ACTH-independent Cushing's syndrome (AICS) accounts for 15–20% of cases of Cushing's syndrome, with <1% due to abnormal receptors. Our aim is to study the presence of abnormal receptors in subjects diagnosed with AICS with nodular adrenal hyperplasia in a 14-year period (2002–2016), as well as its clinical–biological and evolutive characteristics.
Material and methodsA multicentre descriptive study of a 15-case series of AICS with nodular adrenal hyperplasia (study period: 2002–2016). In these cases, abnormal receptor screening was performed by means of stimulation tests, with a plasma cortisol increase of ≥25% from baseline being considered pathologic.
ResultsOf the 15 cases, 13 were female, with a mean age at diagnosis of 56.8 years. In 12 of the 15 cases studied, positivity was detected with stimulation tests, and, of them, 25% were positive for the meal test, 58.3% for posture walking test, 33.3% for desmopressin; 25% for terlipressin; 33.3% for GnRH; 25% for LH and 50% for metoclopramide. Regarding treatment, bilateral adrenalectomy was performed in 16.7% and unilateral adrenalectomy in 41.7%.
The rest continue under observation with periodic follow-up (41.7%).
ConclusionsIn most of the cases studied with AICS and nodular adrenal hyperplasia (80%), an abnormal cortisol response is detected due to the presence of abnormal receptors. The test with the highest percentage of positivity was the postural walking test (58.3%).
El síndrome de Cushing ACTH-independiente (SCAI) supone el 15–20% de los casos de síndrome de Cushing, de los cuales <1% son debidos a receptor anómalo. Nuestro objetivo es estudiar la presencia de receptor anómalo en los sujetos diagnosticados de SCAI con hiperplasia nodular suprarrenal en un período de 14 años (2002-2016), así como sus características clínico-biológicas y evolutivas.
Material y métodosEstudio descriptivo multicéntrico de una serie de 15 casos de SCAI con hiperplasia nodular suprarrenal (período de estudio: 2002-2016). En ellos se hizo el despistaje de receptor anómalo, mediante pruebas de estimulación, considerando patológico un aumento de cortisol plasmático ≥del 25% respecto al valor basal.
ResultadosDe los 15 casos, 13 fueron mujeres, con una edad media al diagnóstico de 56,8 años. En 12 de los 15 casos estudiados se detectó positividad de las pruebas de estimulación. De ellos, fueron positivos para comida de prueba el 25%, para test postural de deambulación el 58,3%, para desmopresina el 33,3%, para terlipresina el 25%, para GnRH el 33,3%, para LH el 25% y para metoclopramida el 50%. En cuanto al tratamiento, se llevó a cabo suprarrenalectomía bilateral en el 16,7% y unilateral en el 41,7%. El resto continúan en observación con revisiones periódicas (41,7%).
ConclusionesEn la mayor parte de los casos estudiados con SCAI e hiperplasia nodular suprarrenal (80%) se detecta una respuesta de cortisol anormal debida a la presencia de receptor anómalo. La prueba con mayor porcentaje de positividad fue el test postural de deambulación (58,3%).
Adrenocorticotropic hormone (ACTH)-independent Cushing's syndrome (AICS) accounts for 15–20% of all cases of Cushing's syndrome. Most cases of AICS are due to unilateral adrenal tumors (≥90%), while the remainder are due to bilateral adrenal lesions. The causes of AICS secondary to bilateral adrenal lesions include pigmented micronodular adrenal hyperplasia and bilateral macronodular adrenal hyperplasia (BMAH) of unknown cause or secondary to the expression of aberrant receptors.
Pigmented micronodular hyperplasia is a very rare cause of Cushing's syndrome. The adrenal glands are usually normal or slightly enlarged, and are occupied by pigmented nodules measuring 2–4mm in size, with an atrophic inter-nodular cortex. Approximately 50% of all cases are sporadic, while the rest are familial cases that may be associated with Carney complex with an autosomal dominant hereditary trait. The symptoms of hypercortisolism always appear before 30 years of age, and under 15 years of age in half of the cases.
Bilateral macronodular adrenal hyperplasia, formerly known as ACTH-independent macronodular adrenal hyperplasia, is the cause of less than 1–2% of all cases of Cushing's syndrome.1,2 However, its prevalence may be underestimated because of the few symptoms it causes in many cases, and the difficulties involved in diagnosing the condition.3 Bilateral macronodular adrenal hyperplasia most often occurs in the fifth or sixth decade of life and is characterized by enlarged adrenal glands containing non-pigmented nodules measuring over 10mm in diameter, with hypertrophic inter-nodular tissue.4 The condition is usually associated with moderate urinary cortisol elevation as a result of inefficient steroidogenesis, and manifests as a discrete and long-standing clinical syndrome.
Bilateral macronodular adrenal hyperplasia is a heterogeneous disorder whose diagnosis is usually delayed because of its clinical diversity and slow course. The most common presentation is diagnosis established in the context of the study of a patient with bilateral adrenal gland incidentalomas, where a differential diagnosis with malignancy is sometimes required in view of the radiological findings.5 The physiopathology underlying BMAH is not fully known. Germline mutations of the armadillo repeat containing 5 (ARMC5) gene have recently been reported in approximately half of all cases, and steroidogenesis in BMAH is known to be regulated by non-ACTH dependent circulating factors, resulting from the anomalous expression of receptors of various hormones in the adrenocortical cell membrane.6,7
The presence of anomalous receptors in adrenal tissue was first described in 1971 by Schorr and Ney,8 but it was not until 1987 that the function of these receptors could be shown in the studies published by Hamet et al.9 In these cases, excessive cortisol production may be attributed to the abnormal expression of hormone receptors, particularly in BMAH.
Two types of receptors are distinguished:
- -
Ectopic receptors not normally expressed in the adrenal cortex. These include receptors for:
- •
Gastric inhibitory peptide (GIP): these receptors are stimulated by oral intake, particularly of carbohydrates and lipids.
- •
Catecholamines: these receptors are stimulated by a variety of factors such as the standing position or hypoglycemia.
- •
Vasopressin: there are three different types of receptors for vasopressin. Of these, receptor V1 can be found in healthy cells of the adrenal cortex, but V2 and V3 are ectopic receptors. They can be stimulated by common vasopressin releasing stimuli such as the standing position (orthostatism).
- •
Serotonin (5-HT7 receptor).
- •
Glucagon.
- •
- -
Eutopic receptors with an abnormal function due to the increased expression of receptors that are common in the adrenal cortex, such as:
- •
Vasopressin: as mentioned above, receptor V1 can be found in cells of the healthy adrenal cortex.
- •
Luteinizing hormone (LH): the LH receptor is over-stimulated in situations of important LH elevation, as occurs in primary hypogonadism (particularly in men) or in women after menopause.
- •
Human chorionic gonadotropin (hCG) in pregnant women.10
- •
Serotonin (5-HT4 receptor).
- •
Angiotensin II.
- •
All of these will cause cortisol elevation with a characteristic pattern, depending on the circumstances that increase the expression of the corresponding factor.11
Most anomalous receptors are part of the family of G protein-coupled receptors, which through the stimulation of adenylate cyclase activate steroidogenesis in the way the ACTH receptor would do under normal conditions.4 Lacroix has developed a diagnostic protocol for the presence of anomalous receptors in adrenal tissue that is used as a reference in studies in this field.11 According to this protocol, screening tests are initially made, followed, if positive, by other tests targeting specific ligands.
The objective of the present study was to explore the presence of anomalous receptors in subjects diagnosed with AICS with adrenal nodular hyperplasia over a 14-year period (from 2002 to 2016), and to describe our experience in the management and follow-up of these cases.
Material and methodsA descriptive study was made of a series of cases of AICS with adrenal nodular hyperplasia selected through consecutive sampling during the period 2002–2016 at the Endocrinology and Nutrition Outpatient Clinics of several centers in the Valencian Community (Spain) 1: Hospital Clínico Universitario de Valencia, Hospital Francesc de Borja (Gandia), Hospital de Dénia and Hospital Marina Baixa (Villajoyosa). A total of 15 cases were diagnosed.
The following criteria were used to diagnose AICS:
- -
Cushing's syndrome: two of the following three anomalous tests:
- •
24-h urinary free cortisol >450μg/24h in at least two measurements.
- •
Salivary cortisol at 23:00h >145ng/dl.
- •
Plasma cortisol after nocturnal suppression with dexamethasone 1mg at 23:00h (or the Nugent test) ≥2μg/dl.
- •
Plasma cortisol after weak suppression with dexamethasone 0.5mg every 6h for two days ≥2μg/dl.
- •
- -
ACTH-independent: ACTH <10pg/ml. At least three determinations were made to establish independence.
Adrenal nodular hyperplasia was diagnosed using imaging techniques (CAT or MRI), including those cases in which multiple lesions were seen in the adrenal glands.
Anomalous receptor screening was performed in the 15 patients meeting these criteria. We followed the protocols of studies based on the recommendations of Lacroix et al. to determine these receptors.1,11 The study was carried out in each patient in the Functional Tests Unit of Hospital Clínico Universitario (Valencia, Spain) on three alternate days of the same week, and involved the sequential application of different stimulus tests. The sequence, dose, administration route of the stimulus used, and the timings of each of them were those recommended by Outeirino et al.12 With regard to the test meal, at 8:00h, and after overnight fasting, the patient consumed: 100g of bread, 250ml of coffee with milk, 20g of butter and 20g of jam. The tests lasted 2h, and 5 cortisol measurements were taken at time 0 and every 30min, until the end of the test. An increase in plasma cortisol ≥25% from baseline was considered pathological.
The following tests were performed in our study:
- -
First diagnostic step: this included some screening tests, the positivity of which could correspond to the presence of different receptors.
- •
The test meal, assessing GIP or GLP-1.
- •
The postural test, evaluating receptors for angiotensin II, vasopressin, catecholamines and atrial natriuretic peptide.
- •
The GnRH test, evaluating receptors for LH, FSH or GnRH.
- •
The TRH test, evaluating receptors for TRH, TSH or prolactin.
- •
The glucagon test.
- •
The metoclopramide test, evaluating the 5-HT4 serotonin receptor.
- •
- -
In the case of positivity in any of the above tests, confirmatory tests were performed to establish the receptor involved:
- •
The desmopressin test, which evaluates V2 receptors.
- •
The terlipressin test, which evaluates V1 receptors.
- •
The LH test.
- •
The FSH test.
- •
In some cases the tests varied due to the characteristics and limitations of the patient (such as limited mobility for performing the postural test) or because of changes in the Department protocols over time, since these were adapted over the years based on the previous results obtained (those tests not proving positive in any of the cases were eliminated). Thus, the test meal and metoclopramide test were performed in all 15 patients, the postural test in 13 patients, the GnRH test in 11 patients, the desmopressin test in 7, the TRH test and glucagon test in 5, the terlipressin test in four, the LH test in two, and the FSH test in two patients.
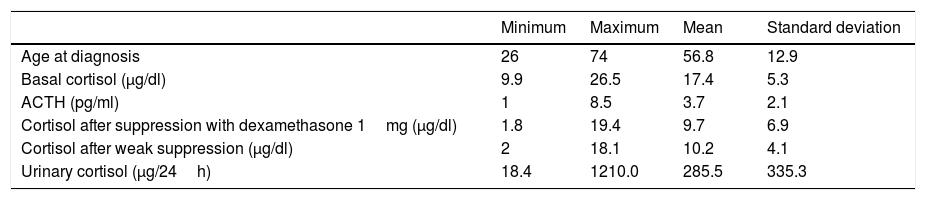
ResultsIn all the patients enrolled, the study was started after the incidental imaging test finding of bilateral adrenal masses (except in patient 6, who presented a unilateral lesion), all of them suggesting a benign nature, with incomplete suppression after the administration of dexamethasone 1mg. Of the 15 cases, 13 were women, with a mean age at diagnosis of 56.8±12.9 years. The mean basal cortisol values at diagnosis were 17.4±5.3μg/dl, while those of ACTH were 3.7±2.1pg/ml. The cortisol values after suppression with 1mg of dexamethasone were 9.7±6.9μg/dl, with cortisol after weak suppression 10.2±4.1μg/dl and cortisol in 24-h urine 285.5±335.3μg/24h (Table 1).
Baseline characteristics.
| Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|
| Age at diagnosis | 26 | 74 | 56.8 | 12.9 |
| Basal cortisol (μg/dl) | 9.9 | 26.5 | 17.4 | 5.3 |
| ACTH (pg/ml) | 1 | 8.5 | 3.7 | 2.1 |
| Cortisol after suppression with dexamethasone 1mg (μg/dl) | 1.8 | 19.4 | 9.7 | 6.9 |
| Cortisol after weak suppression (μg/dl) | 2 | 18.1 | 10.2 | 4.1 |
| Urinary cortisol (μg/24h) | 18.4 | 1210.0 | 285.5 | 335.3 |
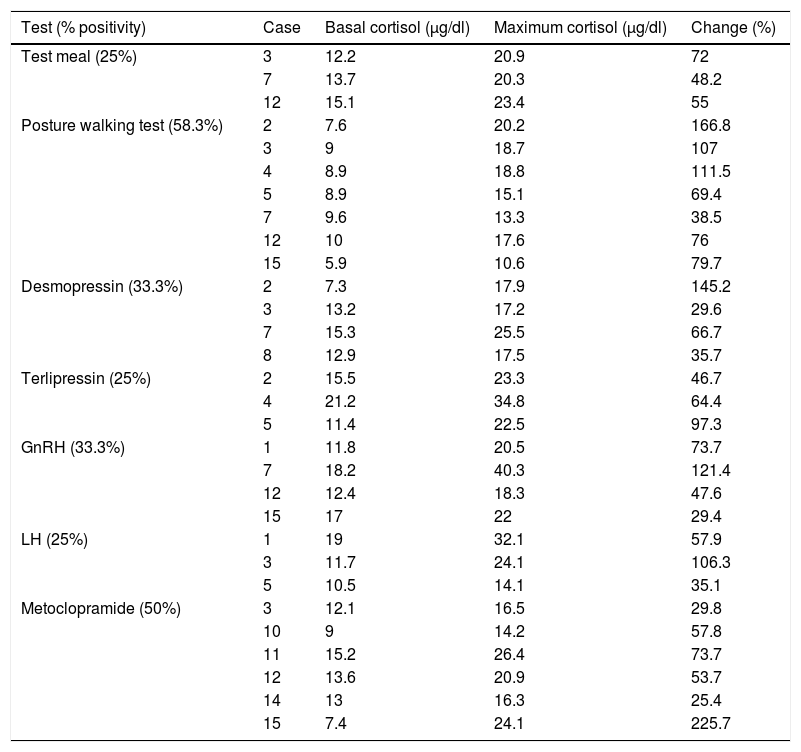
Anomalous receptor testing proved positive in 12 of the 15 cases (80%). Of these, test meal positivity was observed in cases 3, 7 and 12 (25%), with posture walking test positivity in cases 2, 3, 4, 5, 7, 12 and 15 (58.3%), desmopressin positivity in cases 2, 3, 7 and 8 (33.3%), terlipressin positivity in cases 2, 4 and 5 (25%), GnRH positivity in cases 1, 7, 12 and 15 (33.3%), LH positivity in cases 1, 3 and 5 (25%), and metoclopramide test positivity in cases 3, 10, 11, 12, 14 and 15 (50%). Thus, the most frequently positive test was the posture walking test, which was found to be positive in 58.3% of the cases in which an anomalous receptor was detected, and reached a maximum percentage change of 166.8% in case number 2 (Table 2).
Cases with positive test results.
| Test (% positivity) | Case | Basal cortisol (μg/dl) | Maximum cortisol (μg/dl) | Change (%) |
|---|---|---|---|---|
| Test meal (25%) | 3 | 12.2 | 20.9 | 72 |
| 7 | 13.7 | 20.3 | 48.2 | |
| 12 | 15.1 | 23.4 | 55 | |
| Posture walking test (58.3%) | 2 | 7.6 | 20.2 | 166.8 |
| 3 | 9 | 18.7 | 107 | |
| 4 | 8.9 | 18.8 | 111.5 | |
| 5 | 8.9 | 15.1 | 69.4 | |
| 7 | 9.6 | 13.3 | 38.5 | |
| 12 | 10 | 17.6 | 76 | |
| 15 | 5.9 | 10.6 | 79.7 | |
| Desmopressin (33.3%) | 2 | 7.3 | 17.9 | 145.2 |
| 3 | 13.2 | 17.2 | 29.6 | |
| 7 | 15.3 | 25.5 | 66.7 | |
| 8 | 12.9 | 17.5 | 35.7 | |
| Terlipressin (25%) | 2 | 15.5 | 23.3 | 46.7 |
| 4 | 21.2 | 34.8 | 64.4 | |
| 5 | 11.4 | 22.5 | 97.3 | |
| GnRH (33.3%) | 1 | 11.8 | 20.5 | 73.7 |
| 7 | 18.2 | 40.3 | 121.4 | |
| 12 | 12.4 | 18.3 | 47.6 | |
| 15 | 17 | 22 | 29.4 | |
| LH (25%) | 1 | 19 | 32.1 | 57.9 |
| 3 | 11.7 | 24.1 | 106.3 | |
| 5 | 10.5 | 14.1 | 35.1 | |
| Metoclopramide (50%) | 3 | 12.1 | 16.5 | 29.8 |
| 10 | 9 | 14.2 | 57.8 | |
| 11 | 15.2 | 26.4 | 73.7 | |
| 12 | 13.6 | 20.9 | 53.7 | |
| 14 | 13 | 16.3 | 25.4 | |
| 15 | 7.4 | 24.1 | 225.7 |
In cases 6, 9 and 13, no positivity was detected for any of the tests. The TRH, FSH and glucagon tests were not positive in any of the cases.
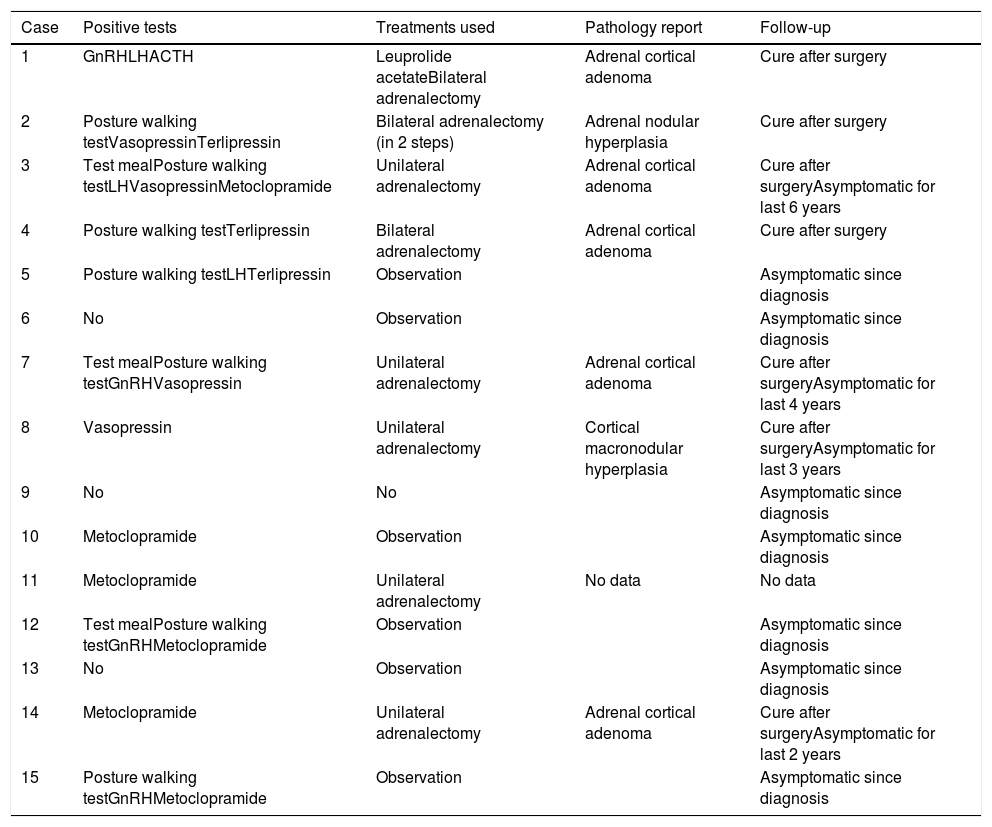
Management of the patients with positive screening comprised bilateral or unilateral adrenalectomy, or observation (monitoring) (Table 3). Bilateral adrenalectomy was performed in those patients with a clear Cushing phenotype and associated comorbidities such as diabetes or arterial hypertension (cases 1, 2, and 4; 25%). Those patients with a Cushing phenotype but no other comorbidities underwent unilateral adrenalectomy, with the largest gland being removed (cases 3, 7, 8, 11, and 14; 41.7%). The remaining patients with positive tests, who only had subclinical Cushing's disease, continue to undergo periodic monitoring (cases 5, 10, 12 and 15; 33.3%).
Summary of the cases studied.
| Case | Positive tests | Treatments used | Pathology report | Follow-up |
|---|---|---|---|---|
| 1 | GnRHLHACTH | Leuprolide acetateBilateral adrenalectomy | Adrenal cortical adenoma | Cure after surgery |
| 2 | Posture walking testVasopressinTerlipressin | Bilateral adrenalectomy (in 2 steps) | Adrenal nodular hyperplasia | Cure after surgery |
| 3 | Test mealPosture walking testLHVasopressinMetoclopramide | Unilateral adrenalectomy | Adrenal cortical adenoma | Cure after surgeryAsymptomatic for last 6 years |
| 4 | Posture walking testTerlipressin | Bilateral adrenalectomy | Adrenal cortical adenoma | Cure after surgery |
| 5 | Posture walking testLHTerlipressin | Observation | Asymptomatic since diagnosis | |
| 6 | No | Observation | Asymptomatic since diagnosis | |
| 7 | Test mealPosture walking testGnRHVasopressin | Unilateral adrenalectomy | Adrenal cortical adenoma | Cure after surgeryAsymptomatic for last 4 years |
| 8 | Vasopressin | Unilateral adrenalectomy | Cortical macronodular hyperplasia | Cure after surgeryAsymptomatic for last 3 years |
| 9 | No | No | Asymptomatic since diagnosis | |
| 10 | Metoclopramide | Observation | Asymptomatic since diagnosis | |
| 11 | Metoclopramide | Unilateral adrenalectomy | No data | No data |
| 12 | Test mealPosture walking testGnRHMetoclopramide | Observation | Asymptomatic since diagnosis | |
| 13 | No | Observation | Asymptomatic since diagnosis | |
| 14 | Metoclopramide | Unilateral adrenalectomy | Adrenal cortical adenoma | Cure after surgeryAsymptomatic for last 2 years |
| 15 | Posture walking testGnRHMetoclopramide | Observation | Asymptomatic since diagnosis |
Four of the cases were initially monitored and started to present clinical symptoms during follow-up. Surgery was therefore performed later. Likewise, in one case with a positive test for GnRH and LH, medical treatment was attempted with leuprolide acetate (an LHRH agonist) for four months: the basal cortisol levels were initially normalized and the urinary cortisol levels decreased, but without reaching normal levels. In addition, we recorded a worsened control of diabetes mellitus and arterial hypertension, as well as of proximal myopathy. Bilateral adrenalectomy was therefore finally performed.
Over subsequent follow-up, those patients with bilateral adrenalectomy met the cure criteria and are currently subjected to follow-up, with the administration of corticosteroid replacement therapy. Those patients with unilateral adrenalectomy remain under follow-up with regular check-ups and are clinically asymptomatic after a mean follow-up period of 41.2±41.6 months. Likewise, those patients in whom no procedure was performed remain for the time being with subclinical Cushing's syndrome after 45±9.9 months of follow-up (Table 3).
DiscussionThe present study describes 12 patients with AICS due to an anomalous receptor presence. Adrenocorticotropic hormone (ACTH)-independent Cushing's syndrome is a low prevalence disorder; as a result, the available clinical and biological information is based on studies of case series. The aberrant expression of ectopic and eutopic receptors in adrenal tissue, coupled to G protein, is present in most cases of BMAH, but also in some unilateral adrenal tumors, as in one of our cases.13 Alterations have also been described in the cyclic AMP/protein kinase A signaling pathway mediated by phosphodiesterases that are implicated in patient susceptibility to develop adrenal hyperplasia and tumors,14 and in the regulation of cortisol secretion.15
Our case series shows the existence of at least one of the anomalous receptors studied in 80% of the patients analyzed. In 8 of the 15 cases (53.3%), positivity for more than one receptor was detected. The results obtained are similar to those of other previous series. The most frequently positive test was the posture walking test, as seen in previous studies.13,16,17 On the other hand, the most common pathological test was the metoclopramide test, which is also described as being frequently positive in other series.13 By contrast, in our series the glucagon test was not positive in any of the cases, which agrees with some previous studies.18,19 However, other studies have shown that some cases of AICS may express anomalous glucagon receptors, stimulating cortisol secretion under fasting conditions.13,20
The posture walking test evaluates the potential modulation of steroidogenesis by angiotensin II, vasopressin, catecholamines and atrial natriuretic peptide. Other studies reported in the literature perform specific a posteriori tests to determine which of these receptors is present in the adrenal tissue of the patients. We have not performed these measurements in our center due to their complexity and their possible side effects. We cannot therefore rule out that hypercortisolism was due to the presence of multiple anomalous receptors in the same adrenal tissue, with a common response to the posture walking test.21
The presence of anomalous receptors in adrenal tissue has been demonstrated in different studies by both the response to stimuli and the use of molecular biology techniques.11 It has been suggested that these receptors may be an effective pharmacological target in some patients, as an alternative to surgery.22 In our series, drug treatment with leuprolide acetate (an LHRH agonist) was attempted in a patient with a positive LH and GnRH test, an intramuscular dose of 3.75mg every four weeks being administered for four months, with a partial response. Definitive treatment in the form of bilateral adrenalectomy was therefore finally decided upon. This treatment has been used by other authors with good results.22 Other proposed pharmacological treatments include propranolol in catecholamine-dependent AICS,23 or pasireotide or octreotide in GIP-dependent AICS.24
Of the 15 patients included in our series, 14 had BMAH. The only patient with unilateral hyperplasia (case 6) did not show positivity with any of the tests. These findings suggest a possible genetic relationship in the pathogenesis of this disorder. Bilateral macronodular adrenal hyperplasia was initially considered a sporadic disease characterized by somatic mutations in adrenal progenitor cells occurring during embryogenesis.11 However, familial cases with an autosomal dominant hereditary pattern indicative of a genetic etiology have been reported. In addition, specific anomalous receptors have been described in the members of the same family with BMAH, including vasopressin and 5-HT4 receptors.25,26 Furthermore, recent studies have shown a relationship between AICS in the context of BMAH and mutations of the ARMC5 gene in approximately one-half of the cases, with a dominant autosomal hereditary pattern. This gene is located in the 16p11.2 genomic region, which had already been associated with BMAH in previous studies.6 The role of ARMC5 is not fully understood, but it has been suggested that it possibly acts as a tumor suppressor gene inducing apoptosis in adrenal cortical cell lines. Although the relationship between variations in the ARMC5 gene and the presence of anomalous receptors has not been fully established, preliminary studies have found that the carriers of mutations of this gene can present aberrant responses to postural tests and to vasopressin and metoclopramide testing.7,27 It has also been suggested that the ARMC5 gene plays a role in the development of other tumors, since mutations of this gene have been described in patients with meningiomas.3,6 Some authors therefore recommend screening for mutations of the ARMC5 gene in patients with BMAH and AICS, as well as in the relatives of carriers.2
Another gene that has been associated with AICS in the context of BMAH is the GNAS1 gene.28,29 No familial clustering or aggregation was seen in our series, and no genetic study was therefore made.
Our study has some limitations. First, the availability of patient information was not complete, since some individuals were referred from other centers, and some data were not adequately collected, particularly as regards the family history. However, the entire study was conducted at our center; we therefore do have the data referring to the stimulation tests. Another important limitation is the fact that not all screening tests were performed in all patients. In some cases, this was due to the clinical characteristics of the patient (seeking to prevent adverse effects), while in other cases it was due to changes in the center protocols, since these patients were studied over an extensive 14-year period (2002–2016).
Another controversial issue is the cut-off point from which a stimulus test is considered to be positive. In this regard we followed the criteria of Lacroix et al.,30 who established a 25–49% change as representing a partial response and a ≥50% change as indicating a complete response. These criteria have also been subsequently accepted by other authors,12 though a firm consensus is lacking.
There are many publications in the medical literature related to this disease condition, though most of them are isolated cases or small case series. The generation of new hypotheses with descriptive studies of this kind is quite limited, though our series largely follows the trends seen in previous studies.
In conclusion, the results of our series are consistent with anomalous receptor expression in subjects with AICS and BMAH, and in line with the observations of other case series. The regulatory mechanisms involved in cortisol secretion in AICS include genetic and molecular factors that are not fully known. The identification of aberrant regulation offers the perspective of possible diagnostic and pharmacological treatment options targeted to hormone receptors coupled to G protein or its ligands, in an attempt to avoid bilateral adrenalectomy, which is the current standard therapy for BMAH. Protocolized study in reference centers and units, as well as the creation of registries, can help in the compilation of information, allowing us to better characterize the expression of anomalous hormone receptors coupled to G protein and to advance in the management of these patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Sergio Martínez-Hervás is an investigator with the Juan Rodés program (JR18/00051) funded by the Carlos III Health Institute and the European Regional Development Fund (ERDF).
Please cite this article as: Ferri J, Perelló E, Lorente RI, Argente C, Rossetti P, Pedro T, et al. Estudio de receptor anómalo suprarrenal en sujetos con síndrome de Cushing ACTH-independiente e hiperplasia nodular suprarrenal. Endocrinol Diabetes Nutr. 2020;67:245–252.