Head and neck cancer patients have a high rate of complications during the postoperative period that could increase their morbidity rate. Arginine has been shown to improve healing and to modulate inflammation and immune response. The aim of our study was to assess whether use of arginine-enriched enteral formulas could decrease fistulas and length of stay (LoS).
MethodsA retrospective study was conducted in patients who had undergone head and neck cancer surgery and were receiving enteral nutrition through a nasogastric tube in the postoperative period between January 2012 and May 2018. The differences associated to use of immunoformula vs. standard formulas were analysed. Sociodemographic, anthropometric, and nutritional intervention variables, as well as nutritional parameters, were recorded during the early postoperative period. Occurrence of complications (fistulas), length of hospital stay, readmissions, and 90-day mortality were recorded.
ResultsIn a univariate analysis, patients who received nutritional support with immunonutrition had a lower fistula occurrence rate (17.91% vs. 32.84%; p=0.047) and a shorter mean LoS [28.25 (SD 16.11) vs. 35.50 (SD 25.73) days; p=0.030]. After adjusting for age, energy intake, aggressiveness of surgery and tumour stage, fistula occurrence rate and LoS were similar in both groups irrespective of the type of formula.
ConclusionsUse of arginine-enriched enteral nutrition appears to decrease the occurrence of fistulas in the postoperative period in patients with head and neck cancer, with a resultant reduction in length of hospital stay. However, the differences disappeared after adjusting for age, tumour stage, or aggressiveness of the surgery.
El postoperatorio de los pacientes con cáncer de cabeza y cuello presenta una alta tasa de complicaciones. Esta circunstancia podría aumentar la morbilidad en estos pacientes. La arginina ha demostrado mejorar la curación y modular la inflamación y la respuesta inmune.
Nuestro planteamiento es valorar si el uso de fórmulas de alimentación enteral enriquecidas con arginina podría reducir la aparición de fístulas y la duración de la estancia hospitalaria.
MétodosEstudio retrospectivo en pacientes intervenidos de cáncer de cabeza y cuello que recibieron nutrición enteral a través de una sonda nasogástrica en el periodo postoperatorio entre enero de 2012 y mayo de 2018. Se analizaron las diferencias asociadas a la utilización de inmunofórmula vs. fórmulas estándar. Se recogieron variables sociodemográficas, antropométricas, de intervención nutricional y de parámetros nutricionales durante el postoperatorio inmediato, así como la aparición de complicaciones (fístulas), la duración de la estancia hospitalaria, los reingresos y la mortalidad a 90 días.
ResultadosEn el análisis univariante los pacientes que recibieron apoyo nutricional con inmunonutrición presentaron menor tasa de aparición de fístulas (17,91 vs. 32,84%; p=0,047) y menor estancia hospitalaria (28,25 [DE 16,11] vs. 35,50 [DE 25,73] días; p=0,030).
Después de ajustar por edad, aporte calórico, agresividad de la cirugía y estadio del tumor, la incidencia de fístula y la estancia hospitalaria fueron similares entre los grupos, independientemente del tipo de fórmula.
ConclusionesEl uso de nutrición enteral enriquecida con arginina en pacientes con cáncer de cabeza y cuello intervenidos podría reducir el desarrollo de la fístula y la duración de la estancia hospitalaria; sin embargo, las diferencias observadas desaparecen después de ajustar por edad, estadio tumoral o agresividad de la cirugía.
Malnutrition occurs at the time of diagnosis in up to 50% of patients with head and neck neoplasia.1–3 This fact is promoted by a delayed diagnosis, as 75% of patients with head and neck cancer have their first consultation in advanced stages of the disease.4 Malnutrition in cancer has a multifactorial origin, including catabolic factors secreted by the tumour, compressive effects at local level that can lead to alterations in swallowing, tumour induced cachexia and anorexia or poor previous dietary habits and excessive alcohol consumption.5
During the postoperative period for patients with head and neck cancer, a high complication rate (20–50%) has been reported.6 These patients are generally malnourished even before starting the treatment. Poor nutritional status is known to have an adverse impact on postsurgical outcomes in this group of patients7 with a higher incidence of poor wound healing, hospital-acquired infection and increased length of hospital stay (LoS). Furthermore, postoperative complications can adversely affect long-term survival.8
Malnutrition is associated with defects in immune function that may impair the host response to malignancy9 and could also play a role in the appearance of complications. The use of perioperative nutritional supplements with immuno-nutritional additives could favourably modulate the immune and inflammatory response, something observed both in vitro and in patients with trauma, burns or undergoing oncological surgery. Specifically, the important role of some amino acids, dietary nucleotides and lipids in the modulation of immune function has been recognised. Arginine is a conditionally essential amino acid that serves as a substrate for nitric oxide synthesis and regulates T-lymphocytes action.10 Its supplementation could have positive effects on immune function and reparative synthesis,11 and arginine could enhance wound healing in situations that compromise immune function, such as surgery. The addition of omega-3 fatty acids to enteral nutrition also appears to have anti-inflammatory effects, possibly by interfering with macrophage eicosanoid production and reducing proinflammatory mediators in stressed patients, which may reduce infections.12
Standard commercial, nutritional formulas are described as polymeric, which means they contain whole protein, partially digested starch and triglycerides, along with electrolytes, trace elements and vitamins. Specific nutritional components have been combined with standard polymeric enteral feeds with the aim to specifically improve immune function. Immunonutrition describes enteral feeding formulas usually supplemented with combinations of the amino acids arginine or glutamine, omega-3 fatty acids and nucleic acids.
Based on these postulations, a new perioperative protocol was implemented in our centre in 2014. This protocol prioritises the use of enteral immunonutrition in the postoperative period, specifically the use of a commercial enteral formula containing arginine, Impact® (Nestlé Healthcare Nutrition, Switzerland) [kcal/mL: 1.01. Caloric distribution (% of kcal): protein: 22%, carbohydrate: 53%, fat: 25%. Supplemental l-arginine: 12.5g/L, dietary nucleotides: 1.2g/L, NPC: N ratio: 71:1, n6:n3 ratio: 1.4:1, EPA+DHA: 1.7g/L), with the objective to detect malnourishment early in patients with malignant head and neck cancer and provide the best evidence-based nutritional support to these patients.
The aim of our study was to determine whether the use of immunonutrition enteral feed improved recovery (led to a shorter LoS and fewer complications) when compared with a standard enteral feed.
Materials and methodsA retrospective observational study was conducted with patients undergoing head and neck surgery who received nutritional support with enteral nutrition via a nasogastric tube between January 2012 and August 2018.
Patients with less than 4 days of enteral nutritional feeding, patients transferred from or to other services or from another hospital centre and patients under 18 years old were excluded from this study.
Every patient admitted during the period of the study was retrospectively evaluated, and patients receiving immunonutrition were compared to those receiving standard formula (control group). As a new protocol prioritising the use of immunonutrition was implemented from 2014, most of the patients included in the control group were those admitted before that date.
Sociodemographic, anthropometric and nutritional intervention variables were recorded during the immediate postoperative period. Fasting blood samples were drawn weekly to measure nutritional parameters. The range values of these parameters were 3.5–4.5g/dL for albumin and 2.0–6.0mg/dL for retinol-binding protein (RBP). Fistula appearance, LoS, readmissions and 90-day mortality were also assessed.
Tumour staging was defined based on American Joint Committee on Cancer (AJCC) Cancer Staging Manual 8th edition.13 Laryngectomy and pharyngo-laryngectomy were considered as aggressive surgical techniques.
The previous nutritional status of the patient was defined based on International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version 201614 and using SENPE-SEDOM15 criteria of hospital malnutrition.
Nutritional requirements were established by the Harris–Benedict equation using real weight or adjusted body weight for each patient. Protein requirements were calculated as 1.3g protein/kg. Enteral feeding was started within 24h of surgery at a rate of 31mL/h, via a nasogastric tube placed during the surgery. The infusion rate was progressively increased every 24h until the calculated energy and protein requirements for each patient were met.
The data analysis was carried out using the SPSS 15.0 programme (SPSS Inc., Chicago, IL, 2008). To compare qualitative variables, the Chi-squared test was used. In the case of quantitative variables, to compare the differences between independent means, parametric or non-parametric tests were used according to the conditions of application: Student's t-test or Mann–Whitney U test was used for variables of 2 categories and ANOVA or Kruskal–Wallis was used for variables of more than 2 categories. Finally, the relationship between quantitative variables was assessed using Pearson or Spearman correlation tests. The level of significance for all the study tests was 5%, using bilateral tests. Logistic regression analysis was performed to adjust by age, tumour stage, malnutrition and aggressiveness of the surgery. Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test, 60 subjects are necessary in first group and 60 in the second to find as statistically significant a proportion difference, expected to be of 0.1 in group 1 and 0.3 in group 2. It has been anticipated a drop-out rate of 0%.
The study was approved by the Ethics in Clinical Research Committee of the University Hospital of León.
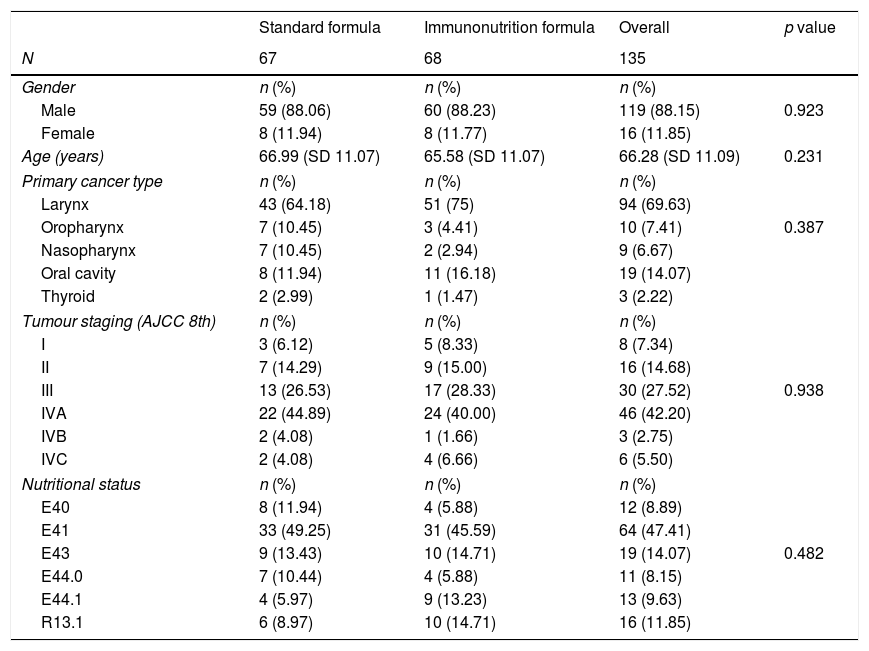
ResultsBaseline characteristics135 patients were included in this study, where 68 patients (50.37%) received nutritional support with immunonutrition. Table 1 summarises demographic characteristics, previous nutritional status, primary tumour location and disease staging. The study population was homogenously distributed into two groups, with no significant differences found between the control and intervention groups (Table 1).
Demographic characteristics of the study population. Nutritional status is defined by ICD-10 version. E40: Kwashiorkor. E41: Marasmus. E43: severe protein-energy malnutrition. E44.0: moderate protein-energy malnutrition. E44.1: mild protein-energy malnutrition. R13.1: Dhysphagia.
| Standard formula | Immunonutrition formula | Overall | p value | |
|---|---|---|---|---|
| N | 67 | 68 | 135 | |
| Gender | n (%) | n (%) | n (%) | |
| Male | 59 (88.06) | 60 (88.23) | 119 (88.15) | 0.923 |
| Female | 8 (11.94) | 8 (11.77) | 16 (11.85) | |
| Age (years) | 66.99 (SD 11.07) | 65.58 (SD 11.07) | 66.28 (SD 11.09) | 0.231 |
| Primary cancer type | n (%) | n (%) | n (%) | |
| Larynx | 43 (64.18) | 51 (75) | 94 (69.63) | |
| Oropharynx | 7 (10.45) | 3 (4.41) | 10 (7.41) | 0.387 |
| Nasopharynx | 7 (10.45) | 2 (2.94) | 9 (6.67) | |
| Oral cavity | 8 (11.94) | 11 (16.18) | 19 (14.07) | |
| Thyroid | 2 (2.99) | 1 (1.47) | 3 (2.22) | |
| Tumour staging (AJCC 8th) | n (%) | n (%) | n (%) | |
| I | 3 (6.12) | 5 (8.33) | 8 (7.34) | |
| II | 7 (14.29) | 9 (15.00) | 16 (14.68) | |
| III | 13 (26.53) | 17 (28.33) | 30 (27.52) | 0.938 |
| IVA | 22 (44.89) | 24 (40.00) | 46 (42.20) | |
| IVB | 2 (4.08) | 1 (1.66) | 3 (2.75) | |
| IVC | 2 (4.08) | 4 (6.66) | 6 (5.50) | |
| Nutritional status | n (%) | n (%) | n (%) | |
| E40 | 8 (11.94) | 4 (5.88) | 12 (8.89) | |
| E41 | 33 (49.25) | 31 (45.59) | 64 (47.41) | |
| E43 | 9 (13.43) | 10 (14.71) | 19 (14.07) | 0.482 |
| E44.0 | 7 (10.44) | 4 (5.88) | 11 (8.15) | |
| E44.1 | 4 (5.97) | 9 (13.23) | 13 (9.63) | |
| R13.1 | 6 (8.97) | 10 (14.71) | 16 (11.85) | |
SD: standard deviation.
The most common malignancy in this study was carcinoma of the larynx (69.62%), 77.8% of the patients had advanced tumour staging (Tumour Node and Metastasis, TNM≥3). 18 patients underwent resection of a tumour located only in the oral cavity, while 115 patients underwent laryngectomy or pharyngo-laryngectomy.
Most of the patients were malnourished at admission (88.15%), with energy malnutrition being the most common malnutrition status (47.41%). 43.70% of the patients suffered protein malnutrition on admission.
Nutritional treatmentBefore January 1, 2014, all patients included were fed using standard formula. After January 1, 2014, 68 out of 97 patients were fed using immunonutrition formula. The mean duration of tube feeding was 19.12 days [standard deviation (SD) 6.31]. No differences were found regarding the need of parenteral nutrition during admission (6.15% in standard feed group vs. 8.88% in immunonutrition group; p=0.527). No significant differences were found in time of use of tube feeding between both groups (p=0.294).
A higher mean energy intake was found when using the standard formula compared to immunonutrition formula [32.39kcal/kg (SD 7.71) vs. 25.45kcal/kg (SD 4.59); p<0.001]. 20.65% of all patients did not reach their calculated energy requirements, and this prevalence was higher in the immunonutrition group (4.54% vs. 35.42%; p<0.001). Protein intake was lower in the standard feed group [1.32g/kg (SD 0.30) vs. 1.41g/kg (SD 0.25); p=0.029]. 43.07% of patients did not reach their calculated protein requirements (1.3g/kg), with no differences observed between the two groups (p=0.311).
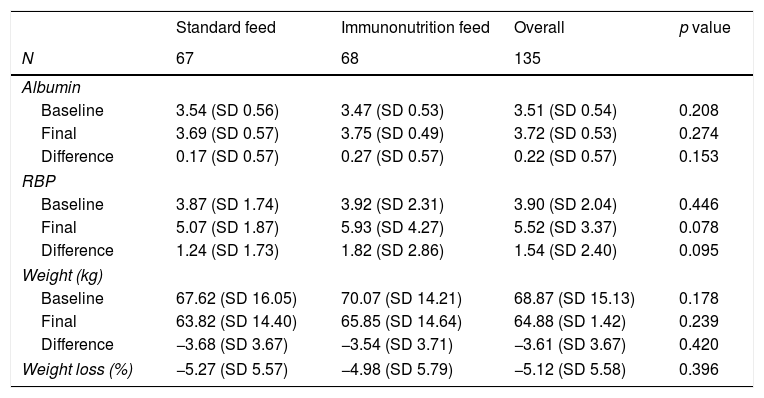
As shown in Table 2, there were no differences in either weight or albumin values, although the immunonutrition group had a statistically insignificant tendency to have a higher RBP at the end of hospitalisation (p=0.078).
Visceral serum protein and anthropometric parameters during hospitalisation. Baseline, before surgery. Final, last parameter during hospitalisation stay.
| Standard feed | Immunonutrition feed | Overall | p value | |
|---|---|---|---|---|
| N | 67 | 68 | 135 | |
| Albumin | ||||
| Baseline | 3.54 (SD 0.56) | 3.47 (SD 0.53) | 3.51 (SD 0.54) | 0.208 |
| Final | 3.69 (SD 0.57) | 3.75 (SD 0.49) | 3.72 (SD 0.53) | 0.274 |
| Difference | 0.17 (SD 0.57) | 0.27 (SD 0.57) | 0.22 (SD 0.57) | 0.153 |
| RBP | ||||
| Baseline | 3.87 (SD 1.74) | 3.92 (SD 2.31) | 3.90 (SD 2.04) | 0.446 |
| Final | 5.07 (SD 1.87) | 5.93 (SD 4.27) | 5.52 (SD 3.37) | 0.078 |
| Difference | 1.24 (SD 1.73) | 1.82 (SD 2.86) | 1.54 (SD 2.40) | 0.095 |
| Weight (kg) | ||||
| Baseline | 67.62 (SD 16.05) | 70.07 (SD 14.21) | 68.87 (SD 15.13) | 0.178 |
| Final | 63.82 (SD 14.40) | 65.85 (SD 14.64) | 64.88 (SD 1.42) | 0.239 |
| Difference | −3.68 (SD 3.67) | −3.54 (SD 3.71) | −3.61 (SD 3.67) | 0.420 |
| Weight loss (%) | −5.27 (SD 5.57) | −4.98 (SD 5.79) | −5.12 (SD 5.58) | 0.396 |
RBP: retinol binding protein.
The incidence of fistula after surgery was 34/135 (25.19%). Fistula appearance was similar irrespective of whether the surgery was performed before or after 2014. The presence of fistula was higher in the standard formula group with an Odds ratio (OR) of 2.24 [95% confidence interval (CI) 1.04–5.52; (32.84% vs. 17.91%; p=0.047)]. By group, a higher mean energy intake was found in patients with fistula compared to patients without fistula [31.17kcal/kg (SD 8.75) vs. 27.96kcal/kg (SD 6.34); p=0.012], but no differences were found in the immunonutrition group. No differences in fistula development were found related to protein intake.
After adjustments based on age, tumour stage, aggressiveness of surgery, energy intake and preoperative malnutrition status, no differences were found by enteral formula used, and only preoperative malnutrition status was associated with an increase in the incidence of fistula after multivariate analysis, with an OR of 8.94 [95% CI 1.09–73.15 (29.36% in standard formula vs. 4.76% in immunonutrtition formula; p=0.041)].
LoS was 28.25 (SD 16.11) days in the immunonutrition group and 35.50 (SD 25.73) days in the standard group (p=0.030). Patients with fistula increased the LoS to an average of 23.1 (SD 15.57) days (p<0.001). The increase in LoS in relation to the appearance of fistula was higher in the standard feed group when compared with the immunonutrition group [27.11 (SD 24.83) days vs. 15.49 (SD 19.42) days, with a mean difference of −12.83 days, 95% CI −35.42 to 9.75], although no statistical difference was found (p=0.126). After adjusting based on age, tumour stage, aggressiveness of surgery and the development of fistula, this difference between types of formula disappeared, and fistula was the only significant factor increasing the LoS (p<0.001).
During the three months after hospital discharge, 29.01% of patients were readmitted. After adjustments were made based on age, tumour stage, enteral formula, aggressiveness of surgery and preoperative malnutrition, the development of fistula with an OR equal to 6.55 [95% CI 2.54–16.95] (p<0.001) was associated with an increase in risk of readmission, no statistically significant differences were observed by enteral formula group.
10 patients died during this period, with no differences observed between groups (5.97% in the immunonutrition group and 8.96% in the standard formula group). No differences in mortality were observed based on fistula development.
DiscussionMany patients with head and neck cancer are malnourished for several reasons including mechanical obstruction, tumour induced cachexia, poor dietary habits and excessive alcohol consumption. Malnutrition has been associated with the occurrence of major postoperative complications and shorter survival.16 Improvement in nutritional status of these patients seems to be associated with better clinical and survival results.17
Recently, a Cochrane Database systematic review18 has analysed the use of immunonutrition in patients with head and neck cancer. This review included 19 studies that recruited 1099 participants.
According to the Cochrane Database systematic review18 postoperative fistula was previously evaluated in 10 studies, with a total of 747 patients. The absolute risk of postoperative fistula in previous studies was 5.4% [range 0% (0/23) to 7% (7/105)] in the immunonutrition groups and 11.3% (range 2% (1/47) to 29% (2/7)] in the control groups. Our results show a higher incidence of fistula in both groups, which might be explained by a poorer, previous nutritional status (88.15% of malnutrition at admission) and more aggressive surgical interventions in our patients. It should be considered that the multivariate analysis of the data from our study did not find significant differences in the development of fistula based on the enteral formula used.
LoS was reported in 10 studies in Cochrane Database systematic review,18 with a total of 757 participants. The mean LoS in the immunonutrition groups ranged from 15.3 to 31.1 and from 17.4 to 36.1 days in the control groups (mean difference −2.5 days, 95% CI −5.11 to 0.12 (p=0.06)).
In comparison to the Cochrane review, we observed a greater reduction in LoS associated with the use of immunonutrition feed (−7.25 days in our analysis versus −2.5 days in theirs), but we must consider that the average stay of our patients is prolonged in both groups, probably in relation to the high incidence of fistula observed in our results in comparison with previous studies.
Another systematic review and meta-analysis,19 including data of four head and neck cancer surgery studies with 277 participants, found a significantly shorter LoS in relation to immunonutrition [MD=−6.8 days (95% CI −12.6 to −0.9), p=0.023], similar to the difference observed in our study results. Perhaps the difference observed between the meta-analysis and the Cochrane review results could be explained by the heterogeneity of baseline characteristics of the patients and the use of different enteral formulas in the studies, taking into account that Impact® formula was only used in the study by Snyderman et al.20 and should consider that Impact® formula used in 46% of total patients included in the meta-analysis and only in 17% in the case of Cochrane review.
As in previous studies, we did not observe differences in the readmission rate or in mortality in favour of immunonutrition use. However, as mentioned before, the heterogeneity between the studies is vast and the timeframes vary considerably, with a range from 30 days to 16 months, therefore limiting the conclusions that can be made.
L-arginine is classified as a nonessential amino acid but may be considered essential or semiessential under conditions of stress when the capacity of endogenous arginine synthesis is exceeded, including during periods of growth (childhood, pregnancy) or trauma (liver disease, severe sepsis, wound healing, cancer).21 Increased concentrations of l-arginine may improve vascular reperfusion by increasing nitric oxide levels22 and has demonstrated anabolic and immunostimulatory properties that could improve postoperative evolution.23 Studies of malnourished patients with head and neck cancer given arginine-enhanced enteral nutrition showed lower fistula rates, decreased length of hospital stay and the magnitude of the effect was related previously with higher dose of l-arginine24,25 both clinical and biochemical effects. According these findings we employed a high l-arginine dose in this work [l-arginine daily mean intake: 21.52 (SD 3.40)g and no-patients received less than 17g daily] close previous high dose reported in comparative studies (18.9–20g). Must take into account that our results shows worse results than previous studies with high l-arginine dose with 17.91% of fistula prevalence in our immunonutrition feed group in contrast with previous study with high dose (17g/day) that reported as low as 2.8%.24 It is possible that in the immunonutrition “cocktail” each component plays a role and different composition of enteral formula employed can explain in part the differences observed, for example the n6:n3 ratio in our formula was 1.4:1 meanwhile it was 5:1 in reported study.
The main limitation is that this is a retrospective study. Our patients come from different periods of time, where the patients from more recent time periods are those that have received immunonutrition. As a result, we cannot ensure that the differences observed are not influenced by the greater experience of the surgical team or any other circumstances. Regarding other limitations of our work, we did not include data about feed tolerance (diarrhoea) or analysis regarding cost effectiveness. Another limitation must be noted is related with fact that our work shows a deficit in the achievement of nutritional requirements. An explanation could be the presentation of the immunoformula used in 500mL packs, so there is a tendency in clinical practice to use 3 packs a day to avoid waste of material assuming a contribution of 1515kcal/day that is insufficient in a high percentage of patients, and could influence the results of this study.
On the other hand, the main strength of our study is bringing new evidence about the relationship between previous malnutrition and postoperative fistula, giving support for performing nutritional intervention prior to surgery in these patients.
ConclusionsThe use of arginine-enriched enteral nutrition in head and neck cancer patients undergoing surgery might reduce fistula development and the length of hospital stay, but the difference disappeared after adjusting by age, tumour stage or aggressiveness of the surgery. Previous nutritional status might be the main condition for the subsequent development of fistula, which highlights the importance of nutritional assessment prior to surgery in patients with head and neck tumours.
FundingThis investigation did not receive any public subsidy or commercial sectors.
Authors contributionDavid E. Barajas-Galindo and Alfonso Vidal-Casariego conceived and designed the study. David E. Barajas-Galindo analysed the data and wrote the document. All authors contributed to the acquisition of data, to the writing of the manuscript and approved the final version thereof.
Conflict of interestNone of the authors present a conflict of interest in relation to the study.