Implementation of the breakpoints established in the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines in comparison with those of the Clinical and Laboratory Standards Institute (CLSI) means that the criteria for interpreting the susceptibility of some antimicrobials have been modified, resulting in changes in the reports of accumulated antibiotic susceptibility.
MethodsThe effect of applying EUCAST breakpoints in 10,359 clinical isolates of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus and Enterococcus spp. was analysed.
ResultsBy applying EUCAST breakpoints, most antimicrobial susceptibility percentages did not change or changed very slightly. However, a decrease in aminoglycoside susceptibility was observed in Gram-negative bacilli, mainly for amikacin and Pseudomonas aeruginosa (23.2%), although only 5.7% were completely resistant; a notably decrease in the percentage of isolates susceptible to aztreonam was also observed. There was also a marked increase in the number of Staphylococcus aureus strains resistant to clindamycin (51.5%) and aminoglycosides (gentamicin 43.1%).
ConclusionsSwitching from CLSI to EUCAST criteria in some pathogens alters the percentages of resistance to several antimicrobials, and therefore the local epidemiology of the resistance. These changes should be implemented by a multidisciplinary group in order to analyse the influence of the new data on the empirical treatment protocols of each centre.
La aplicación de los puntos de corte establecidos por el European Committee on Antimicrobial Susceptibility Testing (EUCAST) en comparación con los del Clinical and Laboratory Standards Institute (CLSI) modifica los criterios de interpretación de la sensibilidad de algunos antimicrobianos y esto conduce a cambios en los informes de sensibilidad antibiótica acumulada.
MétodosAnálisis de la influencia de la aplicación del EUCAST en 10.359 aislados clínicos de Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus y Enterococcus spp.
ResultadosAl aplicar los puntos de corte del EUCAST, la mayoría de los porcentajes de sensibilidad a antimicrobianos no se alteró o lo hizo de forma muy leve; sin embargo, se observó una disminución de la sensibilidad a los aminoglucósidos en bacilos gramnegativos, especialmente a la amicacina en Pseudomonas aeruginosa (23,2%), aunque solo el 5,7% fueron totalmente resistentes; además, disminuyó notablemente el porcentaje de aislados sensibles a aztreonam. Es de destacar el aumento de cepas de Staphylococcus aureus resistentes a clindamicina (51,5%) y a aminoglucósidos (gentamicina 43,1%).
ConclusionesEl cambio de los criterios del CLSI a los de EUCAST en algunos patógenos supone una alteración en los porcentajes de resistencia a algunos antimicrobianos y, por tanto, en la epidemiología local de la resistencia. Estos cambios deben realizarse por un grupo multidisciplinar, que analice la influencia de los nuevos datos en los protocolos de tratamiento empírico de cada centro.
Antibiotic resistance is a serious public health problem, whose approach requires a multidisciplinary work. One of the basic pillars is the adequate empirical treatment of patients based on data from local epidemiology.1 As a result of the work of the Spanish Antibiogram Committee (COESANT), there is a clear tendency among Spanish hospitals to substitute the regulations of the United States’ Clinical and Laboratory Standards Institute (CLSI) with those established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) when interpreting the data provided by studies of antibacterial sensitivity. Our study aims to compare the changes in resistance rates caused by this modification in our environment.
Material and methodsThe sensitivity patterns of 10,359 clinical isolates (first isolate of each patient) of Escherichia coli (n=4987), Klebsiella pneumoniae (n=1324), Pseudomonas aeruginosa (n=1159), Staphylococcus aureus (n=1066), Enterococcus faecalis (n=1522) and Enterococcus faecium (n=301), isolated during a 17-month period in the Hospital General Universitario de Alicante (hospitalised and primary care patients) were analysed using both criteria. The minimum inhibitory concentration values were studied by the commercial microdilution method (MicroScan WalkAway 96 Plus, Siemens, Germany) and the sensitivity percentages obtained according to the cut-off points of CLSI and EUCAST (2017) were compared using the Microb Dynamic computer system (Soria Melguizo, Spain).
To compare the results, the kappa coefficient was used.
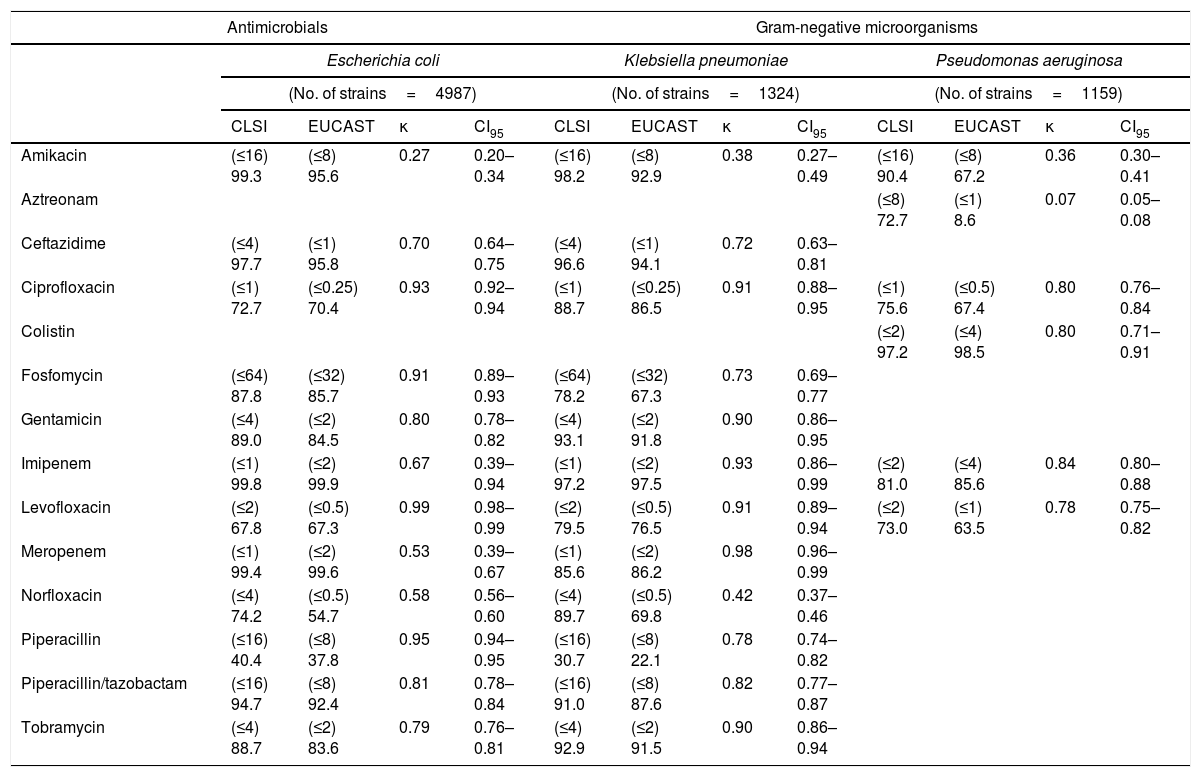
ResultsThe interpretation criteria for most microorganism-antibiotic combinations were the same for both criteria. In enterobacteria, the change of criteria did not lead to a change in sensitivity data for several antimicrobials. Thus, for Escherichia coli/Klebsiella pneumoniae the sensitivity percentages for both criteria were: ampicillin (43.3–0%), cefotaxime (87.8–77.9%) and trimethoprim/sulfamethoxazole (68.3–86.5%); in Pseudomonas aeruginosa were: cefepime (82.3%), ceftazidime (86.8%), meropenem (78.9%), piperacillin (70.9%), piperacillin/tazobactam (84.5%), ticarcillin (43.9%) and tobramycin (88.7%); in Staphylococcus aureus the sensitivity percentages were: ciprofloxacin (90.8%), daptomycin (100%), levofloxacin (91.9%), linezolid (100%), oxacillin (84.9%), trimethoprim/sulfamethoxazole (99.1%) and vancomycin (100%), and in Enterococcus spp., the change did not produce variations in the sensitivity percentage to vancomycin (100%). It was not possible to evaluate the activity of amoxicillin/clavulanic acid since the concentration of clavulanic acid recommended by the 2 committees is different. In other antibiotic-microorganism combinations, some differences that may have clinical significance were observed. The comparison of these data is detailed in Table 1.
Comparison of the percentages of antimicrobial sensitivity of gram-negative microorganisms and the percentages of antibiotic sensitivity in gram-positive bacteria according to CLSI and EUCAST criteria.
| Antimicrobials | Gram-negative microorganisms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Klebsiella pneumoniae | Pseudomonas aeruginosa | ||||||||||
| (No. of strains=4987) | (No. of strains=1324) | (No. of strains=1159) | ||||||||||
| CLSI | EUCAST | κ | CI95 | CLSI | EUCAST | κ | CI95 | CLSI | EUCAST | κ | CI95 | |
| Amikacin | (≤16) 99.3 | (≤8) 95.6 | 0.27 | 0.20–0.34 | (≤16) 98.2 | (≤8) 92.9 | 0.38 | 0.27–0.49 | (≤16) 90.4 | (≤8) 67.2 | 0.36 | 0.30–0.41 |
| Aztreonam | (≤8) 72.7 | (≤1) 8.6 | 0.07 | 0.05–0.08 | ||||||||
| Ceftazidime | (≤4) 97.7 | (≤1) 95.8 | 0.70 | 0.64–0.75 | (≤4) 96.6 | (≤1) 94.1 | 0.72 | 0.63–0.81 | ||||
| Ciprofloxacin | (≤1) 72.7 | (≤0.25) 70.4 | 0.93 | 0.92–0.94 | (≤1) 88.7 | (≤0.25) 86.5 | 0.91 | 0.88–0.95 | (≤1) 75.6 | (≤0.5) 67.4 | 0.80 | 0.76–0.84 |
| Colistin | (≤2) 97.2 | (≤4) 98.5 | 0.80 | 0.71–0.91 | ||||||||
| Fosfomycin | (≤64) 87.8 | (≤32) 85.7 | 0.91 | 0.89–0.93 | (≤64) 78.2 | (≤32) 67.3 | 0.73 | 0.69–0.77 | ||||
| Gentamicin | (≤4) 89.0 | (≤2) 84.5 | 0.80 | 0.78–0.82 | (≤4) 93.1 | (≤2) 91.8 | 0.90 | 0.86–0.95 | ||||
| Imipenem | (≤1) 99.8 | (≤2) 99.9 | 0.67 | 0.39–0.94 | (≤1) 97.2 | (≤2) 97.5 | 0.93 | 0.86–0.99 | (≤2) 81.0 | (≤4) 85.6 | 0.84 | 0.80–0.88 |
| Levofloxacin | (≤2) 67.8 | (≤0.5) 67.3 | 0.99 | 0.98–0.99 | (≤2) 79.5 | (≤0.5) 76.5 | 0.91 | 0.89–0.94 | (≤2) 73.0 | (≤1) 63.5 | 0.78 | 0.75–0.82 |
| Meropenem | (≤1) 99.4 | (≤2) 99.6 | 0.53 | 0.39–0.67 | (≤1) 85.6 | (≤2) 86.2 | 0.98 | 0.96–0.99 | ||||
| Norfloxacin | (≤4) 74.2 | (≤0.5) 54.7 | 0.58 | 0.56–0.60 | (≤4) 89.7 | (≤0.5) 69.8 | 0.42 | 0.37–0.46 | ||||
| Piperacillin | (≤16) 40.4 | (≤8) 37.8 | 0.95 | 0.94–0.95 | (≤16) 30.7 | (≤8) 22.1 | 0.78 | 0.74–0.82 | ||||
| Piperacillin/tazobactam | (≤16) 94.7 | (≤8) 92.4 | 0.81 | 0.78–0.84 | (≤16) 91.0 | (≤8) 87.6 | 0.82 | 0.77–0.87 | ||||
| Tobramycin | (≤4) 88.7 | (≤2) 83.6 | 0.79 | 0.76–0.81 | (≤4) 92.9 | (≤2) 91.5 | 0.90 | 0.86–0.94 | ||||
| Antibiotic | Gram-positive microorganisms | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecium | Enterococcus faecalis | ||||||||||
| (No. of strains=1066) | (No. of strains=301) | (No. of strains=1522) | ||||||||||
| CLSI | EUCAST | κ | CI95 | CLSI | EUCAST | κ | CI95 | CLSI | EUCAST | κ | CI95 | |
| Ampicillin | (≤8) 14.4 | (≤4) 11.2 | 0.866 | 0.78–0.95 | (≤8) 100 | (≤4) 99.7 | 0 | 0.00–0.00 | ||||
| Ciprofloxacin | 0.00 | 0.00–0.00 | (≤1) 58.3 | (≤4) 100 | 0 | 0.00–0.00 | ||||||
| Clindamycin | (≤0.5) 95.9 | (≤0.25) 44.4 | 0.07 | 0.05–0.09 | ||||||||
| Erythromycin | (≤0.5) 76.9 | (≤1) 77.7 | 0.98 | 0.96–0.99 | ||||||||
| Gentamicin | (≤4) 94.6 | (≤1) 51.5 | 0.12 | 0.09–0.14 | ||||||||
| Levofloxacin | (≤2) 15.3 | (≤4) 23.4 | 0.75 | 0.65–0.84 | (≤2) 65.4 | (≤4) 66.6 | 0.97 | 0.96–0.98 | ||||
| Linezolid | (≤2) 100 | (≤4) 100 | (≤2) 100 | (≤4) 100 | ||||||||
| Teicoplanin | (≤8) 100 | (≤2) 99.0 | 0 | 0.00–0.00 | (≤8) 100 | (≤2) 100 | (≤8) 100 | (≤2) 100 | ||||
| Tetracyclines | (≤4) 94.0 | (≤1) 86.2 | 0.57 | 0.49–0.65 | ||||||||
| Tobramycin | (≤4) 90.9 | (≤1) 53.0 | 0.20 | 0.17–0.24 | ||||||||
CI95: 95% confidence interval; κ: kappa coefficient.
The value of the minimum inhibitory concentration considered as sensitive by the different criteria is shown in parentheses.
For gram-negative microorganisms, CLSI only allows the fosfomycin cut-off point for urinary isolates and in uncomplicated urinary tract infections for enterobacteria. There is no cut-off point for Pseudomonas spp. in any of the committees.
For gram-positive microorganisms, the cut-off points for the 2 quinolones in Enterococcus spp. are only for urinary isolates.
The sensitivity cut-off points established by CLSI and EUCAST are shown in parentheses.
The most prominent phenomenon is that the application of EUCAST caused a significant decrease in sensitivity to aminoglycosides (gentamicin and amikacin), both in enterobacteria (3.7 points in Escherichia coli and 5.3 points in Klebsiella pneumoniae for amikacin) and in Pseudomonas aeruginosa (23.2 points for the same antibiotic). However, the vast majority of these isolates did not become completely resistant, since the percentages of amikacin resistance rose much less: Escherichia coli (0.4%), Klebsiella pneumoniae (1.6%) and Pseudomonas aeruginosa (5.7%).
In relation to the activity of fosfomycin, the application of EUCAST caused an increase in resistance in Escherichia coli (2.1%) and in Klebsiella pneumoniae (10.9%).
The percentage of strains sensitive to fluoroquinolones in enterobacteria showed little variation, but in the case of Pseudomonas aeruginosa, the application of EUCAST significantly reduced sensitivity to these antimicrobials (8.2%). The percentage of strains of Pseudomonas aeruginosa sensitive to aztreonam also markedly decreased (64.1%).
In Staphylococcus aureus the decrease in sensitivity to gentamicin (43.1%) and tobramycin (37.9%) is very notable, a phenomenon that was also observed for clindamycin (51.5%). Regarding the sensitivity of Enterococcus spp., much less marked changes were observed except for ciprofloxacin, which saw a 100% sensitivity when applying the EUCAST criteria.
DiscussionOne of the main contributions of clinical microbiology to the improvement of the treatment of infectious processes, in addition to their fast and correct diagnosis, is the study of the local epidemiology of antibiotic resistance, a cornerstone of the local protocols of empirical therapy.2 These data have become much more important with the increase of multiresistance, which requires that they undergo a rigorous and exhaustive analysis.3,4
The gradual incorporation of the EUCAST criteria in European hospitals is forcing the evaluation of the clinical impact of this change in each geographical environment.5,6 In general, changes in the rates of resistance applying the 2 criteria are small in our environment, so the change of standards does not have to significantly alter the hospital's antibiotic policy. These changes reflect the complexity of antibiotic resistance, especially the mechanisms that cause low levels of resistance to antimicrobials.7
However, the change that can have a greater clinical impact when applying the EUCAST criteria is the reduction of the sensitivity rates of Pseudomonas aeruginosa to amikacin, which could require rethinking the association of this antibiotic with carbapenems as an empirical treatment of serious infections with suspicion of this pathogen. However, it must not be forgotten that most of the isolates do not fit into the resistant category, so the most appropriate therapy should be evaluated when strains with these characteristics are involved.8 In relation to the carbapenems, the differences of cut-off points when applying both criteria do not vary and do not suppose a clinical transcendence in our environment, either in the case of enterobacteria or in Pseudomonas aeruginosa.9,10
Another remarkable fact is the decrease in the percentages of sensitivity to fosfomycin in enterobacteria and in Pseudomonas aeruginosa. This fact may be more important in the future because the need to use this antimicrobial in empirical treatments of infections caused by multiresistant strains is increasingly growing.11,12
In relation to the decrease in the percentages of sensitivity to clindamycin in Staphylococcus aureus, it may also require a review of some empirical therapies, especially in the treatment of skin and soft tissue infections, in which this drug is one of those considered to be a drug of choice.
It is advisable to change to the EUCAST criteria, but the changes in the sensitivity percentages that it causes must be analysed jointly by all the specialists involved in the management of the infectious disease, since the variations that can appear in the local epidemiology of resistance may have clinical implications in the practice of care.13–15
FundingThis work has been done thanks to the help of the Fundación Soria Melguizo and the Fundación FISABIO (UGP-14-270).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez-Bautista A, Coy J, García-Shimizu P, Rodríguez JC. Cambio de CLSI a EUCAST en la interpretación de la sensibilidad a antimicrobianos: ¿cómo influye en nuestro medio? Enferm Infecc Microbiol Clin. 2018;36:229–232.