patient diagnosis (Fig. 1).
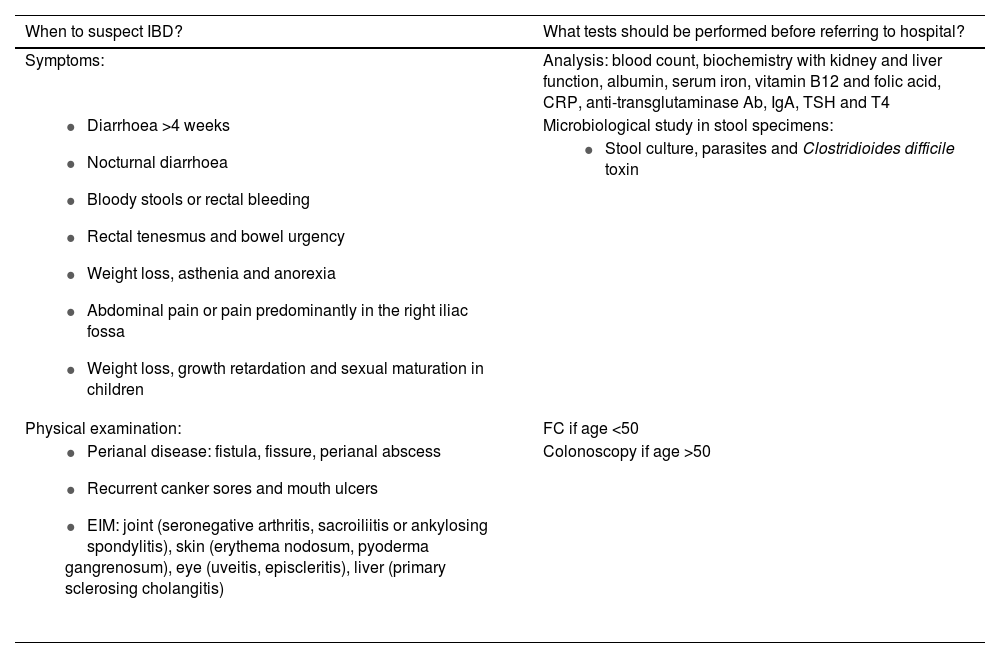
Diagnostic processGastrointestinal symptoms (diarrhoea, abdominal pain or rectal bleeding) are not specific to IBD and occur in other more prevalent diseases, such as irritable bowel syndrome, coeliac disease, lactose intolerance or benign anal disorders (fissures and haemorrhoids). IBD can present in different ways, and should be suspected when some of the signs or symptoms listed in Table 1 are chronic or recurrent.11 Acute symptoms do not exclude IBD, but we need to rule out other causes. In medical records, inquiries should be made about associated diseases, the use of drugs that can cause symptoms such as diarrhoea (for example, non-steroidal anti-inflammatory drugs [NSAID], antibiotics, metformin, colchicine), drug use (alcohol, cocaine), travel to areas endemic for parasitosis, and the possibility of food poisoning or infections.15 Characteristics of patients with UC are bloody diarrhoea (mixed with the stools), stools with mucus, pus and blood, tenesmus, and defecation urgency. Symptoms of CD are more heterogeneous, but usually include diarrhoea lasting more than four weeks with abdominal pain and weight loss. In ileal CD, diarrhoea is not usually bloody and tenesmus and urgency will be less marked. The pain is referred to the area of the right iliac fossa, and occasionally a mass effect can be palpated during abdominal examination. In colonic CD, the presentation is similar to UC. There are patients who present a general syndrome, with weight loss and systemic involvement, with anaemia or anorexia.11
Symptoms of suspected IBD and initial tests.
| When to suspect IBD? | What tests should be performed before referring to hospital? |
|---|---|
| Symptoms: | Analysis: blood count, biochemistry with kidney and liver function, albumin, serum iron, vitamin B12 and folic acid, CRP, anti-transglutaminase Ab, IgA, TSH and T4 |
| Microbiological study in stool specimens: |
| |
| Physical examination: | FC if age <50 |
| Colonoscopy if age >50 |
CRP: C-reactive protein; EIM: extraintestinal manifestations; FC: faecal calprotectin.
Prepared by the authors, adapted from Martín-de-Carpi et al.11
The initial study from PC should include an analysis with a complete blood count, biochemistry, C-reactive protein (CRP), thyroid profile and a coeliac disease study with anti-transglutaminase and IgA antibodies. If the symptoms are diarrhoea, infectious aetiology should be ruled out with a microbiological study in stool specimens (Table 1), and in the case of rectal symptoms, infectious proctitis due to sexually transmitted diseases should be considered.
Utility of faecal calprotectin in the diagnosis of IBDBased on GETECCU recommendations, FC is a good marker of intestinal inflammation. In this group of patients, it could differentiate between functional and organic disease, making it possible to avoid referrals and unnecessary colonoscopies.4 The value of FC for distinguishing between functional and organic gastrointestinal symptoms has been analysed in numerous studies. A meta-analysis that included 2475 patients determined a sensitivity and specificity of 83% and 84% respectively for differentiating organic disease from functional disease.16 The main drawback of FC in this context is its low accuracy in detecting colorectal cancer (CRC). For this reason, in a study population at risk for CRC (for example, patients aged >50 or with a family history of CRC) FC will not be the appropriate test and they will be candidates for investigation by colonoscopy. It is recommended to request FC in the case of clinical suspicion in patients aged <50 with no family history of CRC. A recent publication reported that the detection of high FC levels by PC is associated with a shorter time to diagnosis of IBD, compared to cases where FC is not determined.17 It is important to learn aspects of how to collect the stool sample and know how to interpret the results (Table 2). The FC marker is not specific to IBD and may be elevated in other inflammatory situations, such as diverticulitis or infectious colitis and in patients on treatment with NSAID (as they can cause gastrointestinal lesions) or proton pump inhibitors. Discontinuing NSAID two weeks prior to FC determination is recommended.4 Unfortunately, this marker is not available in all primary healthcare centres. A recent survey of primary care physicians in our area found that one third did not have access.7 We believe that FC should be a test available in primary care centres as an aid to diagnosis in those patients with symptoms, signs and/or suspicious analytical results.
Interpretation of FC determination in the differential diagnosis of gastrointestinal symptoms.
| FC <100 µg/g: inflammation unlikely, not suggestive of IBD, invasive tests not indicated |
| FC 100−150 µg/g: suggestive of low-level inflammation, interpret with caution, consider alternative conditions, repeat in 4–8 weeks |
| FC > 150 µg/g: suggestive of inflammation, request referral or colonoscopy |
FC: faecal calprotectin: IBD: inflammatory bowel disease.
Prepared by the authors, adapted from Guardiola et al.4
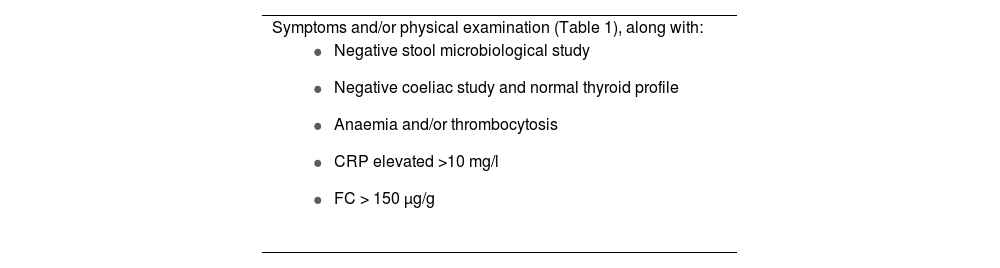
The definitive diagnosis of IBD will be based on clinical and physical examination data, analysis and endoscopy results, pathology reports and imaging tests. However, early diagnosis of IBD will be a determining factor in the response to treatment and requires a high degree of suspicion to begin the diagnostic process.18 To increase the PC physician's level of diagnostic suspicion and improve early referral to the gastroenterologist, several simple tools have been proposed, including alarm signals, allowing for a timelier referral and a quicker diagnosis. The study by Danese et al.19 was based on asking patients with recently diagnosed CD or irritable bowel syndrome and a healthy control population about their gastrointestinal symptoms. After multivariate analysis, eight symptoms, known as Red Flags or alarm symptoms, were detected for identifying patients with CD: presence of complex fistulas or perianal abscesses; history of a first-degree relative with IBD; nocturnal diarrhoea; weight loss or fever in the last three months; abdominal pain for more than three months; and absence of rectal urgency. Each symptom can score 2–5 points, and a Red Flags index greater than or equal to 8 suggests a high probability of the patient having CD. A recently published Italian study attempted to validate this index with 112 patients referred from PC, with much lower sensitivity and specificity (50% and 58% respectively). By adding the determination of FC above 250 µg/g to the Red Flags index, sensitivity and specificity reached 100% and 72% respectively, with a negative predictive value of 100%.20 Another study relating to the screening of cases at risk of IBD is the IBD-REFEER, which, unlike the previous study, includes both CD and UC, and also has the advantage of being applicable to the paediatric population. It uses major and minor clinical criteria (one of them being elevated FC). If patients have one symptom from the major criteria or two symptoms from the minor criteria, they should be referred to Gastroenterology to rule out IBD. This index has a sensitivity and specificity of 98% and 96% respectively.21 Pending validation indices in our setting, we suggest the referral criteria for patients with suspected new-onset IBD shown in Table 3.
Criteria for referral of patients with suspected IBD.
| Symptoms and/or physical examination (Table 1), along with: |
|
CRP: C-reactive protein; FC: faecal calprotectin.
Prepared by the authors.
Not only must GPs make an appropriate referral when they suspect IBD, they should also recognise signs and symptoms that can identify disease recurrence or possible associated complications in a patient already diagnosed with IBD. It should be noted that when patients with IBD come to primary care, they do not always have a relapse of the IBD, so it is essential to perform a differential diagnosis with other diseases, such as gastrointestinal infections, disease of the anal canal, functional gastrointestinal problems, adverse drug effects, concomitant gastrointestinal diseases or intercurrent disease. In the event of increased bowel movement frequency, decreased stool consistency, abdominal pain or the appearance of blood in the stools in a patient with IBD, a full medical history should be obtained, a physical examination carried out, the course of the disease, treatment and comorbidities reviewed, an analysis performed with biomarkers, such as FC and CRP, and a stool culture, including the detection of Clostridioides difficile, even in the absence of prior antibiotic therapy.22
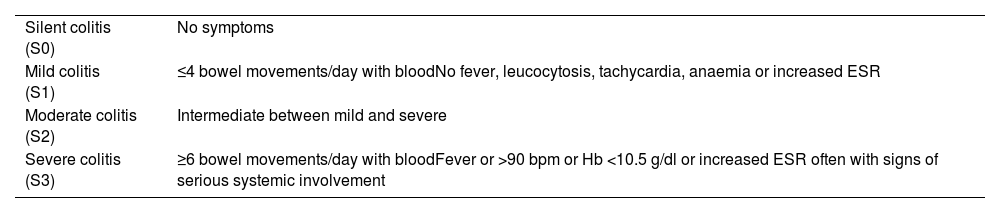
If a flare-up of activity or a complication is suspected, the severity should be assessed to decide the course of action to follow and the treatment. There are different scales for assessing the severity of the flare-up, such as the Montreal classification in UC (Table 4) and the Harvey Bradshaw index in CD (Table 5). Depending on the severity and availability of assessment by Gastroenterology, the GP can treat the flare-up, start treatment in the meantime until the patient is assessed by their specialist, refer to the IBD unit/specialist for them to decide on a change of treatment, or refer the patient directly to the Accident and Emergency department.
Montreal classification for the severity of ulcerative colitis.23
| Silent colitis (S0) | No symptoms |
| Mild colitis (S1) | ≤4 bowel movements/day with bloodNo fever, leucocytosis, tachycardia, anaemia or increased ESR |
| Moderate colitis (S2) | Intermediate between mild and severe |
| Severe colitis (S3) | ≥6 bowel movements/day with bloodFever or >90 bpm or Hb <10.5 g/dl or increased ESR often with signs of serious systemic involvement |
bpm: beats per minute; ESR: erythrocyte sedimentation rate; Hb: haemoglobin.
Harvey Bradshaw Index for Crohn's disease.24
| Variables | Points |
|---|---|
| 1. General condition | 0 Very well1 Slightly below par2 Poor3 Very poor4 Terrible |
| 2. Abdominal pain | 0 None1 Mild2 Moderate3 Severe |
| 3. Number of liquid stools per day (n points) | |
| Abdominal mass | 0 None1 Dubious2 Definite3 Definite and tender |
| Complications | 1 Arthralgia1 Uveitis1 Erythema nodosum1 Aphthous ulcers1 Pyoderma gangrenosum1 Anal fistula1 Other fistulas1 Abscesses |
Score: <6 mild; 6–12 moderate; >12 severe.
Patients with a severe flare-up of UC or CD, complications of CD, such as intestinal occlusion or perianal abscess, severe extraintestinal manifestations (EIM), such as pyoderma gangrenosum or uveitis, or suspected adverse effects or complications associated with treatments, such as high fever in immunosuppressed patients, should be referred urgently (to the IBD unit if open access is available, or to Accident and Emergency). Patients on treatment with an immunosuppressive drug, a biologic or a JAK-kinase inhibitor who develop activity due to loss of treatment efficacy or an associated complication should be assessed by an IBD specialist to decide whether to intensify or change the treatment.
In patients with UC who are being treated with mesalazine and have a mild/moderate flare-up, treatment adherence and the prescribed dose should be checked. In a flare-up of ulcerative proctitis, treatment can be started or optimised with a 1 g mesalazine suppository daily, and if there is no response, oral mesalazine can be added. In left UC and pancolitis, the dose of oral and rectal mesalazine can be optimised25,26 (in many cases, flare-ups coincide with a lack of adherence to the prescribed treatment). If no response is achieved, or if the patient was on high doses of mesalazine, they should be referred to Gastroenterology. If there is a delay with the clinic appointment, topical oral corticosteroids, such as beclomethasone dipropionate, or oral prednisone, may be added, depending on the severity.27 In a mild/moderate flare-up of CD, treatment can be started with oral budesonide in ileal or ileocolic involvement, or with oral prednisone in colonic forms28,29 if there is a delay in assessment by the specialist (see the section "Treatments used in inflammatory bowel disease, Corticosteroids"). Lastly, in patients with CD who consult their doctor for pain or perianal suppuration, an examination of the anal, perianal and perineal region should be performed, describing the number of external orifices and their location, establishing whether there is suppuration and checking for abscesses (which is a reason for referral to Accident and Emergency for drainage and subsequent contact with the unit/specialist). In the case of an uncomplicated fistula, treatment can be started with metronidazole 500 mg/8−12 h or ciprofloxacin 500 mg/12 h and the patient can be referred to a specialist for the diagnostic process and treatment of the fistula.30,31
How to refer patients from primary care to the IBD unit?Referral modelsOnce the primary care physician suspects IBD, the patient may be referred to a specialist, who will request a colonoscopy (if there is direct access to endoscopies, the colonoscopy may be requested and the patient referred to the specialist once the diagnosis has been confirmed). The form of referral from PC to Gastroenterology is highly heterogeneous, depending on where the PC physician works. There is no uniformity in accessibility to testing, in the way of establishing contact with specialised care, or in waiting times. All of this creates a barrier to the referral and follow-up of these patients, as well as significant dissatisfaction on the part of the primary care practitioners.32
Open access to specialised care is preferred by both patients with IBD and primary care physicians, and it has been found that personal acquaintance between primary care physicians and gastroenterologists increases the quality of care. Both these areas should therefore be objectives for improvement, as it is essential to acquainted, at least nominally, with the IBD gastroenterologists and how to access the region's IBD reference units.
In the GETECCU IUC programme, one of the indicators for the accreditation of the units consists of having a priority appointment circuit from PC to the IBD units for both recently diagnosed patients and flare-ups of activity.33
CommunicationIn the computer age, e-consultation is a good option. The primary care physician makes an e-consultation addressed to the gastroenterologist through the patient's electronic medical records and the recipient evaluates the case, determining the need for further information or tests by the primary care physician, establishing the diagnostic approach, referral to the IBD unit, consultation with other specialities, or the start of a particular treatment. All of this is done electronically or by arranging a face-to-face appointment for the patient within a time frame appropriate to the severity of the disease.34 To achieve this, it is essential that GPs submit a summary of the case, which is easy to read, and which allows patients to be filtered and prioritised appropriately. If e-consultation is not available, communication can be established by email or telephone, although this method is much more complicated due to accessibility issues. Lastly, contact can be made through the usual referral circuits, using the official form with normal or priority referral, although this involves the familiar problems of lack of interaction between healthcare professionals, excessive delays, inappropriate referrals, poor patient control or healthcare professional dissatisfaction, both in primary care and specialised care.
Block 2. Management of IBD patients. Role of primary careTreatments used in inflammatory bowel diseaseIt is important for the GP to be familiar with the treatments used in patients with IBD and to recognise the possible side effects. Treatment of IBD is personalised and depends on several factors, including location, disease severity, age and comorbidities of the patient, as well as previous response to specific therapies.
SalicylatesThere are different formulations of mesalazine available, both for oral and rectal use. Clinical trials have not demonstrated differences in efficacy or safety with the different oral formulations, so patient preferences should be taken into account when choosing tablets or microgranules.35 The appropriate oral mesalazine dose for induction of remission in patients with mild/moderate UC flare-up is 2.4 g/day, with >3 g/day, in a single daily dose, being optimal.36 Topical mesalazine may be administered via suppositories, foam or rectal suspension (enemas). The choice of one or another depends on the extent of the disease: the rectal suspension can reach up to the splenic flexure, the foam reaches the sigmoid colon/descending colon, while suppositories reach no further than the rectum. In ulcerative proctitis the first-line indication in the flare-up is a suppository of 1 g/24 h, before going to bed, possibly increasing to 3 g per week for maintenance, depending on tolerance.26 For better adherence, there are explanatory videos about the use of topical mesalazine on the website (GEducaEII) https://www.youtube.com/watch?v=oWyfExo8Zj8&t=271s.
Mesalazine is generally well tolerated. The most frequently reported adverse effects are mild, and consist of abdominal pain, nausea and vomiting. There are no differences in safety between the use of low or high doses of mesalazine, and annual monitoring of renal function and liver enzymes is recommended.37
CorticosteroidsThere are two types of corticosteroids: systemic oral corticosteroids, with high bioavailability and significant toxicity; and topical oral corticosteroids, with low systemic availability. The usual dose for the management of a moderate IBD flare-up is 1 mg/kg/day (or 40−60 mg/day) of oral prednisone (or equivalent). Weaning off systemic steroids, once remission is achieved, is usually done reducing by 10 mg each week until reaching 20 mg/day, and then by 5 mg a week. Steroids should not be stopped abruptly in patients who have been on treatment for more than 7–10 days. Topical oral corticosteroids include budesonide, indicated for inducing remission of mild/moderate flare-ups of ileal CD, at doses of 9 mg/day in a single dose and with a progressive decrease once remission is achieved, and beclomethasone dipropionate, indicated in mild/moderate flare-ups of UC, at a dose of 5 mg per day for one month (sometimes they can be used 10 mg/day for one month and 5 mg for a further 2–4 weeks).28,29 Lack of response to full-dose corticosteroids is known as being refractory to corticosteroids. Corticosteroid dependence occurs when corticosteroids cannot be reduced to under 10 mg/day of prednisone or 3 mg/day of budesonide without recurrence of symptoms, in the first three months of treatment, or when a clinical relapse occurs within three months of discontinuing corticosteroids.38 In these cases, an alternative treatment must be sought to avoid the excessive use of corticosteroids, and they should never be considered as maintenance therapy in patients with IBD, given that they have a significant number of side effects, including high blood pressure, water retention, moon face, acne, hirsutism, stretch marks, glucose intolerance, insomnia, emotional lability, infections, cataracts, glaucoma, adrenal atrophy, growth retardation in children, and loss of bone mineral density. Monitoring of blood glucose and blood pressure levels is recommended in patients with previous diabetes mellitus and hypertension. Special attention should be paid to patients with underlying psychiatric disorders, in case steroids unbalance them. The effect of steroids on bone mineral density should be monitored, and it is recommended that they always be co-administered with calcium and vitamin D supplements.39
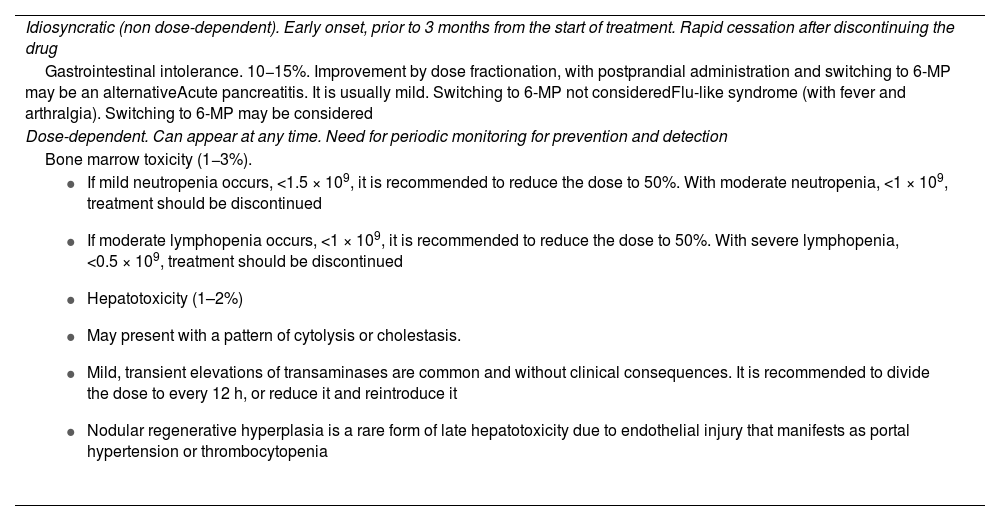
Non-selective immunosuppressantsThiopurine immunosuppressants: azathioprine and mercaptopurine. These are indicated in corticosteroid dependence, and concomitantly with anti-TNF drugs, to prevent immunogenicity. The dose of azathioprine is 2.5−3 mg/kg/day in a single dose (which can be divided if there is poor tolerance). Exposure to sunlight should be limited and high sun protection should be used routinely due to its association with non-melanoma skin cancer. From the start of treatment, a slight decrease in the number of leucocytes (lymphocytes) may occur and the mean corpuscular volume may increase. Adverse effects are very common and lead to withdrawal in 15%–20% of patients (Table 6). Recommendations for analyses during follow-up include monitoring with blood count and liver profile at two weeks, one month, then every three months in the first year, and every four to six months for the duration of the treatment period.40
Considerations on the adverse effects of thiopurine immunosuppressants.
| Idiosyncratic (non dose-dependent). Early onset, prior to 3 months from the start of treatment. Rapid cessation after discontinuing the drug |
| Gastrointestinal intolerance. 10−15%. Improvement by dose fractionation, with postprandial administration and switching to 6-MP may be an alternativeAcute pancreatitis. It is usually mild. Switching to 6-MP not consideredFlu-like syndrome (with fever and arthralgia). Switching to 6-MP may be considered |
| Dose-dependent. Can appear at any time. Need for periodic monitoring for prevention and detection |
| Bone marrow toxicity (1−3%). |
|
6-MP: mercaptopurine.
Methotrexate. This can be used subcutaneously on a weekly basis in steroid-dependent CD or in combination with anti-TNF drugs. It is used much less often than thiopurines. Adverse effects include increased infections, risk of teratogenicity, which renders it contraindicated in women and men who want to have children (it should be withdrawn three months in advance if there is a wish for pregnancy), hepatotoxicity and lung toxicity, with risk of pulmonary fibrosis. For monitoring, a monthly blood tests are recommended for the first three months and subsequently every three to four months, with a liver elastography every two years from the fifth year on.29
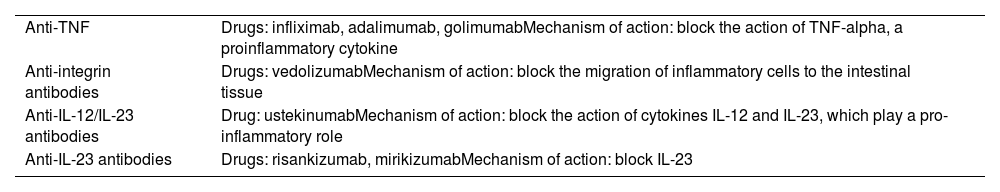
Biologic drugs: monoclonal antibodiesThese are indicated for the induction and maintenance of remission, corticosteroid resistance, corticosteroid dependence, perianal disease or extraintestinal manifestations, especially when other approaches have not been sufficient. Biologic drugs are classified according to their mechanism of action (Table 7). Before starting a biologic drug, the vaccination schedule should be updated and tuberculosis screening should be performed (Mantoux and/or Interferon Gamma Release Assay [IGRA]). IGRA screening is recommended every 12–24 months during treatment.
Classification of biologic drugs.
| Anti-TNF | Drugs: infliximab, adalimumab, golimumabMechanism of action: block the action of TNF-alpha, a proinflammatory cytokine |
| Anti-integrin antibodies | Drugs: vedolizumabMechanism of action: block the migration of inflammatory cells to the intestinal tissue |
| Anti-IL-12/IL-23 antibodies | Drug: ustekinumabMechanism of action: block the action of cytokines IL-12 and IL-23, which play a pro-inflammatory role |
| Anti-IL-23 antibodies | Drugs: risankizumab, mirikizumabMechanism of action: block IL-23 |
Anti-TNF: tumour necrosis factor inhibitors; IL: interleukin.
Adverse effects with anti-TNF treatments include infections (especially in combination therapy with immunosuppressants), hypersensitivity reactions after the infusion, injection site reactions in subcutaneous administration, arthralgia, autoimmune hepatitis, and the appearance of paradoxical reactions such as induced psoriasis or lupus-like phenomena. New biologics, such as vedolizumab, ustekinumab, risankizumab and mirikizumab, have provided a better safety profile with mild side effects, such as headache, nausea, arthralgia, pharyngitis, fatigue and pruritus, which rarely lead to discontinuation of treatment. Biologic drugs have no significant drug interactions.41
JAK-kinase inhibitorsThese are small, chemically synthesised molecules that inhibit JAK receptors, which modulate various inflammatory and immune responses. Three JAK inhibitors are currently approved: tofacitinib, filgotinib, and upadacitinib; the first two for UC, and the third for UC and CD. They are indicated for patients with moderate to severe activity with insufficient response, loss of response or intolerance to conventional treatment or a biologic medication. Latent tuberculosis should be ruled out before and during treatment. An increased risk of herpes zoster has been documented, owing to which vaccination with two doses of the inactivated recombinant vaccine Shingrix® is recommended, if possible, before starting treatment. In patients over the age of 65, with high vascular risk or history of cancer, it can be used as an alternative if other biologic therapies fail. During follow-up, patients should be monitored for possible cytopenias, especially lymphopenia and lipid metabolism.42
Drugs for other indications in patients with IBDNSAIDThe use of short, low-dose regimens is safe, with selective COX-2 inhibitors being the least likely to be associated with adverse events. The general recommendation is to avoid long-term use or high doses, especially in patients with active IBD.43
ProbioticsThere is no evidence to support the use of probiotics, either in induction or maintenance of patients with IBD. The only approved indication is for the prophylaxis of pouchitis with a specific probiotic (De Simone formulation) containing a mixture of Lactobacillus, Bifidobacterium and Streptococcus thermophilus.44
Low-molecular-weight heparin. Indicated for prophylaxis of venous thromboembolism in patients admitted with an IBD flare-up and for one month after discharge after major surgery. There is no evidence for thromboprophylaxis in outpatients with active disease.45
AllopurinolThis is a xanthine oxidase inhibitor and increases the toxicity of thiopurines when used concomitantly. If treatment with allopurinol and thiopurines is required, the dose of thiopurines should be reduced to 25−33% of the original dose to avoid the development of serious adverse effects.40
AntibioticsThere are no comparative studies between different antibiotics that allow us to determine which are most appropriate in the context of IBD. There is also no evidence that antibiotic use can trigger a flare-up or pose any additional risk in patients with IBD.
Pregnancy and IBDContraceptionThe safest and most effective contraceptive option is long-acting reversible contraception, which may include a hormonal or non-hormonal intrauterine device or a contraceptive implant. This is preferable to oral contraception, as active inflammation of the bowel, resection or rapid intestinal transit may reduce its effectiveness. If oral contraceptives need to be used, we would use a contraceptive with low doses of oestrogen, given the risk of venous thromboembolism, or a contraceptive that only contains progestogens. There is no contraindication for emergency contraception.46,47
FertilityIBD frequently affects patients of reproductive age; consequently, information on aspects such as fertility, genetic risk or the influence of the disease on pregnancy is important. Women with IBD have the same fertility rate as age-matched controls, although it may decrease in active disease or with a history of previous pelvic surgery. Reduced desire for motherhood due to fear of transmitting the disease or the effect of treatments, lack of sexual desire or dyspareunia may also have an influence.48 Regarding assisted reproductive therapy or egg freezing, there are no data to suggest that IBD medications have any effect, or that the hormones used in treatment act on the disease.49 Children of parents with IBD have a genetic predisposition to develop the disease. If one parent suffers from CD or UC, the probability is 5% and 3% respectively, and if both parents have it, 30%.46
Preconception, pregnancy and breastfeedingPreconception visits and prior counselling are associated with better pregnancy outcomes. It is important for the patient to know that she should be in remission, ideally for three to six months, before conception. This reduces the risk of flare-ups during pregnancy and postpartum, as well as the risks of miscarriage, low birth weight and premature birth.50 In conjunction with the patient's gastroenterologist, a plan should be established for treatment to maintain the disease inactive and that can be taken during pregnancy. The patient needs to be aware of the risks of discontinuing the prescribed medication, and adherence should be reinforced by primary care. Most medications can be used, except methotrexate, which should be discontinued three months before, in both females and males. Corticosteroids would be indicated in the short term in a flare-up. Biologic drugs can be used throughout pregnancy.47 The use of JAK inhibitors is discouraged due to lack of safety studies. In cases where a pregnancy is considered high risk, a multidisciplinary approach should be taken.46 The usual analytical and ultrasound tests of the pregnancy programme will be requested, incorporating disease monitoring parameters, such as FC, and taking into account that patients with IBD have a higher risk of suffering from iron or vitamin B12 deficiency. During pregnancy, the flu, pneumococcus and hepatitis B vaccines are recommended if there are risk factors, and the diphtheria, tetanus and whooping cough vaccines are recommended between weeks 28 and 36 (ideally at week 32). SARS-CoV-2 vaccination is also recommended during pregnancy or breastfeeding.51
Patients may have a vaginal delivery unless they have active perineal disease, in which case a caesarean section is usually recommended to reduce the risk of injury.47 Breastfeeding should be advised, and the same drugs that are permitted during pregnancy may be administered. If the mother has received biologic therapy during pregnancy, live virus vaccines should be avoided during the first 12 months.51
Vaccination in patients with IBDInfection screening and vaccination programmes are part of the quality standards of IBD units.33 It is recommended to assess the immunisation status of IBD patients at the time of diagnosis before starting immunosuppressive treatment (whenever possible) and to administer the appropriate vaccines to avoid preventable infections. In recent decades, there has been an increase in biologic and immunosuppressive therapies, and the recommendations of vaccination guidelines vary depending on the degree of immunosuppression and safety of the vaccines, taking into account that live virus vaccines are contraindicated in patients on immunosuppressive treatment. Unfortunately, vaccination rates are suboptimal.52 The availability of a checklist in a brief format, with precise indications of mandatory and/or recommended vaccinations adapted to whether or not the patient is immunosuppressed, specifying brands, doses and guidelines, can improve prescribing, compliance, patient information and adherence. The record of administered vaccines must be included in the patient's electronic medical records and the vaccination programme must be kept up to date during follow-up. This checklist or vaccination sheet can be drawn up by the IBD unit and given to the patient so they can contact their primary care centre or preventive service (according to the protocol of each IBD unit) for vaccine administration. GETECCU recommendations have recently been published (https://geteccu.org/formacion/documentos-de-posicionamiento) on screening and vaccination in patients with IBD, where all this information can be consulted.51
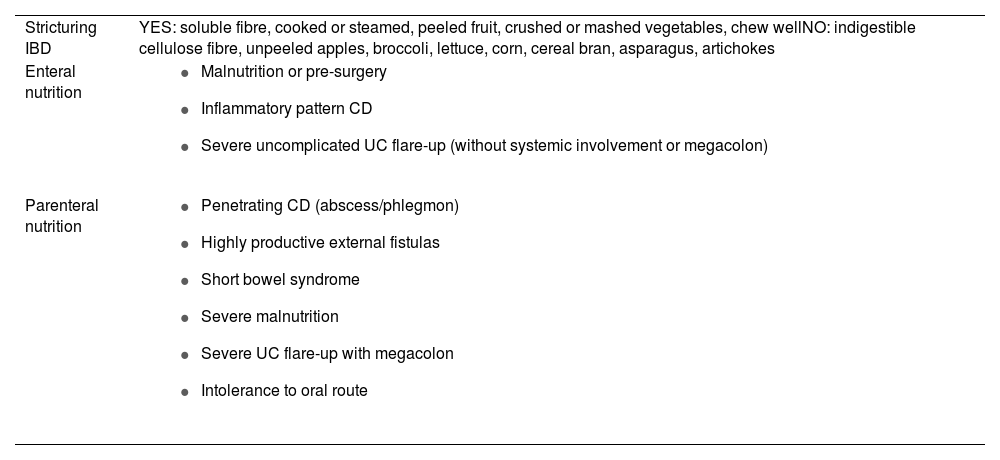
Diet in patients with IBDDiet has been proposed as a possible environmental trigger for IBD in genetically susceptible individuals. Some studies link the development of IBD with diets based on meat, fats and refined sugars. Although the protective role of fibre in the development of IBD is well known, during flare-ups of the disease it is advisable to have a low-residue diet (eliminating insoluble fibre), especially in stricturing forms of CD or in flare-ups of severe or extensive UC. Foods rich in insoluble fibre include whole grain foods, preparations containing cereal bran, and vegetables such as asparagus and artichokes. Soluble fibre contained in fruits and legumes produces less residue and is fermented by the colon flora, owing to which it can cause an increase in abdominal pain and a feeling of flatulence during flare-ups, and should only be limited if it causes such discomfort.53 Patients with CD without a stricturing pattern should not limit dietary fibre. In patients with stricturing CD, it is recommended to modify the texture of foods with fibre by boiling them, blending them, and chewing them well, which will enable better tolerance to a healthy diet (Table 8). In general, it should be recommended that they follow a Mediterranean diet, rich in fresh fruits and vegetables, mono-unsaturated fats, complex carbohydrates and lean fats, and low in ultra-processed foods, which often contain added sugar, excess salt and other food additives.54 Different diet models, depending on whether or not patients are in flare-up, can be consulted on the GETECCU website at https://educainflamatoria.com/dietas-y-nutricion/. There is no consistent evidence to support gluten withdrawal in patients with IBD in the absence of a diagnosis of coeliac disease or suspected gluten sensitivity. IBD patients should only limit dairy consumption during flare-ups if their diarrhoea increases repeatedly. Most patients who are intolerant to milk can tolerate other dairy products, such as yogurt or aged cheeses, which have a much lower lactose content. Restrictive diets should be avoided.54
Recommendations and types of diet according to IBD situations.
| Stricturing IBD | YES: soluble fibre, cooked or steamed, peeled fruit, crushed or mashed vegetables, chew wellNO: indigestible cellulose fibre, unpeeled apples, broccoli, lettuce, corn, cereal bran, asparagus, artichokes |
| Enteral nutrition |
|
| Parenteral nutrition |
|
CD: Crohn's disease; IBD: inflammatory bowel disease; UC: ulcerative colitis.
Prepared by the authors, adapted from AGA recommendations.54
Patients with IBD should be assessed for evidence of malnutrition by assessing signs and symptoms, including unintentional weight loss, oedema and loss of fat and muscle mass. Assessing malnutrition based on protein or albumin levels is not recommended due to their low specificity for nutritional status and high sensitivity due to inflammation. In cases of malnutrition, referral to a dietician or nutrition service for assessment is recommended.
Extraintestinal manifestations and IBD-associated disordersUp to half of patients with IBD have EIM and they are more common in CD, and especially in those with perianal disease and colonic involvement, with an activity parallel to that of the disease itself, except in the case of axial spondyloarthropathy, uveitis, pyoderma gangrenosum and primary sclerosing cholangitis (PSC), which have an independent course.55
Joint manifestations. SpondyloarthropathiesThese are the most common EIM. They belong to the group of spondyloarthritis (SpA); they are divided into axial and peripheral forms, and may appear before or after the diagnosis of IBD.56 It is essential to establish referral criteria, predominantly clinical, between PC, Gastroenterology and Rheumatology (Table 9).57
Criteria for referral to Rheumatology for suspected spondyloarthritis in patients with IBD.
| Chronic lower back pain (lasting more than three months) starting before the age of 45 |
| Inflammatory low back pain, defined as low back pain and four of the following five criteria: |
|
| Peripheral manifestations (arthritis, enthesitis or dactylitis) |
| Sacroiliitis in imaging tests (only if they have already been requested, not essential for screening) |
| HLAB27+ (if available, not essential for screening) |
Prepared by the authors, adapted from Sanz Sanz et al.57
This ranges from inflammatory low back pain, with or without sacroiliitis, to true ankylosing spondylitis. These conditions are less common than peripheral SpA, there are no gender differences, and they follow a course independent of the activity of IBD. Sacroiliitis may be present radiologically in 20%–50% of patients with a low percentage of progression, the typical symptom being inflammatory low back pain, and the majority are HLA B27 negative. Ankylosing spondylitis presents as inflammatory low back pain and morning spinal stiffness lasting more than 30 min, persisting over time (more than three months in duration), and HLA-B27 may be positive in 25%–75% of patients with IBD. Suspicion arises from clinical findings and diagnosis is made by lumbar magnetic resonance imaging. Management should be carried out jointly with Rheumatology. Intensive physiotherapy and short-term NSAID are effective. The use of COX-2 inhibitors may be an alternative. Sulfasalazine and methotrexate are not effective. Anti-TNF agents are the treatment of choice for patients intolerant or refractory to NSAID, and JAK inhibitors may be considered as an alternative.36,55,58
Peripheral spondyloarthritisDiagnosis is clinical and based on signs of inflammation and the exclusion of other specific forms of arthritis. Erosion is usually not present in imaging tests (unlike rheumatoid arthritis and connective tissue diseases). There is a higher prevalence in females and in colonic forms. Two different types have been empirically identified. Type 1, or pauciarticular, affects fewer than five, mainly large joints and asymmetrically; symptoms are usually acute and self-limiting and generally correlate with IBD flare-ups. Type 2, or polyarticular, affects more than five small joints of the upper limbs, with a symmetrical distribution. Symptoms persist for months and usually have a course independent of IBD activity. It usually responds to the treatment for IBD.
Rest, exercise, NSAID and intra-articular corticosteroid injections may control symptoms. Oral corticosteroids may be effective, but at the lowest dose and for the shortest duration possible. Methotrexate, sulfasalazine (the only aminosalicylate indicated) and anti-TNF agents are effective in resistant cases.56
Dermatological manifestationsErythema nodosum is the most common cutaneous EIM. It is characterised by the presence of red or purple subcutaneous nodules, 1–5 cm in size, on the extensor surfaces of limbs; the pretibial region is the most common, and they may be painful. The clinical course is usually associated with flare-ups, and the treatment is that for IBD, in addition to rest and analgesia.59
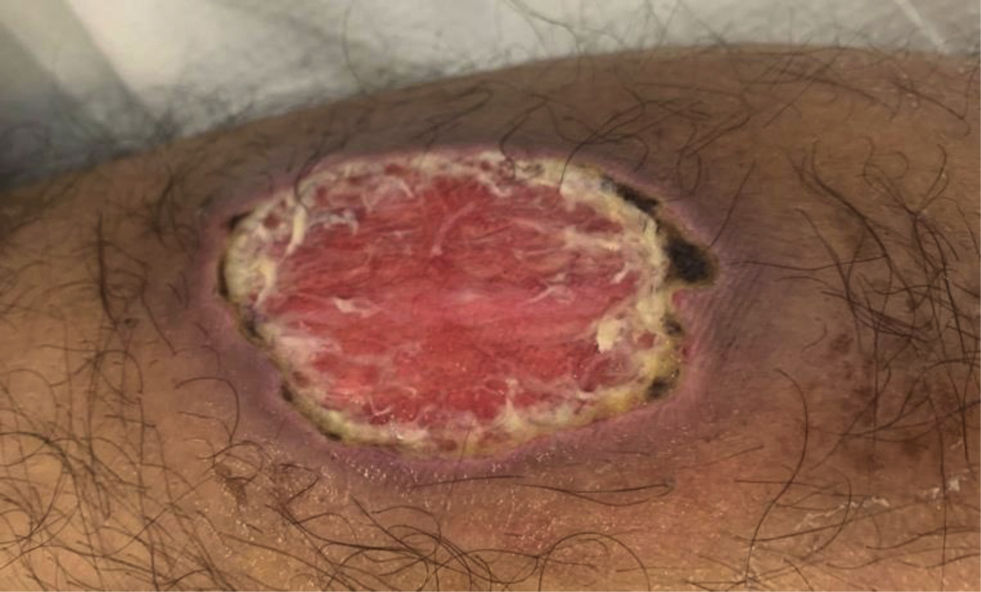
Pyoderma gangrenosum is characterised by the appearance of a pustule or nodule on the skin that quickly transforms into a purple-edged ulcer, which can spread due to a pathergy phenomenon (Fig. 2). It usually appears on the lower limbs or at the edges of the stoma. It may have a course independent of that of IBD, and early referral to dermatology is recommended. Treatment is with topical tacrolimus, corticosteroids or biologic drugs.60
Hidradenitis suppurativa is a disease that affects the folliculopilosebaceous units in different locations, with a variable course, from mild forms with papules, pustules and inflammatory nodules, to severe cases with deep abscesses, cutaneous fistulas and scar formation.61 Patients with IBD (more common in CD) tend to be younger, smokers, and with other associated comorbidities.62 The diagnosis is clinical, and the course is recurrent. In mild stages, topical 15% resorcinol (two applications per day), intralesional corticosteroids (triamcinolone acetonide or betamethasone) and topical clindamycin may be useful. In moderate/severe cases, clindamycin and rifampicin (300 mg of each drug every 12 h for 10 weeks), adalimumab or oral acitretin are indicated. Routine surgical drainage is not recommended. Pain management can include local dry cold, lidocaine 5%, diclofenac gel 1% and abscess drainage.
There are other less common disorders, such as Sweet syndrome and cutaneous metastatic CD.58
Lastly, there are the skin manifestations associated with or induced by the treatments used in IBD. These include non-melanoma skin tumours associated with thiopurine drugs (in these patients it is recommended to limit exposure to the sun and to use sun protection routinely) and anti-TNF-induced eczema and paradoxical psoriasis.59
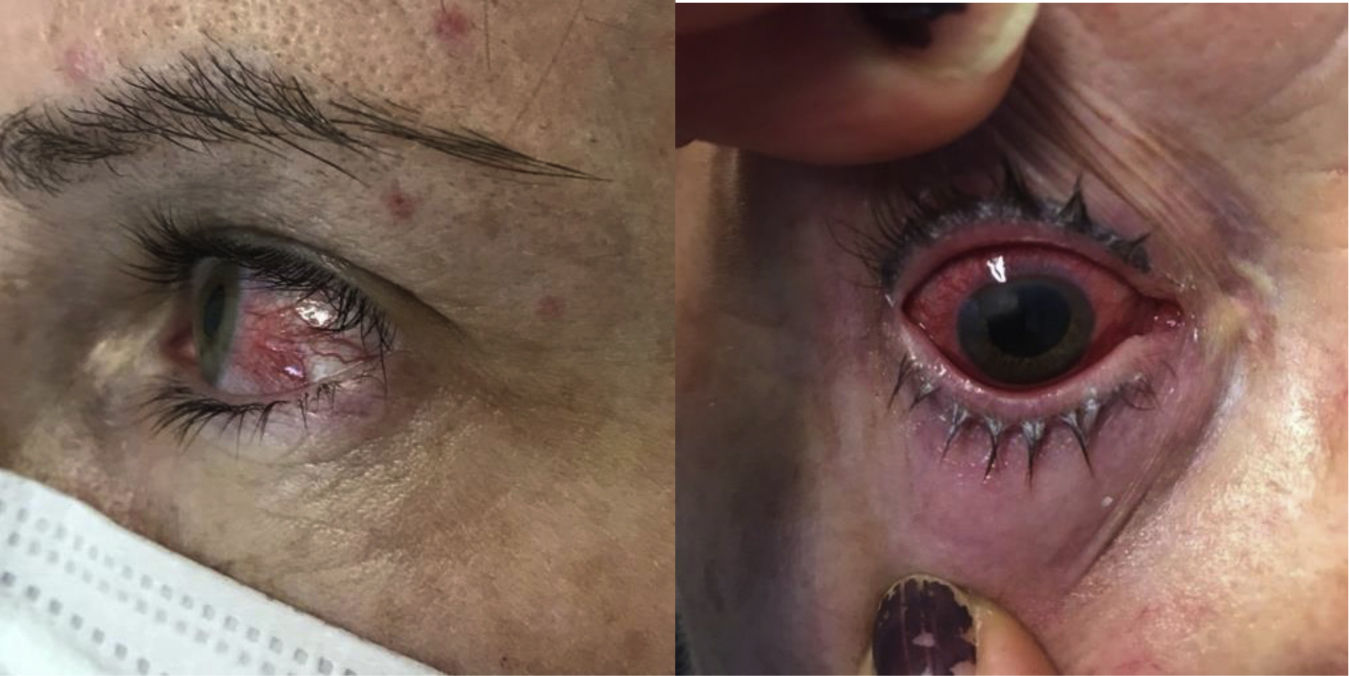
Eye manifestationsThe most common eye EIM are dry eye, blepharitis, episcleritis and anterior uveitis (Fig. 3). Scleritis and intermediate/posterior uveitis are less common, but potentially more serious, and constitute an ophthalmological emergency. Uveitis in CD is usually bilateral, associated with SpA, more common in females, and may present with red eye, eye pain, blurred vision, photophobia and headache. Treatment for uveitis will depend on the type and activity of the IBD. Corticosteroid drops is the initial treatment for anterior uveitis, while intravitreal or systemic corticosteroids should be used for intermediate, posterior or panuveitis. Anti-TNF agents are indicated when eye inflammation persists or there is IBD activity. Complications associated with the use of corticosteroids include cataracts, ocular hypertension and glaucoma.63
Hepatobiliary manifestationsIn patients with IBD, it is common to detect elevated liver enzymes and in 5%–16%, this can be persistent. In these cases, patients should be assessed for viral, autoimmune, metabolic or drug-related causes. Metabolic dysfunction-associated steatotic liver disease is considered the most common and with the highest risk of cardiovascular diseases (CVD), and screening and prevention from primary care is important, regardless of the presence or absence of other classic CVD factors.64
PSC is the most common IBD-specific liver disease (2%), particularly with extensive colonic involvement, and almost 75% of patients with PSC have an associated IBD.65 It is characterised by inflammation, fibrosis and destruction of the bile duct, producing cholestasis, stricture of the bile ducts and liver fibrosis, which can progress to cirrhosis. It is usually asymptomatic in early stages. In advanced stages, it may present with itching, asthenia, jaundice and pain in the right hypochondrium, which may be intermittent and confused with flare-ups of activity. A common finding is a chronic, 3- to 5-fold increase in alkaline phosphatase levels. Diagnosis is made by MR cholangiopancreatography ± liver biopsy. PSC progresses independently of IBD, giving it a worse prognosis. Treatment includes bezafibrate as first line, and cholestyramine resin for biliary pruritus and ursodeoxycholic acid (15−20 mg/kg/day) to improve the liver profile, but these drugs do not modify disease outcome.66 PSC increases the risk of colorectal cancer fourfold in patients with IBD, and an annual screening colonoscopy is recommended. It also increases the risk of gallbladder cancer, and an annual abdominal ultrasound is therefore recommended.55
Drug-induced liver damage can affect 10% of patients treated with methotrexate and 3–15% of patients treated with azathioprine. Azathioprine hepatotoxicity is usually dose-dependent and resolves by reducing or withdrawing the drug, although it can also cause more serious damage, such as veno-occlusive disease, peliosis hepatis and nodular regenerative hyperplasia.67
Cardiovascular and metabolic diseasesChronic inflammation in IBD is considered an independent cardiovascular risk factor and is associated with early atherosclerosis, thrombotic events and rhythm disturbances, especially atrial fibrillation. It is important to detect and optimise cardiovascular risk factors. An electrocardiogram should be requested in patients with a flare-up of activity and tachycardia, to rule out atrial fibrillation.68
Patients with IBD, particularly during the active phase of the disease, have twice the risk of developing deep vein thrombosis (DVT), owing to which it is essential to institute early prophylaxis during medical hospital admissions or admission for major surgery. If a thrombotic event occurs, anticoagulation with low molecular weight heparin should be started, followed by oral anticoagulation for at least three months (unless there is a prior episode, in which case maintaining it indefinitely should be considered).69 It is important that the patient receive information from PC about the risk factors for DVT, including long distance travel and oestrogen oral contraceptives.
Diabetes mellitus has a higher prevalence in patients with IBD and is considered a risk factor for the development of complications.70 The prevalence of overweight/obesity appears to be similar or slightly higher, with a harmful effect on IBD, as adipose tissue is capable of producing proinflammatory cytokines. A consensus document on the management of obesity in patients with IBD has recently been published.71
Lung diseaseRespiratory involvement tends to be uncommon, but includes inflammation of the airways, parenchymal involvement, pulmonary embolisms and respiratory complications caused by medication (sulfasalazine, 5-aminosalicylates, methotrexate, azathioprine or anti-TNF agents).
An increased risk of bronchiectasis, interstitial lung disease and granulomatous lung disease has been found in patients with IBD.55
Haematological manifestationsAnaemia is the most common manifestation, and patients should be checked periodically in view of the impact on quality of life. It can be multifactorial, with iron deficiency and anaemia of chronic disease being the most prevalent causes in IBD, followed by vitamin B12 and folic acid deficiency, and adverse effects of medication. In the presence of inflammation, the criterion for anaemia of chronic disease is ferritin >100€µg/l and a transferrin saturation index <20%. With hypoalbuminaemia, ferritin may be falsely normal. Patients with iron deficiency anaemia should receive iron therapy until haemoglobin and iron deposition reach normal levels. Oral iron is recommended in patients with mild anaemia and no clinical activity, and intravenous iron is recommended in patients with moderate/severe anaemia with clinical activity or previous intolerance to oral iron. Iron levels should be monitored every 3–6 months the first year after supplementation. Leucopenia and thrombocytopenia may occur associated with the use of immunosuppressants.72
Neurological manifestationsThe most common is peripheral and sensory neuropathy, which is usually due to vitamin B12 deficiency or prolonged treatment with metronidazole. Demyelinating diseases may worsen, and are a contraindication to the use of anti-TNF agents.55
Urinary and kidney manifestationsIn exceptional cases, aminosalicylates can cause intestinal nephritis, so kidney function should be monitored regularly. There is an increased risk of kidney stones (7–10%), especially in CD. Renal amyloidosis is a rare but serious complication associated with CD; its most common manifestation is proteinuria.73
Vitamin and mineral deficiencyPatients with IBD are at a high risk of vitamin and mineral deficiencies (especially vitamin D, vitamin B12, iron, calcium and zinc). The location, extension or surgical resection will specifically affect some of these deficiencies (vitamin B12, the terminal ileum; iron and calcium, the small intestine). Flare-ups need to be controlled, a balanced diet recommended, and deficiencies corrected according to individual abnormalities. Monitoring of blood levels is recommended every 6–12 months, including acute phase reactants, albumin, iron deficiency profile (especially in the case of anaemia), folic acid (especially if the patient takes sulfasalazine or methotrexate), vitamin B12 (if there is resection or involvement of the ileum) and ions (calcium, phosphorus, magnesium and potassium) in the case of chronic diarrhoea.55
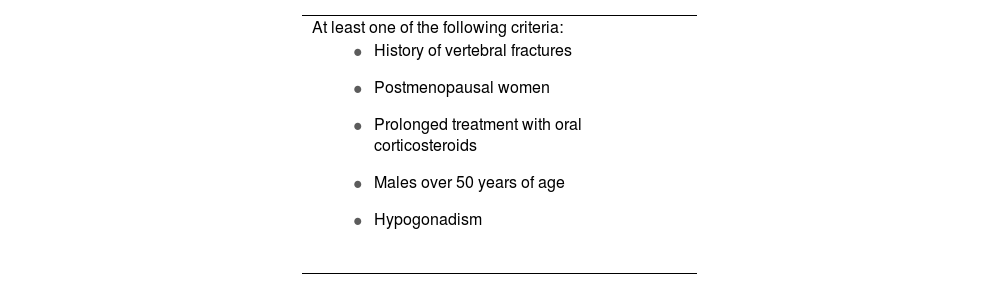
Osteopenia, osteoporosisIn IBD, there is an increased risk of osteopenia, osteoporosis (15%) and fractures (40%) compared to the general population. Factors that influence the reduction in bone mineral density include systemic inflammation, the use of corticosteroids, CD, smoking, malabsorption of vitamin D and calcium, little physical activity and malnutrition. Screening bone densitometry is recommended in patients with risk factors (Table 10), and if normal, the recommendation is to repeat after 2–3 years. Treatment includes hygiene and dietary measures. Calcium and vitamin D should be supplemented in patients on treatment with corticosteroids, with osteopenia or at risk of low bone mineral density. In the case of a high risk of fracture or worsening, bisphosphonates may be useful.74
Indications for densitometry in patients with inflammatory bowel disease.
| At least one of the following criteria: |
|
Prepared by the authors, adapted from Chaparro et al.75
Approximately one third of patients with IBD have symptoms of anxiety or depression and sleep disturbances, increasing to 60–80% during flare-ups, with a greater possibility of relapse and poorer quality of life. In children and adolescents, the disease has a negative impact on their day-to-day activities and those of their parents.76 From PC, it is important to assess in an open and empathetic way how IBD can affect the psychological sphere, identifying the associated psychological comorbidities, referring to Mental Health where necessary, and introducing the figure of the social worker to resolve legal issues and provide information regarding the social resources available.77
IBD is associated with a high prevalence of sexual dysfunction. The chronic and intermittent nature of the disease, its clinical manifestations such as abdominal pain, faecal incontinence and perianal disease, surgery and stomas and affective disorders can all have an impact on sexual health. A position statement has recently been published on sexuality and IBD.78
FatigueFatigue has a prevalence of 40−60% in inactive disease and 80% in active disease. It negatively affects health-related quality of life, regardless of disease activity. It is important to rule out nutritional deficiencies (iron and vitamins) and to explore comorbidities such as pain, anxiety and sleep disorders. A complete and balanced diet should be recommended, as well as gradual aerobic physical exercise of moderate intensity.79
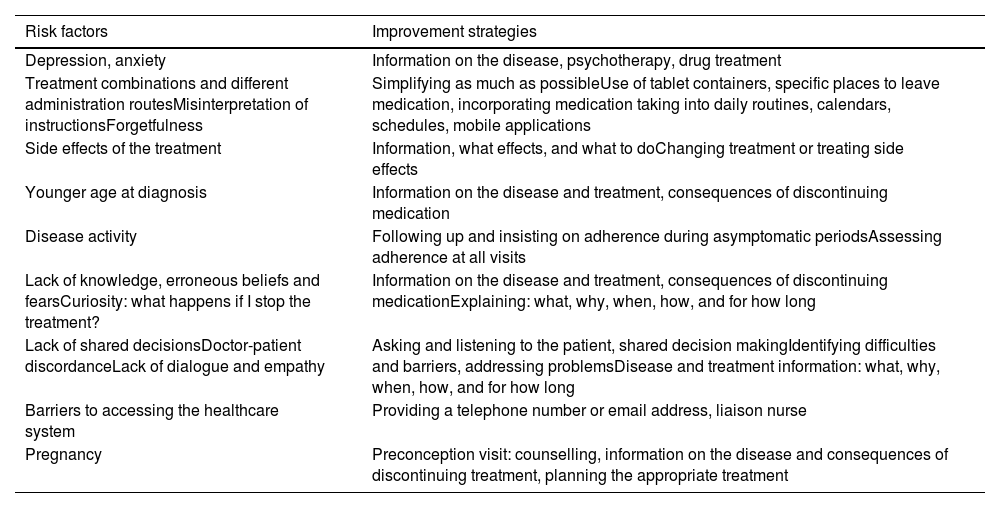
Disease information, self-care, and adherence monitoringIBD is a chronic disease, because of which patients will need to follow a series of interventions including care, monitoring and treatment throughout their lives. This can lead to patients becoming tired and frustrated and abandoning treatment.80 GPs can help by providing information about the disease, the medication and side effects, and promoting patient empowerment, self-care, and lifestyle changes which may improve subjective symptoms and quality of life.81 Lack of adherence can affect quality of life, with more hospital admissions, higher costs and a worse disease prognosis. Recognising and improving adherence therefore becomes a primary goal of treatment.82 Different factors that can trigger poor adherence and possible solutions are shown in Table 11. The approach should be personalised and multidisciplinary (gastroenterologist, pharmacist, GP, nurse and psychologist).83
Factors that can trigger poor adherence and strategies for improvement.
| Risk factors | Improvement strategies |
|---|---|
| Depression, anxiety | Information on the disease, psychotherapy, drug treatment |
| Treatment combinations and different administration routesMisinterpretation of instructionsForgetfulness | Simplifying as much as possibleUse of tablet containers, specific places to leave medication, incorporating medication taking into daily routines, calendars, schedules, mobile applications |
| Side effects of the treatment | Information, what effects, and what to doChanging treatment or treating side effects |
| Younger age at diagnosis | Information on the disease and treatment, consequences of discontinuing medication |
| Disease activity | Following up and insisting on adherence during asymptomatic periodsAssessing adherence at all visits |
| Lack of knowledge, erroneous beliefs and fearsCuriosity: what happens if I stop the treatment? | Information on the disease and treatment, consequences of discontinuing medicationExplaining: what, why, when, how, and for how long |
| Lack of shared decisionsDoctor-patient discordanceLack of dialogue and empathy | Asking and listening to the patient, shared decision makingIdentifying difficulties and barriers, addressing problemsDisease and treatment information: what, why, when, how, and for how long |
| Barriers to accessing the healthcare system | Providing a telephone number or email address, liaison nurse |
| Pregnancy | Preconception visit: counselling, information on the disease and consequences of discontinuing treatment, planning the appropriate treatment |
Living with IBD has a huge impact. In addition to the symptoms of the disease, its chronic nature and the unpredictability of the flare-ups, there is the perception of a social stigma which affects self-esteem and can affect social and interpersonal relationships, leading to isolation.84 Intimate relationships and sexual function may also be affected.78 The implementation of coping strategies and visibility campaigns in the media and social networks, often carried out by patient organisations, are of particular importance here. The main reasons why people with IBD turn to patient associations are: to have verified, reliable and accessible information, in a language adapted to their needs; to connect with people with a similar experience, to learn from them, and to offer the same support to others; and to avoid situations of unwanted loneliness by seeking emotional support, which helps them develop coping tools such as empowerment and resilience.85 Belonging to a social support network helps people face the daily challenges of living with a chronic disease, can reduce the effects of psychological stress, has been directly linked to an improvement in quality of life, promotes normalisation and participation in inclusive leisure activities, and can lead to savings in healthcare costs. Support programmes through mentoring or accompaniment promote the development of self-care behaviours, self-management of one's own health, and improved treatment adherence.86 They can also provide psychosocial support to family members and caregivers of people with IBD.87 For all these reasons, it is essential that healthcare professionals inform their patients about the benefits that patient associations could provide them in coping with IBD, as a complementary resource and support for the medical care they receive.
CRediT authorship contribution statementAll authors participated in the conceptualisation of the project, the drafting and/or the critical review of the manuscript. All authors have given their approval for the final version of the manuscript to be submitted.
Ethical considerationsThis work was carried out in full compliance with the principles established in the latest version of the Declaration of Helsinki, regarding medical research in humans, and in accordance with the applicable regulations on Good Clinical Practices.
FundingThis work received no funding.
This article has been jointly prepared by Gastroenterología y Hepatología, Gastroenterology and Hepatology (English Edition), Medicina de Familia, SEMERGEN and published jointly by Elsevier España S.L.U. The articles are identical, except for minor stylistic and spelling differences in keeping with the style of each journal. Either citation may be used when citing this article.