To assess the effect of postprandial lipemia on endothelial function, insulin resistance, and lipid profile in healthy subjects.
Patients and methodsA prospective, interventional study in 14 healthy young men aged 18–25 years who were given a high-fat meal. Endothelial function was measured using flow-mediated dilation (FMD) in the brachial artery, flow velocity, mean arterial pressure and serum nitrite/nitrate levels (NO2/NO3). Glucose, insulin, total cholesterol, and triglyceride levels were also tested. Insulin resistance was determined by calculating the HOMA-IR index (Homeostatic Model Assessment-Insulin Resistance).
ResultsBaseline FMD was 5.9±1.1%. Postprandial lipemia reduced endothelial function by approximately 50% in the first (3.3±0.5%, p=0.03) and second (3.3±0.4%, p=0.04) moment, respectively. This finding was associated to an increased flow rate in the brachial artery and lower NO2/NO3 levels (p<0.05). Higher cholesterol and triglyceride levels were found 1h and 2h postprandial (p<0.05). HOMA-IR was significantly increased 1h and 2h postprandial (p<0.05).
ConclusionsPostprandial lipemia causes changes in circulating lipid profile and induces endothelial dysfunction and higher insulin resistance.
Evaluar el efecto de la lipemia pos-prandial sobre la función endotelial, la resistencia a la insulina y el perfil de lípidos en sujetos sanos.
Materiales y métodosEstudio de intervención prospectivo en 14 hombres jóvenes sanos entre los 18 y 25 años de edad a los que se administró una comida rica en grasas. La función endotelial se midió a través de vasodilatación mediada por flujo (VMF) en arteria braquial, la velocidad de flujo y los niveles séricos de nitritos/nitratos (NO2/NO3). Se evaluaron los niveles de glucosa, insulina, colesterol total y triglicéridos. La resistencia a la insulina se determinó mediante el cálculo del índice homeostatic model assessment–insulin resistance (HOMA-IR).
ResultadosEl valor basal de la VMF fue de 5,9±1,1%. Se identificó que la lipemia pos-prandial reducía la función endotelial aproximadamente en 50%, en la primera (3,3±0,5%, p=0,03) y segunda hora (3,3±0,4%, p=0,04), respectivamente. Este hallazgo se acompañó de un incremento en la velocidad del flujo braquial, la presión arterial media y menores niveles de NO2/NO3 (p<0,05). Se encontraron mayores niveles de colesterol total y triglicéridos a la 1h y 2h pos-prandial (p<0,05). El HOMA-IR, se encontró significativamente elevado comparado a la 1h y 2h pos-prandial, (p<0,05).
ConclusionesLa lipemia pos-prandial induce cambios en el perfil de lípidos circulantes e induce disfunción endotelial y mayor grado de resistencia a la insulina.
Epidemiological,1,2 clinical,3,4 and experimental5,6 trials have reported an association between postprandrial metabolism of triglyceride rich lipoproteins and cardiovascular and metabolic diseases. Postprandial lipemia is a metabolic state characterized by an exaggerated increase in plasma triglyceride concentrations, due to an increase in their clearance after a high-fat intake.7 An increase in remnant chylomicrons (RCs) and very low density lipoprotein (VLDL) has also been observed during this period, stimulating the formation of low density lipoprotein (LDL), as well as a reduction in concentrations of high density lipoprotein (HDL).8
Over the long term, this metabolic state causes impaired vascular function and increased oxidative stress.9,10 Oxidative stress and endothelial dysfunction are considered significant risk factors for the development and progression of atherosclerosis11 and both conditions have been associated with an increased risk of all-cause morbidity and mortality.12 However, other authors have added that genetic13 and environmental14 factors, including decreased insulin sensitivity secondary to obesity and carbohydrate rich diets, could lead to increased baseline triglyceride levels, thereby also facilitating the development of cardiometabolic diseases.15
Another relevant aspect in the postprandial state is the effect of insulin and insulin sensitivity, considered important biomarkers of cardiovascular risk.16,17 Insulin facilitates the entry of free fatty acids, as well as their esterification and storage in the form of triglycerides in adipose tissue. This does not occur in situations of insulin resistance (IR), so that an inadequate quantity of free fatty acids circulates in the postprandial state to reduce insulin sensitivity and accentuate postprandial lipemia, while also reducing endothelial dependent vasodilation with resulting endothelial dysfunction.18 Taking into account that a large part of the human life cycle is in the postprandial state, this may be considered as a important metabolic factor in the study of cardiovascular risk. The aim of this study was to assess the effect of postprandial lipemia on endothelial function, insulin resistance and lipid profile in healthy subjects.
MethodsThis was a prospective interventional study of 14 apparently healthy young men aged 18–25 years. The subjects included in the study belonged to a higher education institution in the city of Cali (Colombia) and were selected by consecutive sampling during the first quarter of 2011. Subjects with a history of smoking, surgery, or recent major trauma, endocrine, autoimmune, respiratory or heart disease (<1 month at study entry) and body mass index (BMI) greater than 28kg/m2 were excluded. In addition, a survey on nutritional habits, sociodemographic data and informed consent, following approval by the ethics committee (UV 10-09), was carried out. Anthropometric variables (weight, height, BMI, % fat) were measured according to the protocol described by Estrada19 and Ramírez-Vélez et al.20 for the Colombian population.
After approximately a 12h fast, 10ml of blood was collected via puncture of the antecubital vein into tubes without additive for measurement of glucose, total cholesterol and triglycerides (TG) by direct colorimetric method in an automated spectrophotometer (Biosystems, Spain).21 Insulin levels were measured by a chemiluminescence assay (Immulite 1000 kit, San Jose, CA. USA).22 With these results, the index of insulin resistance was calculated (HOMA-IR: Homeostatic Model Assessment-Insulin Resistance) using the formula: HOMA-IR=fasting glucose (mmol/L)×fasting insulin (μU/mL)/22.5.23 Measurement of serum levels of nitric oxide (NO) metabolites nitrite and nitrate (NO2/NO3) was performed by colorimetric assay (Cayman Chemical, USA),24 as an indicator of in vivo NO production and bioavailability.
Endothelial function was evaluated using the flow-mediated vasodilation (FMD) test, a technique that includes visualization of the brachial artery, measurement of its diameter and blood flow velocity using vascular plethysmography Images.25,26 After 5min of rest, the patient was placed in a supine position; in this position, the diameter of the brachial artery was measured both 1min before and after it had been occluded with the sphygmomanometer cuff for 5min (applying a pressure 60mmHg above baseline systolic blood pressure). The 7.5MHz transducer was placed at 60° with respect to the longitudinal axis of the vessel and arterial flow velocity (cms2) was measured. The image was frozen when it coincided with the QRS interval of the electrocardiogram and the intima–lumen interface was identified. The diameter and area of the artery (mm) were measured, and the skin on the arm was marked for subsequent placement of the transducer (in the same position) in subsequent measurements. FMV was expressed as the percent change in arterial diameter calculated with the formula FMV=(peak hyperemic diameter−baseline arterial diameter)/baseline arterial diameter×100. Endothelial sheer stress (ESS) of the brachial artery (dynescm−2) was calculated using the formula: ESS=(VmD−1), where Vm=mean or peak blood velocity (cms−1) and D−1=mean baseline brachial arterial diameter (cm).27 During this measurement, blood pressure was recorded according to the recommendations of the American Heart Association28 with an Omron® automatic sphygmomanometer. Mean arterial pressure was calculated using the formula: (2×diastolic blood pressure+systolic blood pressure)/3. The effect of a high fat intake on vascular and cardiovascular markers was measured after fasting for 8–12h (baseline), and 1 and 2h post-intake, and all subjects received a standard diet low in nitrates 24h before the tests.
To induce the postprandial lipemia state, a menu containing 1049 calories was prepared, containing 31g protein, 79g fat (31g saturated fat), 666mg cholesterol and 69g carbohydrates (Ceres software version 1.02, 1997, FAO). A pilot study confirmed that this dietary intake led to a transient change in endothelial function.
The data were presented as mean±standard error of the mean (SEM) for metabolic and vascular variables, and mean±standard deviation (SD) for the characteristics of the subjects. The Shapiro–Wilk test was used to assess the normality of continuous data. Repeated measure analysis of variance (one-way ANOVA) with Dunnett's T3 post hoc test, a factorial analysis of variance or the Kruskall–Wallis test according to the distribution of the variables, was used to examine the differences between the different times. Statistical significance was assigned to a p-value≤0.05, and data were processed with the SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
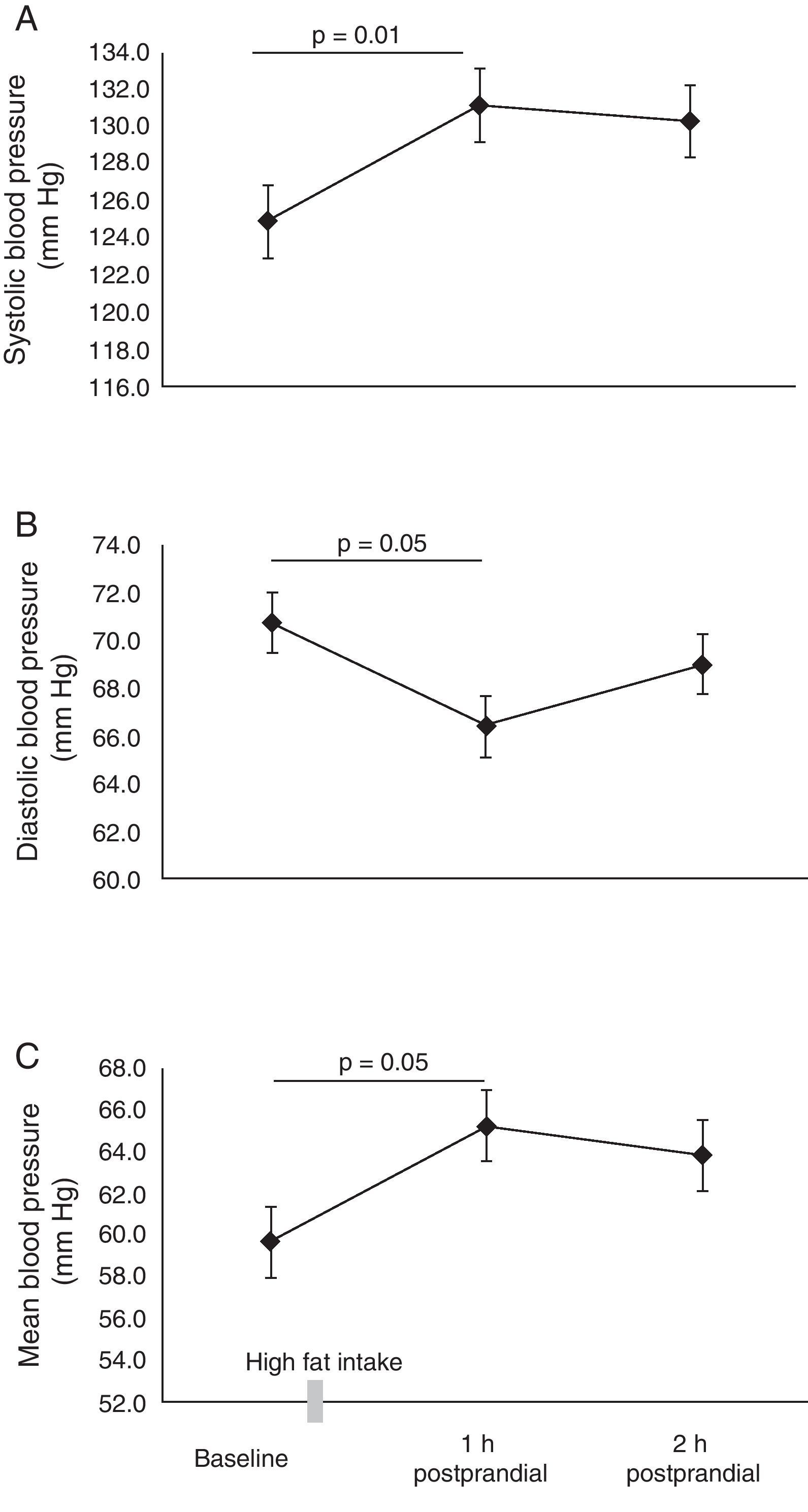
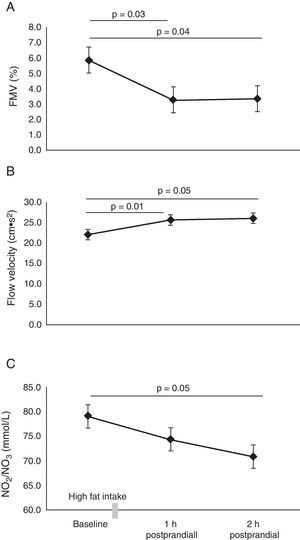
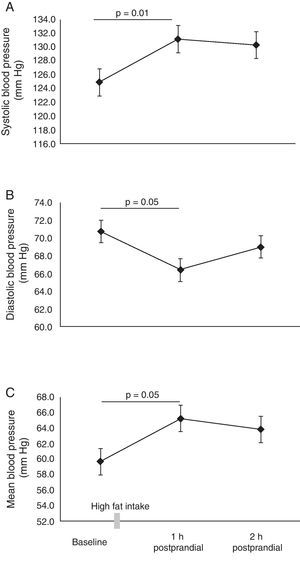
ResultsTable 1 summarizes the baseline characteristics of the participants. All parameters evaluated were within the range considered healthy for this age range. In the subjects evaluated, the value of FMV was 5.9±1.1% at baseline measurement. It was determined that postprandial lipemia reduced endothelial function by approximately 50% in the first (3.3±0.5%, p=0.03) and second hours (3.3±0.4%, p=0.04), respectively (Fig. 1A). This finding was associated with a significant increase in brachial artery flow velocity (Fig. 1B), increasing from 22±1.2cms2 at baseline to 25.6±1.5cms2 at 1h and 26.0±1.7cms2 at 2h postprandial, respectively (p<0.05). When testing the metabolism of NO, lower NO2/NO3 levels were observed 1h (74.4±4.4mmol/L) and 2h (70.9±3.8mmol/L, p=0.05) postprandial vs baseline (79.1±6.0mmol/L) (Fig. 1C). A significant increase was also noted in mean, systolic and diastolic blood pressure 1h postprandial (p<0.05) (Fig. 2A–C).
Baseline characteristics of study subjects.
| Characteristics | Mean±SD |
| Age (years) | 21.0±2.8 |
| Height (cm) | 176.2±15.7 |
| Weight (kg) | 73.8±7.5 |
| BMI (kg/m2) | 23.7±4.3 |
| Body fat (%) | 13.7±8.5 |
| HR at rest (beats/min) | 64.7±8.1 |
| SBP (mmHg) | 127.6±9.2 |
| DBP (mmHg) | 71.1±9.4 |
| MBP (mmHg) | 60.4±11.9 |
| FMV (%) | 5.9±1.1 |
| Brachial diameter (mm) | 3.5±0.2 |
| Flow velocity (cms2) | 23.3±5.5 |
| Endothelial sheer stress | 0.09±0.04 |
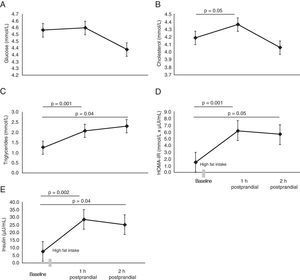
| Glucose (mmol/L) | 4.6±0.4 |
| Cholesterol (mmol/L) | 4.6±1.0 |
| Triglycerides (mmol/L) | 1.6±1.5 |
| Insulin (μU/mL) | 9.5±4.7 |
| HOMA-IR (mmol/L×μU/mL) | 1.9±0.9 |
| NO2/NO3 (mmol/L) | 69.3±15.6 |
HR, heart rate; HOMA-IR, Homeostatic Model Assessment-Insulin Resistance; BMI, body mass index; NO2/NO3, nitrites/nitrates; DBP, diastolic blood pressure; SBP, systolic blood pressure; MBP, mean blood pressure; FMV, Flow mediated vasodilation.
No significant differences were found in glucose concentrations (Fig. 3A). As expected, higher cholesterol and triglyceride levels were found 1h and 2h postprandial (p=0.05) (Fig. 3B and C). HOMA-IR was significantly increased 1h and 2h postprandial (p=0.05) (Fig. 3D). Plasma insulin concentrations were elevated 1h postprandial from baseline status (7.6±1.2μU/mL vs 28.5±5.7μU/mL, p=0.002) (Fig. 3E).
DiscussionThe main finding of this study was that postprandial lipemia causes changes in the circulating lipid profile and induces endothelial dysfunction and higher insulin resistance (IR), measured by the HOMA index. These results agree with prior studies suggesting an association between higher degrees of IR and an increased risk of cardiovascular events in subjects with characteristics similar to those in this study.29,30 Although the mechanism responsible for the reduction in vascular function and IR caused by postprandial lipemia is not known exactly, it is thought that elevated levels of triglyceride rich lipoproteins such as chylomicrons and VLDL, and their remnants, together with a state of oxidative stress are the main mechanisms that could explain this metabolic state.31
The marked reduction (50% reduction) in FMV in the first postprandial determination (p=0.03) in this study was consistent with the results of De Koning and Rabelink,29 Tsai et al.,32 and Kovacs et al.33 However, the differences with other studies that did not find these same changes may be explained by the participants’ age, the lipid composition of the diet administered, the time of vascular measurements and/or sex of the study subjects.10,13 Likewise, a similar finding was reported in diabetic and nondiabetic subjects after a high fat intake. For example, Tsai et al.32 observed that after a high fat meal−similar to that given in this study–the concentration of 8-PFG-2α triglycerides (a marker of oxidative stress) was increased and GSH-Px activity (glutathione peroxidase) was decreased; these findings are consistent with the significant reduction in FMV in this study. In addition, it has been shown that vascular function improves after treatment with ciprofibrate33 or the intake of supplements containing vitamin C and vitamin E.6
There is evidence that increased clearance of triglycerides in plasma can cause endothelial dysfunction and IR, a finding that was confirmed in this study.34 Free fatty acids may lead to endothelial dysfunction due to increased superoxide anion (O2−) production and a subsequent decrease in NO bioavailability,35 as observed in the first and second hour postprandial with NO2/NO3 plasma levels. Oxygen free radicals, such as O2−, can react directly with NO forming peroxynitrites (ONOO−) and neutralizing the biological function of the latter, causing a loss in endothelium dependent vasodilation.36 The accumulation of ONOO− leads to an inhibition of eNOS (nitric oxide synthase; the enzyme responsible for NO production), generating changes in the vascular system that are reflected even in blood pressure levels, as was found in this study.
Multiple markers of IR have been studied as predictors of the risk of cardiovascular events, and their results are heterogeneous and discordant.23,37 The hyperinsulinemic euglycemic clamp is considered the reference test for assessing insulin sensitivity.38 However, due to its technical difficulty, invasiveness and cost, it has not been considered as a tool applicable to large populations, which leads to the use of more practical alternatives for evaluation. HOMA-IR has been used as a clinical and epidemiological tool that can replace the clamp.37 Thus, this model is a good tool for establishing IR. This study found significant increases in this evaluation parameter of IR. Although HOMA-IR, by definition, is used in the fasting state, its use in the postprandial state has been shown to account for some of the mechanisms by which IR attenuates postprandial hypertriglyceridemia. For example, decreased lipoprotein lipase (LPL) activity inhibits the clearance of postprandial triglycerides in individuals with IR.39 On the other hand, IR causes less postprandial suppression of the release from the liver of triglyceride rich particles, such as VLDL that interfere with the insulin signaling pathways.40
As mentioned above, the etiology of cardiometabolic diseases has not been fully established and it has been suggested that the postprandial state would partly explain the process leading to their development.1–7 Recently, it has been shown that subjects who eat high fat diets have impaired endothelial function and higher levels of insulin, tumor necrosis factor-α and C-reactive protein,4 markers that have also been reported in patients with cardiovascular diseases associated with insulin resistance.41 All these mechanisms are the basis for the association between postprandial lipemia and IR.
In general, our results should be interpreted with caution due to the study limitations. First, the small number of subjects and the limited age range should be considered. We did not consider the eating patterns and physical activity of the subjects that may have modulated their metabolic and vascular response. In conclusion, our results suggest that a high fat meal causes changes in the circulating lipid profile and induces endothelial dysfunction and a higher IR in healthy subjects. While not all studies have shown attenuation of FMV and a higher IR in the postprandrial state, our findings may serve as a starting point for the preparation of further studies to investigate and confirm this metabolic phenomenon.
FundingThis study received financial and administrative support from the Program of Medicine of the ICESI University, Cali, Colombia, and the Laboratory of Proteins and Enzymes of Valle University, Cali, Colombia.
Conflicts of interestDuring the preparation of this article, none of the authors had ties to activities that could create conflicts of interest.
Please cite this article as: Ramírez-Vélez R. La lipemia pos-prandial induce disfunción endotelial y mayor grado de resistencia a la insulina en sujetos sanos. Endocrinol Nutr. 2011;58:529–35.