To assess the prevalence of non-Caucasian patients in hospital admissions for onset of symptomatic diabetes mellitus during the 2003–2010 period, and to analyze the characteristics differentiating them from the Caucasian population at diagnosis and 2 years later.
Material and methodsA retrospective, observational study. Inclusion criteria: patients aged 18–40 years admitted for de novo symptomatic diabetes from January 2003 to October 2010. Prevalence of patients of non-Caucasian origin was analyzed, and clinical, biochemical, immunological, and beta-cell function of both populations were compared at diagnosis and 2 years later.
ResultsNineteen percent of patients admitted to hospital for de novo symptomatic diabetes were non-Caucasian, with a progressive increase in recent years. Non-Caucasian patients had milder decompensation (3.0% had ketoacidosis, as compared to 15.2% in the Caucasian group, p<0.05), lower presence of autoimmunity (27.2 vs. 73.1%, p<0.01) and higher stimulated C-peptide levels (0.70±0.56 vs. 0.42±0.39nmol/L, p<0.05), mainly because of the subgroup with negative autoimmunity (0.82 vs. 0.25). Two years after diagnosis, less non-Caucasian patients were on intensified treatment (39.1 vs. 93.8%).
ConclusionsNon-Caucasian patients had a lower prevalence of autoimmunity, better beta-cell function at diagnosis, particularly due to the subgroup with negative autoimmunity, and less need for intensive treatment 2 years after diagnosis, features which are more characteristic of type 2 diabetes mellitus.
Determinar la prevalencia de pacientes de origen no caucásico en los ingresos hospitalarios por inicio de diabetes mellitus durante el periodo 2003–2010 y analizar las características diferenciales respecto a la población caucásica en el momento del inicio y a los 2 años.
Material y métodosEstudio observacional retrospectivo. Criterios de inclusión: pacientes ingresados por inicio sintomático de diabetes entre enero de 2003 y octubre de 2010, con edad entre 18 y 40 años. Se analizó la prevalencia de pacientes de origen no caucásico, se compararon ambas poblaciones respecto a datos clínicos, bioquímicos, de reserva pancreática e inmunológicos en el momento del inicio y se analizó la evolución a los 2 años.
ResultadosDe los ingresos por inicio sintomático de diabetes, el 19% fueron pacientes no caucásicos, con un aumento progresivo en los últimos años. Estos presentaban un grado de descompensación más leve (3,0% de cetoacidosis respecto al 15,2% en el grupo caucásico, p<0,05), menor autoinmunidad (27,2 vs. 73,1%, p<0,01) y un péptido C estimulado mayor (0,70±0,56 vs. 0,42±0,39nmol/L; p<0,05), básicamente a expensas del grupo con autoinmunidad negativa (0,82 vs. 0,25). A los 2 años del inicio, los pacientes no caucásicos tenían un menor porcentaje de tratamiento intensivo (39,1 vs. 93,8%).
ConclusionesEl grupo de pacientes no caucásicos presenta menor prevalencia de autoinmunidad, mejor funcionalismo celular beta al diagnóstico, sobre todo a expensas del subgrupo de pacientes con autoinmunidad negativa, y menor necesidad de tratamiento intensivo a los 2 años del diagnóstico, comportamiento más característico de la diabetes mellitus tipo 2.
Recent studies estimate a 7.6% prevalence of diagnosed diabetes in the Catalan population1 and a 13.8% prevalence of diabetes (diagnosed and undiagnosed) in the Spanish population.2 Consequently, this disease is considered a major health problem. On the other hand, the proportion of the non-Caucasian population has increased in Catalonia, and specifically in the metropolitan area of Barcelona, due to increased immigration in the years prior to the economic crisis. In the specific case of L’Hospitalet de Llobregat, a town with approximately 250,000 inhabitants in 2010, immigrants account for almost 25% of the total population.3
In developed countries where the Caucasian population is predominant, a type of diabetes mellitus with a symptomatic onset showing characteristics different from those seen in the autochthonous population has been reported in non-Caucasian adults. These patients usually have a symptomatic picture with acute ketotic decompensation (ketosis or ketoacidosis) despite being overweight or obese and frequently having a relatively preserved pancreatic reserve. They also have negative pancreatic autoimmunity4–6 and a course that allows for disease control with no need for insulin in a substantial proportion of cases, and even for remission to normoglycemia.7 This type of diabetes with characteristics halfway between type 1 and type 2 diabetes mellitus has been given different names, including atypical diabetes mellitus,8 ketosis-prone diabetes,9,10 Flatbush diabetes,4 or type one and a half diabetes, and its pathophysiology is not fully known.11
In Spain, despite the increasing percentage of the non-Caucasian population, little attention has been paid to the characteristics of diabetes mellitus at onset in this group. Our objective was to determine the proportion of patients of non-Caucasian origin in hospital admissions for the onset of diabetes mellitus during the 2003–2010 period at the reference area of Hospital Universitari de Bellvitge and to analyze their differences from the Caucasian population regarding clinical, biochemical, immunological, and pancreatic reserve characteristics, both at hospital admission and at 2 years after diabetes onset.
Patients and methodsAn observational, retrospective study was conducted at Hospital de Bellvitge, a non-pediatric teaching hospital attached to Barcelona University and acting as reference in its area for patients with symptomatic diabetes onset, with a reference population for admissions for diabetes of 300,561 inhabitants. In all patients admitted for this disease, an established clinical protocol including supplemental tests and treatment, and subsequent monographic monitoring, is followed.
Study patientsAll patients admitted to the department of endocrinology and nutrition from January 2003 to October 2010 with a diagnosis of the onset of diabetes mellitus, with ages ranging from 18 to 40 years, and for whom autoimmunity and pancreatic reserve data were available were enrolled into the study. Subjects with known diabetes or with known concomitant relevant conditions, such as severe infections or corticosteroid treatment were excluded from the study.
Study proceduresBaseline analysisThe annual prevalence for the non-Caucasian population was determined, and an analysis comparing clinical (sex, age, symptom duration, weight loss, degree of decompensation, the body mass index [BMI], insulin dose at discharge), biochemical (blood glucose, HbA1c, pH, bicarbonate), immunological (anti-GAD and anti-IA-2 antibodies), and pancreatic reserve data between Caucasian and non-Caucasian patients was performed. For this, the baseline C-peptide level was assessed at baseline (normal range at our hospital, 0.26–1.44nmol/L) and after stimulation with 1mg of intravenous glucagon administered 4–5 days after the resolution of acute decompensation, under fasting conditions and with blood glucose levels ranging from 70 to 180mg/dL.
Two-year analysisSubjects with follow-up data available were assessed 2 years after diabetes onset. The clinical data (the BMI, insulin dose, treatment received, and the proportion of patients on intensive treatment) and metabolic control (HbA1c) of the Caucasian and non-Caucasian groups were compared. Intensive treatment was defined as the basal-bolus regimen, either with insulin analogs or with human insulin. The insulin dose at 2 years was estimated only for insulin-treated patients. All the data regarding changes over time were analyzed by subgroups depending on origin (Caucasian and non-Caucasian) and the presence or absence of autoimmunity markers.
Statistical analysisResults are given based on descriptive analysis of the study variables: the frequencies and percentages for categorical variables, and the mean and standard deviation for quantitative variables. Comparative analysis of quantitative variables was performed using parametric tests (Student's t test) or non-parametric tests (Mann–Whitney test) depending on the distribution. Categorical variables were compared using a Chi-square test, which was considered adequate provided the percentage of cells with an expected frequency less than 5 was lower than 20%. Two-side tests with a 5% significance level were used for all variables.
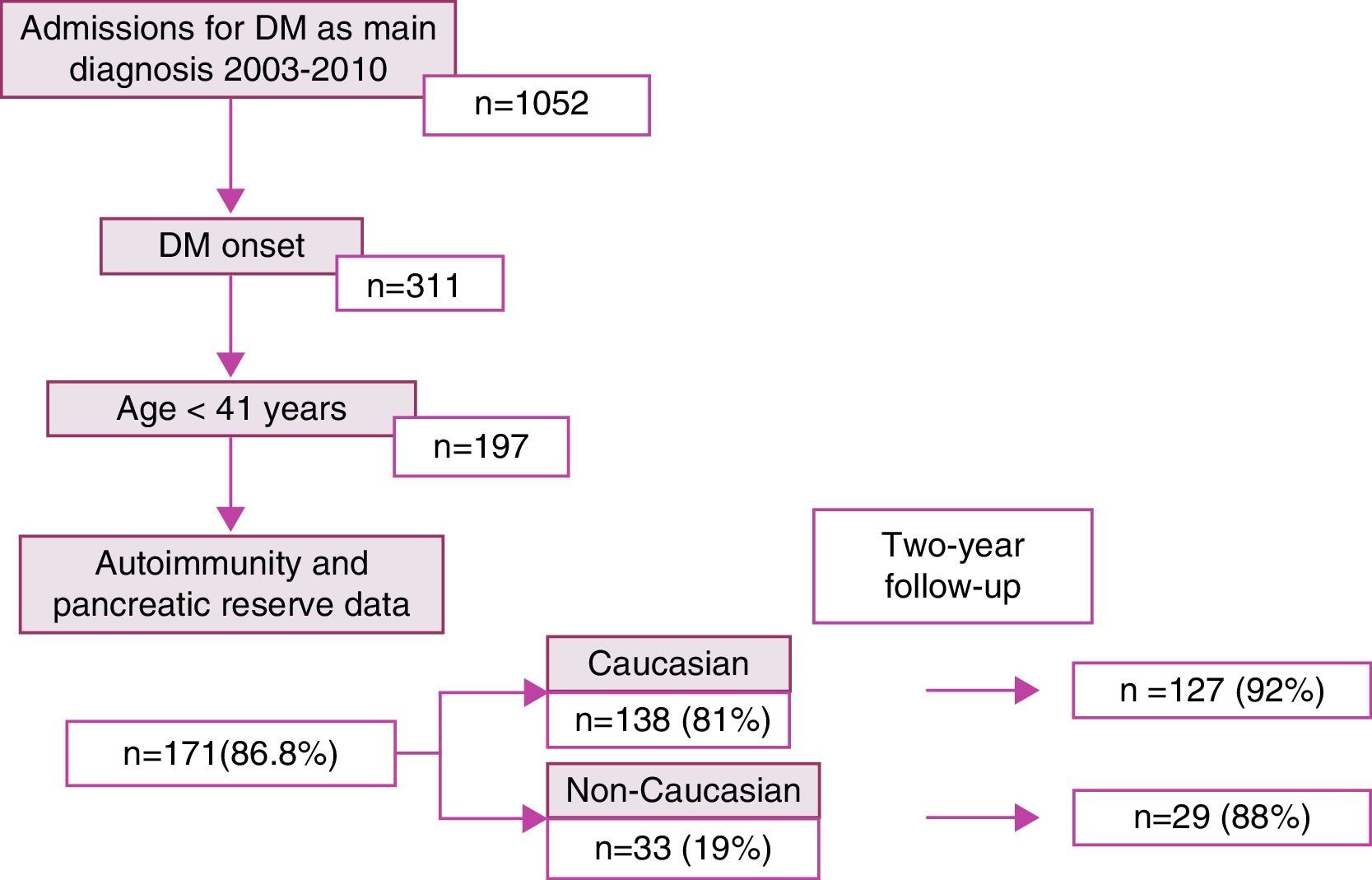
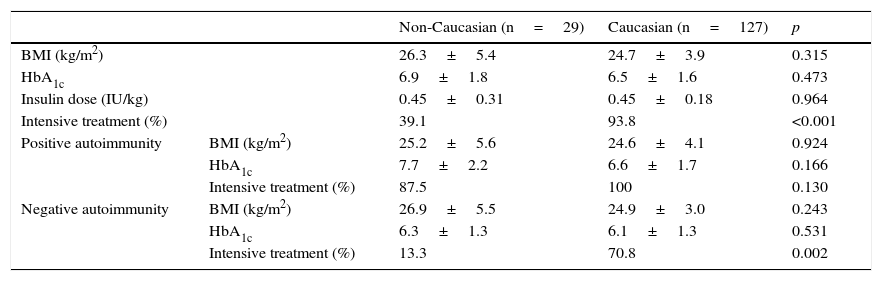
ResultsDuring the study period, 1052 patients with a main diagnosis of diabetes mellitus were admitted to hospital. Of these, 311 were newly diagnosed, including 197 patients aged up to 40 years of age. Autoimmunity and pancreatic reserve data were available for 171 patients (86.8%), who comprised the study sample. Of these, 138 (81%) and 33 (19%) were of Caucasian and non-Caucasian origin respectively. After 2 years, monitoring data were available for 92% of the Caucasian patients and 88% of the non-Caucasian patients (Fig. 1).
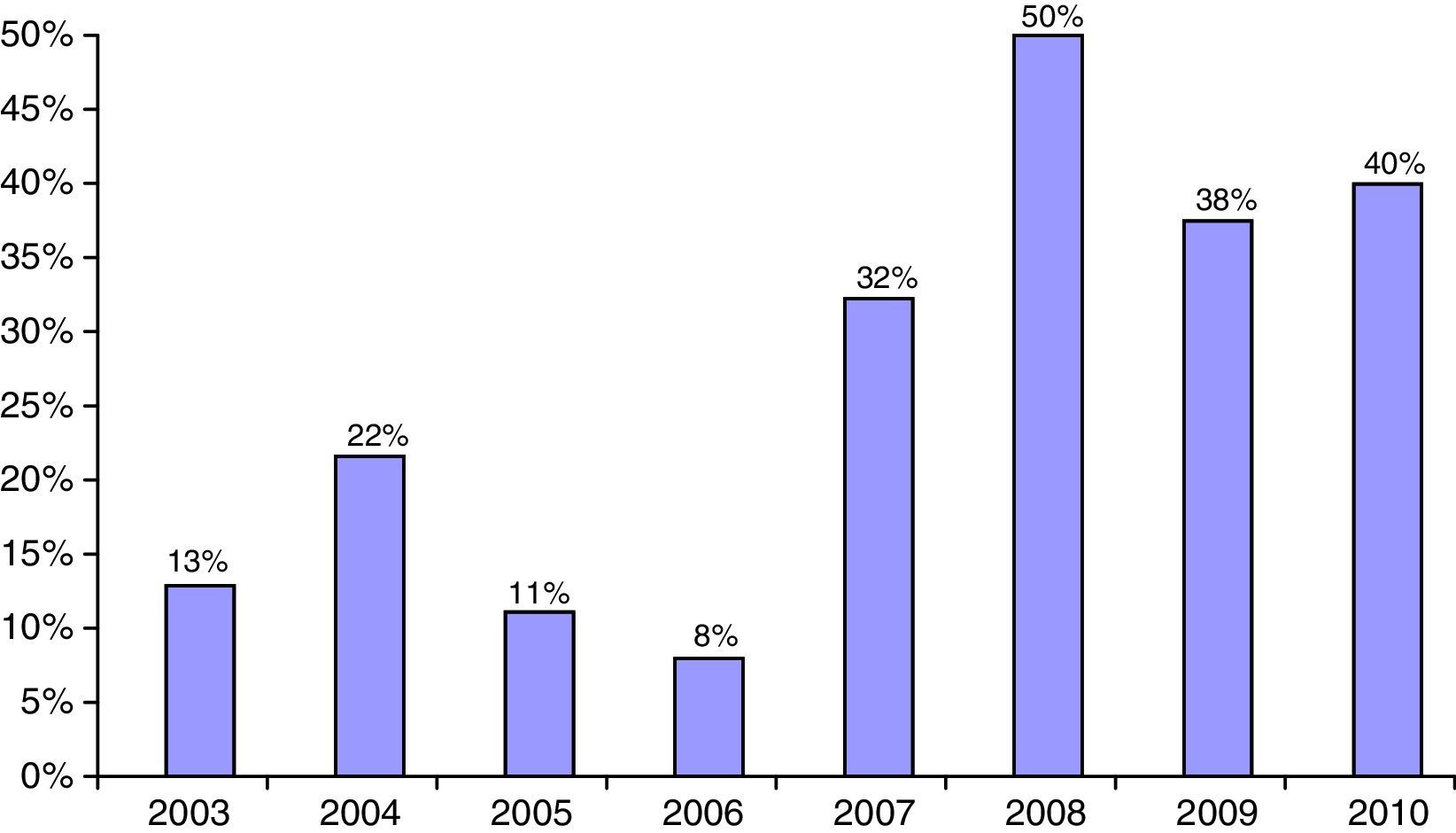
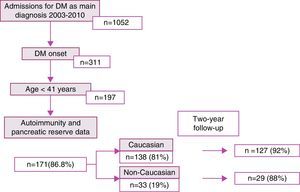
The proportion of non-Caucasian patients reached a maximum of 50% in 2008 (Fig. 2). As regards the origin of the non-Caucasian population, 47.9% came from Central and South American countries, mainly Ecuador, 33.3% from the Maghreb area (mostly from Morocco), and 18.8% came from other regions, mainly in Asia.
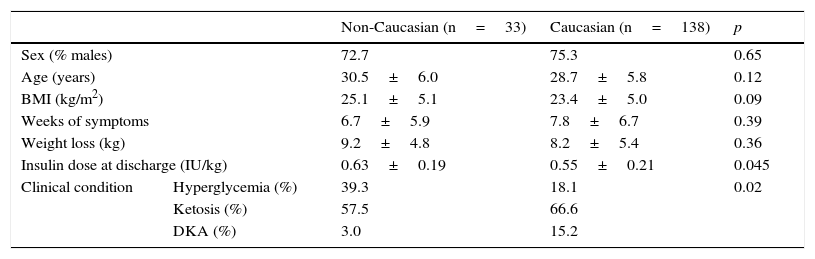
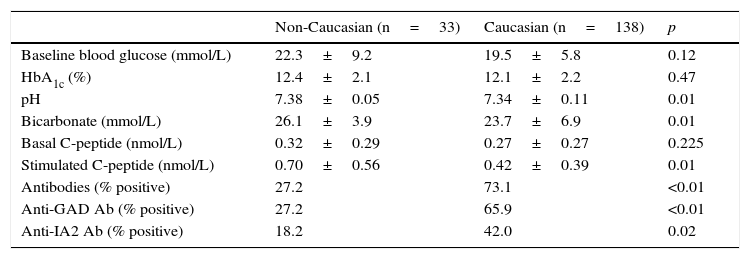
Baseline dataTable 1 shows the epidemiological and clinical data. Although non-Caucasian and Caucasian patients did not differ in sex, age, the BMI, weeks with symptoms or weight loss, the clinical condition at admission was more severe in Caucasian patients, 15.2% of whom had ketoacidosis, as compared to 3.0% of non-Caucasian patients. As shown in Table 2, there were no differences at diabetes onset in baseline blood glucose or HbA1c, but Caucasian patients had a greater impairment of the acid–base balance. However, the insulin dose at discharge was slightly higher in the non-Caucasian group.
Epidemiological and clinical variables at diabetes onset.
| Non-Caucasian (n=33) | Caucasian (n=138) | p | ||
|---|---|---|---|---|
| Sex (% males) | 72.7 | 75.3 | 0.65 | |
| Age (years) | 30.5±6.0 | 28.7±5.8 | 0.12 | |
| BMI (kg/m2) | 25.1±5.1 | 23.4±5.0 | 0.09 | |
| Weeks of symptoms | 6.7±5.9 | 7.8±6.7 | 0.39 | |
| Weight loss (kg) | 9.2±4.8 | 8.2±5.4 | 0.36 | |
| Insulin dose at discharge (IU/kg) | 0.63±0.19 | 0.55±0.21 | 0.045 | |
| Clinical condition | Hyperglycemia (%) | 39.3 | 18.1 | 0.02 |
| Ketosis (%) | 57.5 | 66.6 | ||
| DKA (%) | 3.0 | 15.2 | ||
Data given as mean±standard deviation.
Biochemical and immunological data at diabetes onset.
| Non-Caucasian (n=33) | Caucasian (n=138) | p | |
|---|---|---|---|
| Baseline blood glucose (mmol/L) | 22.3±9.2 | 19.5±5.8 | 0.12 |
| HbA1c (%) | 12.4±2.1 | 12.1±2.2 | 0.47 |
| pH | 7.38±0.05 | 7.34±0.11 | 0.01 |
| Bicarbonate (mmol/L) | 26.1±3.9 | 23.7±6.9 | 0.01 |
| Basal C-peptide (nmol/L) | 0.32±0.29 | 0.27±0.27 | 0.225 |
| Stimulated C-peptide (nmol/L) | 0.70±0.56 | 0.42±0.39 | 0.01 |
| Antibodies (% positive) | 27.2 | 73.1 | <0.01 |
| Anti-GAD Ab (% positive) | 27.2 | 65.9 | <0.01 |
| Anti-IA2 Ab (% positive) | 18.2 | 42.0 | 0.02 |
Table 2 shows differences in pancreatic beta cell function between the two groups at admission. Non-Caucasian patients had higher baseline C-peptide levels (although the difference was not statistically significant) and significantly higher stimulated C-peptide levels as compared to the Caucasian group.
Table 2 shows the prevalence of autoantibodies in Caucasian and non-Caucasian patients. Anti-pancreatic beta cell antibodies were found in 73.1% and 27.2% of Caucasian and non-Caucasian patients respectively. Patients with positive autoimmunity had stimulated C-peptide levels lower than patients with negative autoimmunity, and no differences were seen between Caucasian and non-Caucasian patients. Among patients with negative autoimmunity, however, non-Caucasians had a clearly greater pancreatic reserve (0.93 vs. 0.57nmol/L) (Table 3).
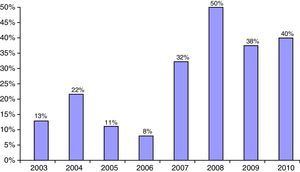
Two-year dataAt 2 years of follow-up, patients in both groups showed no differences in the BMI and HbA1c, but the proportion of patients with intensified treatment was significantly greater in Caucasian patients (93.8% vs. 39.1%) (Table 4). Treatment with metformin was also more common in non-Caucasian patients: 17.4% vs. 1.8% as a single treatment and 21.7% vs. 3.6% in combination with insulin.
Clinical variables and metabolic control 2 years after onset.
| Non-Caucasian (n=29) | Caucasian (n=127) | p | ||
|---|---|---|---|---|
| BMI (kg/m2) | 26.3±5.4 | 24.7±3.9 | 0.315 | |
| HbA1c | 6.9±1.8 | 6.5±1.6 | 0.473 | |
| Insulin dose (IU/kg) | 0.45±0.31 | 0.45±0.18 | 0.964 | |
| Intensive treatment (%) | 39.1 | 93.8 | <0.001 | |
| Positive autoimmunity | BMI (kg/m2) | 25.2±5.6 | 24.6±4.1 | 0.924 |
| HbA1c | 7.7±2.2 | 6.6±1.7 | 0.166 | |
| Intensive treatment (%) | 87.5 | 100 | 0.130 | |
| Negative autoimmunity | BMI (kg/m2) | 26.9±5.5 | 24.9±3.0 | 0.243 |
| HbA1c | 6.3±1.3 | 6.1±1.3 | 0.531 | |
| Intensive treatment (%) | 13.3 | 70.8 | 0.002 | |
In the patient subgroup with positive autoimmunity, no differences were seen between patients of Caucasian and non-Caucasian origin in the BMI, HbA1c, or the proportion with treatment intensification. By contrast, in the subgroup with negative autoimmunity, although no differences were found in the BMI or metabolic control, a difference was seen in the proportion of patients with treatment intensification, which was significantly higher in the Caucasian group (70.8% vs. 13.3%) (Table 4).
ConclusionsThe number and proportion of patients of non-Caucasian origin admitted to our hospital for adult-onset diabetes has significantly increased in recent years, reaching a maximum of 50% in 2008. This progressive increase has been in line with the increase in the non-Caucasian population in the districts forming the reference area of our hospital. According to the official statistics web site of Catalonia (idescat), the immigrant population increased in the 2003–2010 period from 10.48% to 23.26% of the total population in L’Hospitalet de Llobregat and from 6.27% to 11.96% in the Baix Llobregat region.
Non-Caucasian patients have characteristics similar to those of the Caucasian population as regards age at diagnosis, the BMI, weeks of symptoms, and weight loss, which makes differentiation based on clinical history and physical examination impossible. Sex distribution is also similar in both groups and agrees with that already reported at diabetes onset, especially type 1 diabetes. Despite this, patients of non-Caucasian origin have less severe laboratory test results at diagnosis, a lower prevalence of autoimmunity, and better pancreatic beta cell function. In addition, the overall non-Caucasian group has a course more characteristic of type 2 diabetes, with good metabolic control despite a low rate of treatment intensification and wide metformin use.
There are differences between our study and other studies based on subjects from other non-Caucasian populations. In those studies, adult patients with symptomatic onset who had ketotic decompensation had a phenotype more characteristic of type 2 diabetes, typically with obesity.4,5 By contrast, in our study sample patients of non-Caucasian origin had from the phenotypic viewpoint characteristics similar to Caucasian patients, so making the initial classification of diabetes more difficult.
Different ways have been proposed to classify the different subgroups of atypical diabetes mellitus, of which the Aβ system best predicts the persistence of pancreatic function,12–14 which is in turn the best long-term marker of insulin dependence.15,16 Prior studies have shown that better baseline pancreatic function data correlate to a trend to insulin dependence over time.8 This model is based on the presence or absence of autoantibodies (A+ or A−) and pancreatic reserve (β+ or β−) and classifies patients into one of four different subgroups. Pancreatic beta function is “preserved” if the baseline C-peptide level is ≥1ng/mL (0.33nmol/L) or the stimulated C-peptide level is ≥1.5ng/mL (0.495nmol/L), and “absent” if a lower level is found.12,14 A+ β− subjects have a complete defect of pancreatic function and the presence of autoantibodies, corresponding to cases of type 1A diabetes mellitus. A− β− subjects have complete β cell failure, but an absence of immunological markers, corresponding to the group of patients with type 1B diabetes mellitus, also with poor long-term prognosis in terms of insulin dependence. A+ β+ subjects have preserved pancreatic function and positive autoantibodies; approximately half of this group have a course similar to patients with type 1 diabetes mellitus, while the others have a clinical course similar to type 2, with the long-term preservation of pancreatic function. Finally, A− β+ subjects have preserved β cell function and no autoimmunity, corresponding to cases of type 2 diabetes of atypical onset.
In our study, Caucasian patients with positive autoimmunity, who form a majority, had a course characteristic of type 1A diabetes, with treatment intensification in 100% at 2 years. In addition, a substantial proportion of Caucasian patients with negative autoimmunity also were on intensified treatment at 2 years (70.8%) and had baseline stimulated C-peptide levels which, although higher than those of patients with positive autoimmunity, were clearly lower than those of non-Caucasian patients with negative autoimmunity. It can thus be stated that a high proportion of these patients behave as if they had type 1B diabetes.
Our most relevant findings were made in the non-Caucasian population, in which the presence or absence of autoantibodies was determinant. Patients with positive autoimmunity, who were a minority (28%), had pancreatic reserve data and a 2-year course similar to Caucasian patients and, thus, a diabetes 1A phenotype. By contrast, the absence of autoimmunity virtually ensured, unlike in the Caucasian population, a course characteristic of type 2 diabetes, with baseline stimulated C-peptide levels four times higher than those seen in patients with positive autoimmunity and a low need for treatment intensification at 2 years. From a practical viewpoint, it may be stated that a course characteristic of type 1B diabetes (clear insulin dependence with negative autoimmunity data) is virtually nonexistent in non-Caucasian patients.
Our study had some limitations. Our patients were adults aged 18–40 years, and the findings cannot therefore be extrapolated to the pediatric population as a whole or to elderly subjects, in whom the epidemiology of diabetes in the non-Caucasian population is little known. Moreover, the non-Caucasian group had a heterogeneous origin, thus preventing the extrapolation of conclusions for specific ethnic groups. On the other hand, the need for initial admission may induce selection bias in study cases, a factor which could be especially relevant at centers that have an outpatient department where patients may be cared for without being admitted to a general ward. In this regard, the diabetes outpatient department of our center has only been available since the end of 2010, so that admission to a general ward was the only form of treatment available for our study patients. In addition, no data were available on pancreatic reserve at 2 years. Therefore, patient categorization based on the course of the disease was mainly based on the type of treatment they required. This may induce bias if the trend of the medical team is to a decreased intensification in the non-Caucasian population due to aspects derived from the social environment and existence of a language barrier. However, a comparability of the degree of metabolic control at 2 years in Caucasian and non-Caucasian subjects does not suggest the existence of such a bias. On the other hand, only anti-GAD and anti-IA-2 antibodies were considered for the detection of autoimmunity. Other autoantibodies used for the classification of autoimmune diabetes mellitus were not measured, including anti-insulin, zinc transporter 8 (ZnT8 Ab) or IA-2β antibodies, although the two later antibodies are not usually expressed alone, but only when at least the other three types are positive,17 so that the rate of false negative results would not have been so important. Finally, most studies previously published were conducted on populations of African or Chinese origin, and few studies are available in Maghrebi and Hispanic subjects, who were the main population groups in our study, so that the populations were not fully comparable.
To sum up, the proportion of non-Caucasian patients admitted to hospital for diabetes onset has significantly increased. This group has a lower prevalence of pancreatic autoimmunity and better beta cell function at onset, and a course of disease more similar to that of type 2 diabetes. This is particularly evident in non-Caucasian patients with negative autoimmunity data who, unlike Caucasian patients with negative autoimmunity, show a course of disease closer to that of type 2 diabetes.
Conflict of interestThe authors state that they have no conflicts of interest.
Please cite this article as: San José P, Guerrero M, García-Martín I, Caballero J, Pérez-Maraver M. Impacto y características diferenciales de la población de origen no caucásico en los ingresos por inicio de diabetes durante el periodo 2003–2010. Endocrinol Nutr. 2016;63:285–290.