To study plasma adiponectin levels in women diagnosed with polycystic ovary syndrome given omega-3 fatty acid supplements.
Patients and methodsA study was conducted in 195 women diagnosed with polycystic ovary syndrome treated with omega-3 fatty acids for 12 weeks (n=97; group A) and control women given placebo (n=98, group B). General characteristics, metabolism, lipid profile, and hormone and adiponectin levels were compared.
ResultsThere were no significant differences between the two groups in general characteristics. No significant differences were also found in hormone, blood glucose, and HOMA levels between the groups. Women in study groups A and B showed no statistically significant differences in total calorie, carbohydrate, protein, and total fat intake between the baseline and final values. Decreased total cholesterol, low-density lipoprotein, and triglyceride levels were found in group A women (p<0.0001). Mean of adiponectin levels also showed a statistically significant increase after treatment (p<0.0001). There were no statistically significant differences in the mean values of the different variables in group B women.
ConclusionOmega-3 fatty acid supplementation for 12 weeks caused a significant increase in plasma adiponectin levels in women with polycystic ovary syndrome.
Estudiar las concentraciones plasmáticas de adiponectina en mujeres con diagnóstico de síndrome de ovario poliquístico (SOPQ) tratadas con suplementación de ácidos grasos omega-3.
Material y métodosSe realizó un estudio en 195 mujeres con diagnóstico de SOPQ que fueron tratadas con ácidos grasos omega-3 durante 12 semanas (n=97; grupo A) y controles tratados con placebo (n=98, grupo B). Se compararon las características generales, las concentraciones hormonales, el perfil lipídico y la adiponectina.
ResultadosNo se encontraron diferencias significativas entre ambos grupos con relación a las características generales. Tampoco se encontraron diferencias significativas en las concentraciones hormonales, glucemia y HOMA entre los grupos. Las mujeres de los grupos A y B no mostraron diferencias estadísticamente significativas en la ingesta total, ingesta de hidratos de carbono, proteínas y grasas totales entre los valores al inicio y al final del estudio. Las mujeres del grupo A presentaron disminución en las concentraciones de colesterol total, de lipoproteínas de baja densidad y de triglicéridos (p<0,0001). Los valores promedio de adiponectina también mostraron aumento estadísticamente significativo luego del tratamiento (p<0,0001). No se encontraron diferencias estadísticamente significativas en los valores promedio de las diferentes variables en las mujeres del grupo B.
ConclusiónLa suplementación de ácidos grasos omega-3 durante 12 semanas produce aumento significativo en las concentraciones plasmáticas de adiponectina en mujeres con SOPQ.
Polycystic ovary syndrome (POS) is a common endocrine-metabolic disorder in women of reproductive age, and is characterized by chronic anovulation and hyperandrogenism. The syndrome affects 5–10% of all women and is the cause of 50–70% of all cases of anovulatory infertility. Patients with POS suffer oligomenorrhea or amenorrhea and hyperandrogenism (with hirsutism, acne, increased plasma androgen levels, or a combination of such findings).1 Obesity and insulin resistance are known to be frequent in women with POS, and both conditions are related to hormone changes.2,3
Adipose tissue is active from the endocrine perspective and produces a number of peptides (including adipokines and growth factors), lipids and steroids. Adipokines exert autocrine and paracrine action upon the adipose tissue itself, as well as endocrine actions upon other organs and tissues such as skeletal muscle, adrenal glands and the central nervous system, modulating different functions, including insulin action.4 Hypoadiponectinemia has been associated with the physiopathology and metabolic complications of the syndrome.5 A number of studies have demonstrated a decrease in plasma adiponectin concentration in women with POS.4,5
In women with POS, many nutritional studies have focused on the effects of energy restriction and weight loss upon different metabolic and hormone parameters.6 Omega-3 fatty acids exert effects against obesity, inflammation and insulin resistance.7 Experimental studies have revealed an association between the consumption of these fatty acids and increments in adiponectin concentration.8 However, in humans the findings of clinical trials involving different doses and supplementation periods remain controversial.9
A number of studies have evaluated the effect of omega-3 fatty acids upon the metabolic balance and condition of different diseases,10,11 though few have involved women diagnosed with POS. Understanding the effects of omega-3 fatty acids upon the syndrome and certain adipokines, particularly adiponectin, may be important for women. The present study investigates plasma adiponectin concentrations in women diagnosed with POS and treated with omega-3 fatty acid supplements.
Material and methodsThe present prospective clinical study was carried out in women with POS who attended our hospital in the period between January 2011 and May 2017. All the participants were subjected to clinical evaluation (general and gynecological exploration, including transvaginal ultrasound).
The diagnosis of POS was established according to the criteria of the consensus group12: oligomenorrhea (an interval of ≥35 days) or amenorrhea (an absence of vaginal bleeding for 6 months), hirsutism, an increased LH/FSH ratio, elevated serum testosterone, and the presence of multiple ovarian cysts (over 10 small cysts measuring 2–8mm in diameter) with a peripheral and dispersed distribution throughout the dense stromal nucleus (the presence of a ring of follicular cysts), revealed by transvaginal ultrasound performed by two physicians of the Imaging Diagnosis Department of the hospital and unrelated to the investigation. In addition to the above findings, other similar syndromes were excluded, such as adrenal gland dysfunction, Cushing's syndrome, congenital adrenal hyperplasia, androgen-producing tumors, hyperprolactinemia and untreated hypothyroidism or thyroid gland disease.
Women using oral contraceptives were excluded from the study, as were those receiving antiandrogen medication, insulin sensitizing drugs or any other medication or supplement with effects upon body weight, insulin sensitivity or the normal function of the hypothalamic-hypophyseal-gonadal axis during the three months before the study. We also excluded women with a history of diabetes mellitus, kidney disease, liver and/or gastrointestinal disorders, smoking or the consumption of more than two alcoholic drinks per week, as well as those that failed to comply with the study protocol or could take more than 80% of the administered treatment.
Written informed consent to participation was obtained from all the patients. The study protocol abided with the ethical standards of “Dr. Urquinaona” Central Hospital–University of Zulia, and with the Declaration of Helsinki (1975, revised in 2004). The local Research Ethics Committee approved the study.
The sample size was calculated on the basis of a review of previous investigations that showed a decrease of approximately 20% in adiponectin concentration after supplementing with omega-3 fatty acids. In reaching this objective with an alpha error of 0.05 and a statistical power of 90%, the inclusion of 125 women in each group was considered necessary in order to obtain similar results.
A computer-generated random numbers table was used for patient randomization. The sealed and numbered envelopes were kept by a person unconnected to the study and blinded to the objectives of the trial. Each envelope contained an assignment to a group: group A (cases: supplementing with omega-3 fatty acids) or group B (controls: supplementing with placebo). Each capsule of omega-3 fatty acids contained 180mg of eicosapentaenoic acid and 120mg of docosahexaenoic acid, while each capsule of placebo contained 1g of paraffin. The capsules and containers were all similar. The written records with the codes and respective intervention groups were only opened once all the analyses had been made.
The participants in both groups were consulted weekly by telephone and visited the clinic every four weeks. The women were asked to continue their usual diet and lifestyle. Data regarding the food consumed during a 7 day period were obtained at the start and end of the study, and were analyzed by two nutritionists participating in the study but unaware of the group to which each patient belonged, using Food Processor Nutrition Analysis Software (Esha Research, USA).
Body weight (kg) was recorded twice to the nearest 0.1kg, with the patient barefoot. Body height (m) was recorded twice to the nearest 0.5cm, with the patient barefoot. The body mass index (BMI) was calculated as weight divided by height,2 or kg/square meter. The waist circumference was measured with the patient in the standing position, at the midpoint between the upper margin of the iliac crest and the lower margin of the last rib, using a metric tape (cm). The hip circumference in turn was determined with the patient in the standing position as the greatest distance between the major trochanters. The waist/hip ratio was calculated by dividing the waist circumference (in cm) by the hip circumference (in cm).
The venous blood samples at the start and after 12 weeks of treatment were collected between 8:00–11:00a.m., under fasting conditions. Standard oral glucose tolerance testing (75g) was performed, together with evaluation of the insulin response to glucose loading, after 10–12h of fasting, between 8:30 and 10:30a.m. The results of the glucose tolerance tests were evaluated based on the criteria of the American Diabetes Association (ADA).13 All the samples were stored at −70°C until the time of analysis.
The plasma adiponectin concentrations were measured using a commercial ELISA kit (B-Bridge International, USA). The intra- and inter-assay coefficients of variation were 4.9% and 6.3%, respectively. All hormones were determined based on electrochemiluminescence immunoassays using the Elecsys 2010 autoanalyzer (Boehringer Mannheim, Germany), with specific reagents. The intra- and inter-assay coefficients of variation for each hormone were: FSH (1.7% and 4.7%), LH (1.1% and 3.1%), prolactin (2.9% and 4.1%), TSH (4.2% and 5.2%), estradiol (2.1% and 4.5%), testosterone (2.4% and 3.8%), insulin (3.0% and 4.7%), androstenedione (4.1% and 5.2%), respectively. The concentrations of dehydroepiandrosterone sulfate (intra- and inter-assay coefficients of variation: 7.5% and 5.5%, respectively) and androstenedione (intra- and inter-assay coefficients of variation: 6.8% and 7.2%, respectively) were measured using enzymoimmunoassay tests (Diagnostic Systems Laboratories, USA). In turn, 17-hydroxyprogesterone was measured using a double antibody test (ICN Pharmaceuticals, USA) (intra- and inter-assay coefficients of variation: 5.1% and 7.6%, respectively).
The glucose concentrations were determined using an enzyme method. An autoanalyzer (Hitachi 912, Boehringer Mannheim, Germany), with specific reagents was used. Insulin resistance under fasting conditions was evaluated using the Homeostatic Model Assessment (HOMA-IR), calculated using the following equation: (insulin×glucose)/22.5. Insulin was measured in μU/ml, and glucose in mmol/l.14 A HOMA-IR score of over 3.5 was interpreted as representing insulin resistance. The areas under the insulin and glucose curves were calculated according to the formula applicable to each geometrical figure, representing an increase in postprandial plasma concentrations above basal levels.15
The serum concentrations of total cholesterol (TC), high density lipoprotein cholesterol (HDLc), low density lipoprotein cholesterol (LDLc) and triglycerides (TGs) were recorded using the Abbott Aeroset automatic analyzer (Abbott Diagnostics, USA). The values corresponding to apolipoprotein A (Apo-A) and apolipoprotein B (Apo-B) were recorded by nephelometric assay using an Array 360 system (Beckman Coulter, USA). The intra- and inter-assay coefficients of variation were under 10% for all the tests.
The variables were reported as the mean±standard deviation (SD). After confirming normal data distribution with the Kolmogorov–Smirnov test, the Student t-test for related samples was used to compare values before and after treatment in both groups of patients. The Pearson test was used to correlate the adiponectin values to the values of the different laboratory parameters in each study period. The percentage change in variables after the intervention was determined from the following formula: [(final values−initial values)/initial values]×100. All statistical analyses were made using the SPSS version 19.0 statistical package (SPSS Inc., USA). Statistical significance was considered for p<0.05.
ResultsA total of 250 women diagnosed with POS were selected. Fifty-five participants were excluded from the final analysis (28 in group A and 27 in group B) because they failed to complete the follow-up period, stopped taking the capsules and/or could not provide all the measurements of the different study variables. A total of 195 women were therefore included in the final analysis: 97 treated with omega-3 fatty acids during 12 weeks (group A) and 98 controls (group B). The mean patient age was 23.5±3.6, with a BMI of 26.2±2.9kg/m2 and a waist/hip ratio of 0.80±0.06.
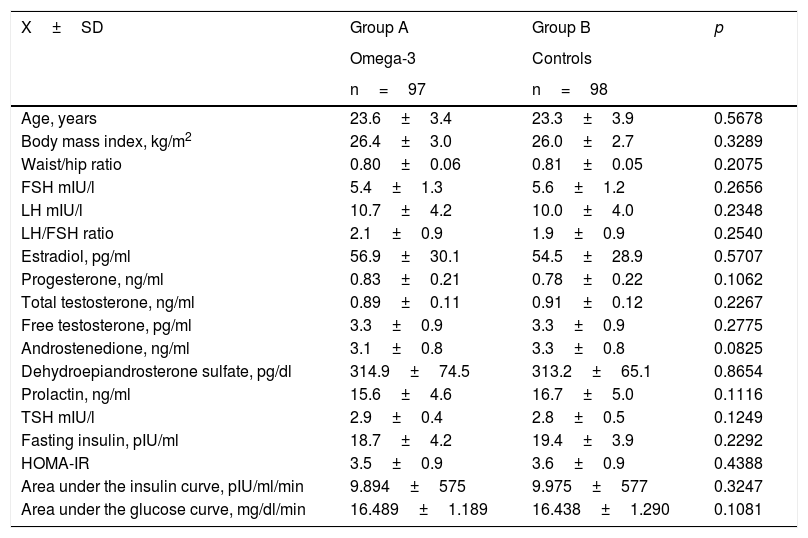
Table 1 shows the general characteristics of each study group. There were no statistically significant differences between the two groups of women in terms of age, the BMI or the waist/hip ratio. Likewise, no significant differences were observed in the concentrations of sex hormones, thyroid hormones or prolactin between groups A and B. In turn, no significant differences were observed between the two groups in relation to fasting insulin concentration, HOMA-IR, or the areas under the insulin and glucose curves.
General characteristics of each study group.
| X±SD | Group A | Group B | p |
|---|---|---|---|
| Omega-3 | Controls | ||
| n=97 | n=98 | ||
| Age, years | 23.6±3.4 | 23.3±3.9 | 0.5678 |
| Body mass index, kg/m2 | 26.4±3.0 | 26.0±2.7 | 0.3289 |
| Waist/hip ratio | 0.80±0.06 | 0.81±0.05 | 0.2075 |
| FSH mIU/l | 5.4±1.3 | 5.6±1.2 | 0.2656 |
| LH mIU/l | 10.7±4.2 | 10.0±4.0 | 0.2348 |
| LH/FSH ratio | 2.1±0.9 | 1.9±0.9 | 0.2540 |
| Estradiol, pg/ml | 56.9±30.1 | 54.5±28.9 | 0.5707 |
| Progesterone, ng/ml | 0.83±0.21 | 0.78±0.22 | 0.1062 |
| Total testosterone, ng/ml | 0.89±0.11 | 0.91±0.12 | 0.2267 |
| Free testosterone, pg/ml | 3.3±0.9 | 3.3±0.9 | 0.2775 |
| Androstenedione, ng/ml | 3.1±0.8 | 3.3±0.8 | 0.0825 |
| Dehydroepiandrosterone sulfate, pg/dl | 314.9±74.5 | 313.2±65.1 | 0.8654 |
| Prolactin, ng/ml | 15.6±4.6 | 16.7±5.0 | 0.1116 |
| TSH mIU/l | 2.9±0.4 | 2.8±0.5 | 0.1249 |
| Fasting insulin, pIU/ml | 18.7±4.2 | 19.4±3.9 | 0.2292 |
| HOMA-IR | 3.5±0.9 | 3.6±0.9 | 0.4388 |
| Area under the insulin curve, pIU/ml/min | 9.894±575 | 9.975±577 | 0.3247 |
| Area under the glucose curve, mg/dl/min | 16.489±1.189 | 16.438±1.290 | 0.1081 |
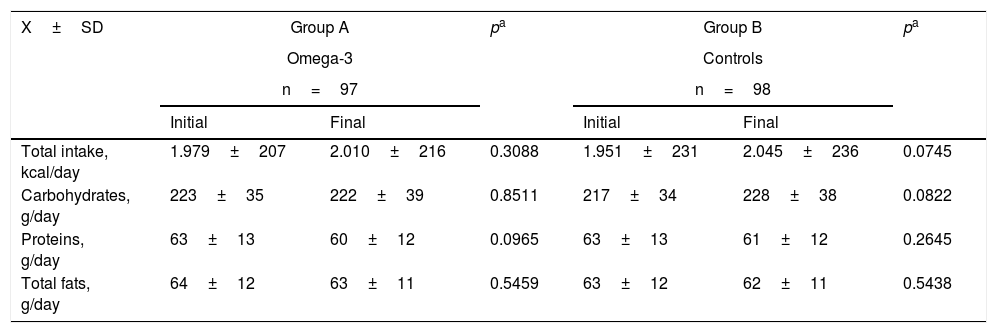
Table 2 shows the food intake values of the two groups at the start and end of the study. There were no statistically significant differences between the two groups in terms of the total intake or the intake of carbohydrates, proteins and fats between the start and end of the study. Likewise, no statistically significant differences were recorded in any of the study variables between the two food intake groups at the end of the study.
Food intake in the two groups at the start and end of the study.
| X±SD | Group A | pa | Group B | pa | ||
|---|---|---|---|---|---|---|
| Omega-3 | Controls | |||||
| n=97 | n=98 | |||||
| Initial | Final | Initial | Final | |||
| Total intake, kcal/day | 1.979±207 | 2.010±216 | 0.3088 | 1.951±231 | 2.045±236 | 0.0745 |
| Carbohydrates, g/day | 223±35 | 222±39 | 0.8511 | 217±34 | 228±38 | 0.0822 |
| Proteins, g/day | 63±13 | 60±12 | 0.0965 | 63±13 | 61±12 | 0.2645 |
| Total fats, g/day | 64±12 | 63±11 | 0.5459 | 63±12 | 62±11 | 0.5438 |
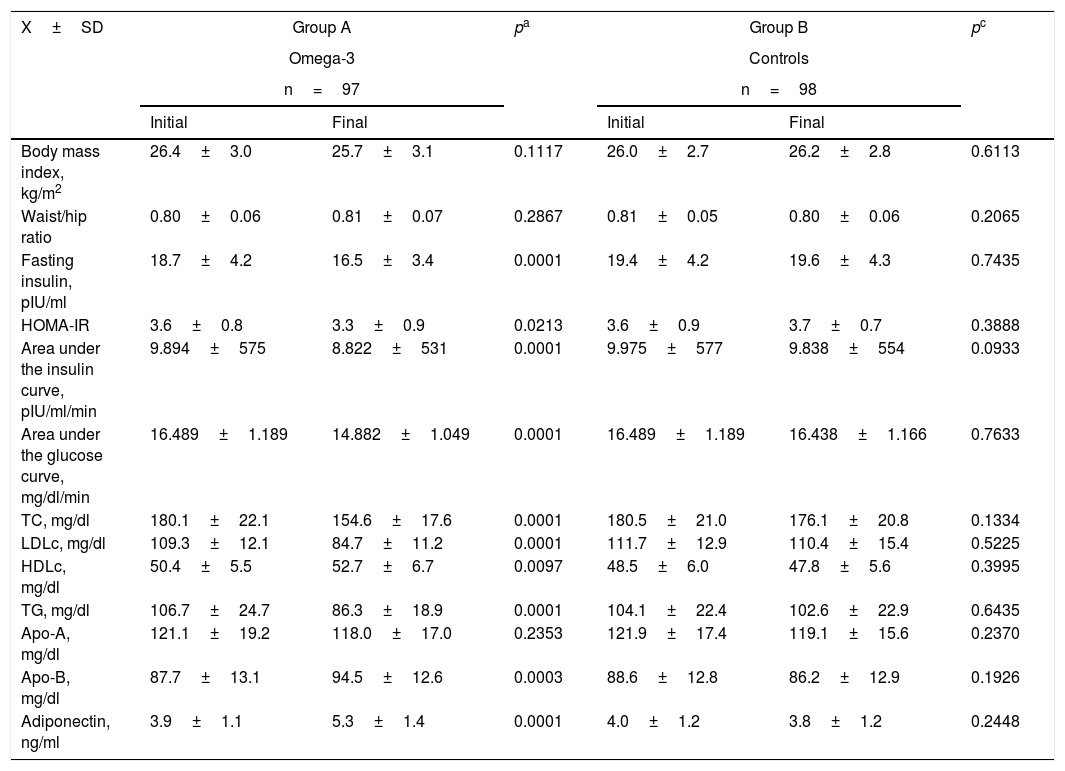
With regard to insulin resistance, lipid profile and adiponectin (Table 3), the women treated with omega-3 fatty acids for 12 weeks showed a decrease in fasting insulin (approximately 12%), HOMA-IR (over 8%), the area under the insulin curve (approximately 10%) and the area under the glucose curve (over 10%). All of these differences were considered statistically significant (p<0.05). The patients in group A also showed a decrease of about 15% in total cholesterol (p<0.0001). This was accompanied by a 23% decrease in LDLc (p<0.0001) and a 25% decrease in triglyceride levels (p<0.0001). We also recorded a significant 4% increase in HDLc concentration (p<0.0097) and a 7% increase in Apo-B concentration (p<0.0001). By contrast, Apo-A levels showed no significant variations (p=0.2353). The mean final adiponectin values showed a statistically significant increase of about 35% versus the initial levels (5.3±1.4ng/ml versus 3.9±1.1ng/ml; p<0.0001). There were no statistically significant differences in the mean values of the different variables in the control group.
Clinical and laboratory test parameters in the two groups at the start and end of the study.
| X±SD | Group A | pa | Group B | pc | ||
|---|---|---|---|---|---|---|
| Omega-3 | Controls | |||||
| n=97 | n=98 | |||||
| Initial | Final | Initial | Final | |||
| Body mass index, kg/m2 | 26.4±3.0 | 25.7±3.1 | 0.1117 | 26.0±2.7 | 26.2±2.8 | 0.6113 |
| Waist/hip ratio | 0.80±0.06 | 0.81±0.07 | 0.2867 | 0.81±0.05 | 0.80±0.06 | 0.2065 |
| Fasting insulin, pIU/ml | 18.7±4.2 | 16.5±3.4 | 0.0001 | 19.4±4.2 | 19.6±4.3 | 0.7435 |
| HOMA-IR | 3.6±0.8 | 3.3±0.9 | 0.0213 | 3.6±0.9 | 3.7±0.7 | 0.3888 |
| Area under the insulin curve, pIU/ml/min | 9.894±575 | 8.822±531 | 0.0001 | 9.975±577 | 9.838±554 | 0.0933 |
| Area under the glucose curve, mg/dl/min | 16.489±1.189 | 14.882±1.049 | 0.0001 | 16.489±1.189 | 16.438±1.166 | 0.7633 |
| TC, mg/dl | 180.1±22.1 | 154.6±17.6 | 0.0001 | 180.5±21.0 | 176.1±20.8 | 0.1334 |
| LDLc, mg/dl | 109.3±12.1 | 84.7±11.2 | 0.0001 | 111.7±12.9 | 110.4±15.4 | 0.5225 |
| HDLc, mg/dl | 50.4±5.5 | 52.7±6.7 | 0.0097 | 48.5±6.0 | 47.8±5.6 | 0.3995 |
| TG, mg/dl | 106.7±24.7 | 86.3±18.9 | 0.0001 | 104.1±22.4 | 102.6±22.9 | 0.6435 |
| Apo-A, mg/dl | 121.1±19.2 | 118.0±17.0 | 0.2353 | 121.9±17.4 | 119.1±15.6 | 0.2370 |
| Apo-B, mg/dl | 87.7±13.1 | 94.5±12.6 | 0.0003 | 88.6±12.8 | 86.2±12.9 | 0.1926 |
| Adiponectin, ng/ml | 3.9±1.1 | 5.3±1.4 | 0.0001 | 4.0±1.2 | 3.8±1.2 | 0.2448 |
Apo-A: apolipoprotein A; Apo-B: apolipoprotein B; HDLc: high density lipoprotein cholesterol; LDLc: low density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides.
In group A, the adiponectin concentrations before treatment were seen to correlate significantly (p<0.05) with the area under the insulin curve (r=−0.245), while the concentrations after treatment only correlated with the triglyceride levels (r=−0.340).
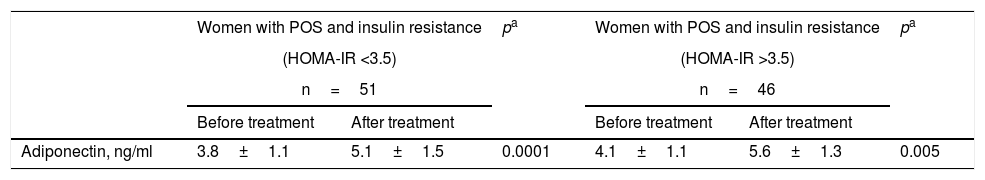
Table 4 shows the adiponectin levels before and after treatment among the patients in group A, stratified according to the presence of insulin resistance (HOMA-IR >3.5). The women with insulin resistance showed a significant increase (34%) in the final adiponectin levels versus the initial values (5.1±1.5ng/ml versus 3.8±1.1ng/ml; p<0.0001). The women without insulin resistance also showed a significant increase (36%) in the final adiponectin levels versus the initial values (5.6±1.3ng/ml versus 4.1±1.1ng/ml; p=0.005). No statistically significant differences were observed on comparing the initial and final concentrations in the two groups.
Plasma adiponectin according to insulin resistance among women in group A at the start and end of treatment with omega-3 fatty acids.
The evidence indicates that POS contributes to the development of a proinflammatory state that in turn correlates with variations in the plasma concentrations of different adipokines.16 Adiponectin exerts important antiinflammatory, vascular protective, antidiabetic and cardioprotective effects.4 The present study investigated the plasma adiponectin concentrations in women diagnosed with POS and treated with omega-3 fatty acid supplements. A significant increase in these concentrations was observed in the women with POS after 12 weeks of supplementing with omega-3 fatty acids.
Different clinical and experimental studies in healthy individuals and subjects with different disease conditions have explored the potential effects of omega-3 fatty acid supplements upon adiponectin concentrations. One such study showed that the consumption of fish oil for 8 weeks increased adiponectin concentrations in non-obese young individuals.17 Likewise, two studies, one involving obese subjects8 and another in women with hyperinsulinemia and overweight,18 found that supplementing with polyunsaturated fatty acids raised adiponectin concentrations. Studies in animals have shown a diet containing fish oil to be associated with increased adiponectin levels.19 However, other earlier studies were unable to demonstrate changes in adiponectin concentration after supplementing with omega-3 fatty acids20; this was possibly attributable to the metabolic state of the selected individuals or to differences in design between the studies.
The increase in adiponectin observed in our study among women with POS after 12 weeks of supplementing with omega-3 fatty acids was similar to that reported by Mohammadi et al.,21 who recorded an increase in adiponectin levels after treatment with omega-3 fatty acids for 8 weeks. This increase was accompanied by benefits in terms of insulin resistance, suggesting a contribution to metabolic control among the women. However, a previous investigation found no significant changes in plasma adiponectin concentrations in women diagnosed with POS after 6 weeks of supplementing with fish oil.22
In the present study, treatment with omega-3 fatty acids resulted in significant reductions in total cholesterol, triglycerides, LDLc and Apo-B, together with an increase in HDLc. These results are consistent with those of previous studies indicating significant reductions in triglyceride levels in women with POS treated with fish oil, although in these cases no significant changes in serum total cholesterol, LDLc or HDLc were noted.23 A decrease in the plasma concentrations of triglycerides has also been reported by other authors.24 A previous study showed that treatment with omega-3 fatty acids resulted in significant reductions in triglyceride levels, together with an increase in HDLc.25 The effect of supplementing with omega-3 fatty acids upon triglyceride levels depends upon the dose administered.24 In this regard, the dosage and duration of treatment with omega-3 fatty acids may have sufficed to produce the significant improvements observed after the intervention in this group of women.
The women with POS subjected to omega-3 fatty acid supplementing showed no significant differences in the BMI versus the initial or baseline values. This observation is consistent with the findings of a previous study in which supplements of this kind exerted scant effects upon the patient anthropometric profile.26 This is an important finding, since no differences in diet were noted during the study, and agrees with the previous observations of Mohammadi et al.,21 who recorded no changes in patient body weight after supplementing for 8 weeks. On the other hand, a number of studies demonstrated a decrease in the BMI after 8 weeks of supplementing with omega-3 fatty acids in women with non-insulin dependent diabetes,22 as well as a decrease in weight among mice.27 Other studies suggest that supplementing exerts this effect, especially when complemented with other weight-losing treatments, though these findings are contradictory.28 Obesity and its comorbidities (diabetes, cancer and heart diseases) are related to inflammation, and omega-3 fatty acids have antiinflammatory properties.
The possible explanation for the findings of this study is that omega-3 fatty acids are natural ligands for four metabolic nuclear receptors: the peroxisome proliferator activated receptor (PPAR) γ, the liver X receptor, the hepatocyte nuclear factor-4α and the farnesol X receptor. The activation of these receptors down-regulates genes encoding for proteins that stimulate lipid synthesis, and regulates genes that improve fatty acid oxidation in liver and muscle.29 Furthermore, certain positive effects upon the lipid profile are mediated by an improvement in AMP-activated protein kinase, an important sensor of cell energy status that regulates the division between lipid oxidation and lipogenesis.27 One of the main reported effects of supplementing with omega-3 fatty acids is the stimulation of the activation of the adiponectin gene in adipose cells, probably acting through ligands of peroxisome proliferator γ, a transcription regulator that interacts with the gene promoter.30
This study has several limitations. The investigation was carried out in women diagnosed with POS and of normal body weight. This can result in under- or overestimation of the changes in plasma adiponectin concentration; since the effects of omega-3 fatty acids are dose dependent, the findings may not be applicable to obese or overweight women, to other types of supplements or to different supplementing periods. In our study, we only measured total adiponectin concentrations, not those corresponding to high molecular weight adiponectin (which is more active and is related to insulin sensitivity). Lastly, although caloric intake was evaluated among the patients, no assessment of the changes in physical activity of the participants during the study period was made.
On the basis of the findings of our study, it can be concluded that supplementing with omega-3 fatty acids for 12 weeks results in a significant increase in plasma adiponectin levels in women with POS. This modulation of adiponectin secretion, directly related to the regulation of metabolism and insulin resistance, could have beneficial effects.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Mejia-Montilla J, Reyna-Villasmil E, Domínguez-Brito L, Naranjo-Rodríguez C, Noriega-Verdugo D, Padilla-Samaniego M, et al. Suplementación de ácidos grasos omega-3 y adiponectina plasmática en mujeres con síndrome de ovarios poliquísticos. Endocrinol Diabetes Nutr. 2018;65:192–199.