Nutritional ultrasound® is a new concept that uses ultrasound to assess body composition. It is composed of the evaluation of fat-free mass and fat mass. It is an emerging, economical, portable, non-invasive technique that evaluates the musculoskeletal area with linear, broadband, multifrequency probes, with a depth field of 20–100mm. It quantifies muscle modifications in malnutrition and provides information on functional changes (echogenicity).
Although there are no validated specific cut-off points, the anterior rectum area of the quadriceps can be used as a criterion for malnutrition. The distribution of adipose tissue provides information on the energy reserve and the inflammatory pattern. It is important to integrate nutritional ultrasound® measures in clinical practice adapted to different settings and pathologies.
It is necessary to establish training plans in nutritional ultrasound® for use by Endocrinology and Nutrition Specialists, with the aim of improving the diagnosis and treatment of their patients.
La ecografía nutritional® es un nuevo concepto que utiliza el ultrasonido para evaluar la composición corporal. Se compone de la evaluación de la masa magra y de la masa grasa. Es una técnica emergente, barata, portátil y no invasiva que evalúa el área musculoesquelética con sondas lineales, de banda ancha, multifrecuencia, con una profundidad de campo de 20 a 100mm. Cuantifica los cambios de las estructuras musculares en desnutrición y proporciona información sobre los cambios funcionales (ecogenicidad).
Aunque no existen puntos de corte específicos validados, el área del recto anterior del cuádriceps se puede utilizar como criterio de desnutrición. La distribución del tejido adiposo proporciona información sobre la reserva energética y el patrón inflamatorio.
Es importante integrar las medidas de ecografía nutricional® en la práctica clínica adaptada a diferentes escenarios clínicos y enfermedades. Es necesario establecer programas de formación en ecografía nutricional® para su uso por especialistas en endocrinología y nutrición, con el objetivo de mejorar el diagnóstico y tratamiento de sus pacientes.
Disease-related malnutrition (DRM) is defined as a subacute or chronic state in which different degrees of overnutrition or undernutrition are combined with an inflammatory pattern that generates changes in body composition and function.1 It is essential to diagnose these changes in body composition (BC) and function to establish a morpho-functional diagnosis of DRM and define the most relevant prognostic factors.2 There is no universally accepted criterion for diagnosing DRM. However, recently different scientific societies at the international level have established the criteria to define it using phenotypic and aetiological criteria.3–6 The muscle has been positioned as crucial aspect for the diagnostic of DRM.7
There are new approaches that include techniques such as bioelectrical impedance analysis (BIA) or Nutritional ultrasound® (NU®) for morphological diagnosis and dynamometry or functional tests to measure functionality that can be applied in clinical practice.8
In nutritional assessment, diagnostic imaging is important when the classic parameters of weight loss or body mass index (BMI) do not provide diagnostic value. There are new lines of investigation in the Oncology Area that provide great value to the diagnosis by computed tomography (CT). They calculate the muscle area and the fat area of the abdomen as a prognostic factor on oncological treatment. The diagnosis of malnutrition in sarcopenic obesity requires an assessment of fat-free mass (FFM) and fat mass (FM)9 together. However, these CT or dual-photon X-ray absorptiometry (DXA) techniques are not very accessible in clinical practice and involve a high healthcare cost.
The objective of this work is to review the value of NU® as a direct morphological technique for the evaluation of body composition (BC) in nutritional assessment.
Fundamentals of the use of nutritional ultrasound®ConceptualisationNU® is a new concept that uses the determination of measurements through ultrasound to evaluate the BC. It includes the evaluation of body compartments such as adipose, muscular, connective, vascular, bone tissue, etc., with the point of view of ultrasound. It is composed of two dimensions focused on the assessment of FFM (muscle ultrasound) and the evaluation of FM (ultrasound of adipose tissue).
Ultrasound is an emerging, cheap, portable, and non-invasive technique since it does not involve ionising radiation for the patient. Muscle ultrasound evaluates different muscle areas in a transverse and longitudinal position with the aim to evaluate muscle volume and area, the length of the fascicles and the angle of muscle pennation (PA).10 Different measurement techniques are being developed with the use of echosonographic contrast that try to evaluate in a more precise way the morphological and functional aspect of muscle mass.11
Both muscle size and structure are correlated with metabolically active FFM while adipose tissue corresponds to the amount of fat deposits (FM) and their distribution. Nutritional ultrasound® includes the assessment of muscle mass (Quadricep Rectus Femoris – QRF) and the subcutaneous (superficial and deep-layer) and visceral adipose tissue. It tries to evaluate fat distribution and correlate the different clinical variables related to nutrition and metabolism.
The subcutaneous adipose tissue (SAT) represents the body's energy deposits. The deep SAT is more metabolically active and is also responsible for neuroendocrine regulation with adipokine expression.12 Intra-abdominal, visceral or perivisceral fat is found within the musculoskeletal area of the abdomen and can be subdivided into intraperitoneal (omental and mesenteric) and extraperitoneal (pre and retroperitoneal). The preperitoneal adipose tissue (PAT), easily identifiable with ultrasound of the abdominal area, represents the distribution of ectopic adipose tissue. The PAT shows a direct correlation with other visceral deposits such as intrahepatic fat.
On the other hand, there are locations of non-visceral internal adipose tissue related to muscle mass (intramuscular, perimuscular and intermuscular) that are evaluated in the study of the muscular component of the leg in NU®.13
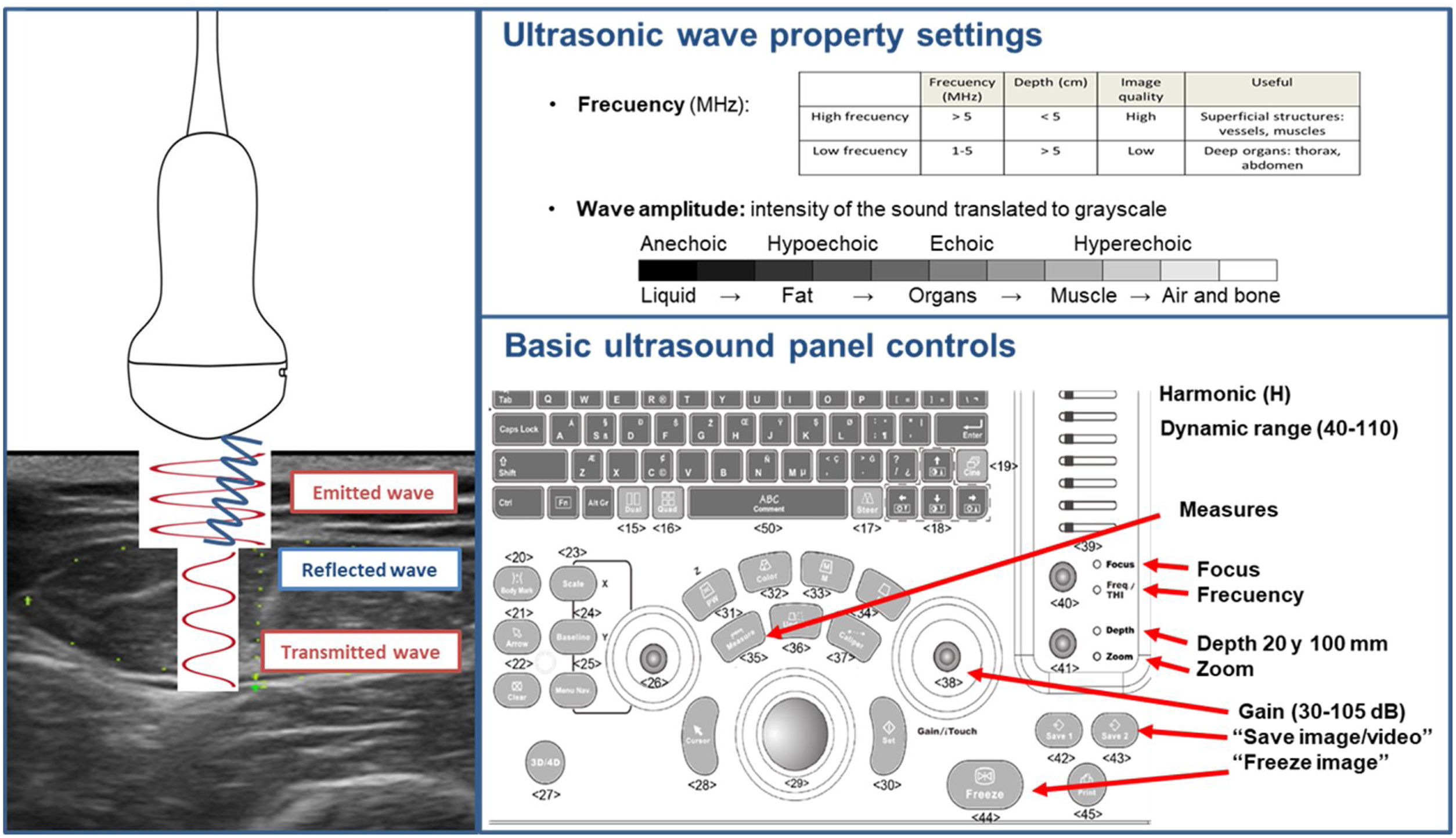
General technical aspects of ultrasoundThe technical aspects of NU® involve the emission of ultrasound in the tissues and the analysis of the echoes reflected to the transducer. Reflected ultrasound appears at the interface, within and between tissues of different acoustic impedance.14 Anisotropy is the tendency of tissues to reflect sound in a directionally dependent manner. Keeping the probe perpendicular minimises his impact.15
The most common format for describing ultrasound scans is mode B (brightness or greyscale mode) in which the various tissues under the transducer produce a greyscale image that includes different echo intensities. Two projections can be made according to the orientation of the transducer, transverse and longitudinal, to the patient's body. There are also other additional scanning modes such as Doppler, Duplex, Power Doppler and elastography that are under development in their application to NU® analysis. The ultrasonographic contrast technique is being evaluated to detect inflammatory changes in clinical investigation studies.16
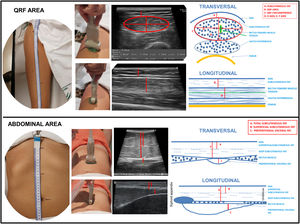
The technical equipment used for NU® is essentially the same as that used in other disciplines related to Endocrinology and Nutrition, such as the thyroid. The transducers are multi-frequency broadband linear matrix, usually in the range of five to 10MHz that adapt to the needs of tissue penetration and axial resolution. You must select the appropriate frequency for the scan. The higher the frequency, the less penetration into the tissues and the higher the resolution. The high-frequency transducers used in this technique achieve an axial resolution of up to 0.1mm and a lateral resolution of 0.2mm. Harmonic imaging (H), gain and dynamic range are the main settings needed for the correct visualisation of NU® (Fig. 1).17
Technical considerations and normality patternHigh-resolution ultrasound of musculoskeletal tissues makes it possible to visualise the epidermis and dermis as a highly hyperechogenic linear layer and subcutaneous cellular tissue as a hypoechogenic layer, separated by hyperechogenic linear septum. In the case of SAT, surface and deep structures with different ultrasonographic densities can be identified.17
Healthy muscle tissue in axial images is shown as echolucent (dark) areas interspersed with small, bright and curved echoes. The fine intramuscular hyperechogenic lines represent the epi- and perimysium. The thicker lines represent the septum and the lining fascia. On the sagittal plane, however, these echoes look like the fibrous tissue surrounding muscle fibres. In bipennate muscles, as the quadriceps rectum femoris (QRF), the central tendon can be identified as an area of hyperechogenic thickened fibrous tissue (Fig. 2).
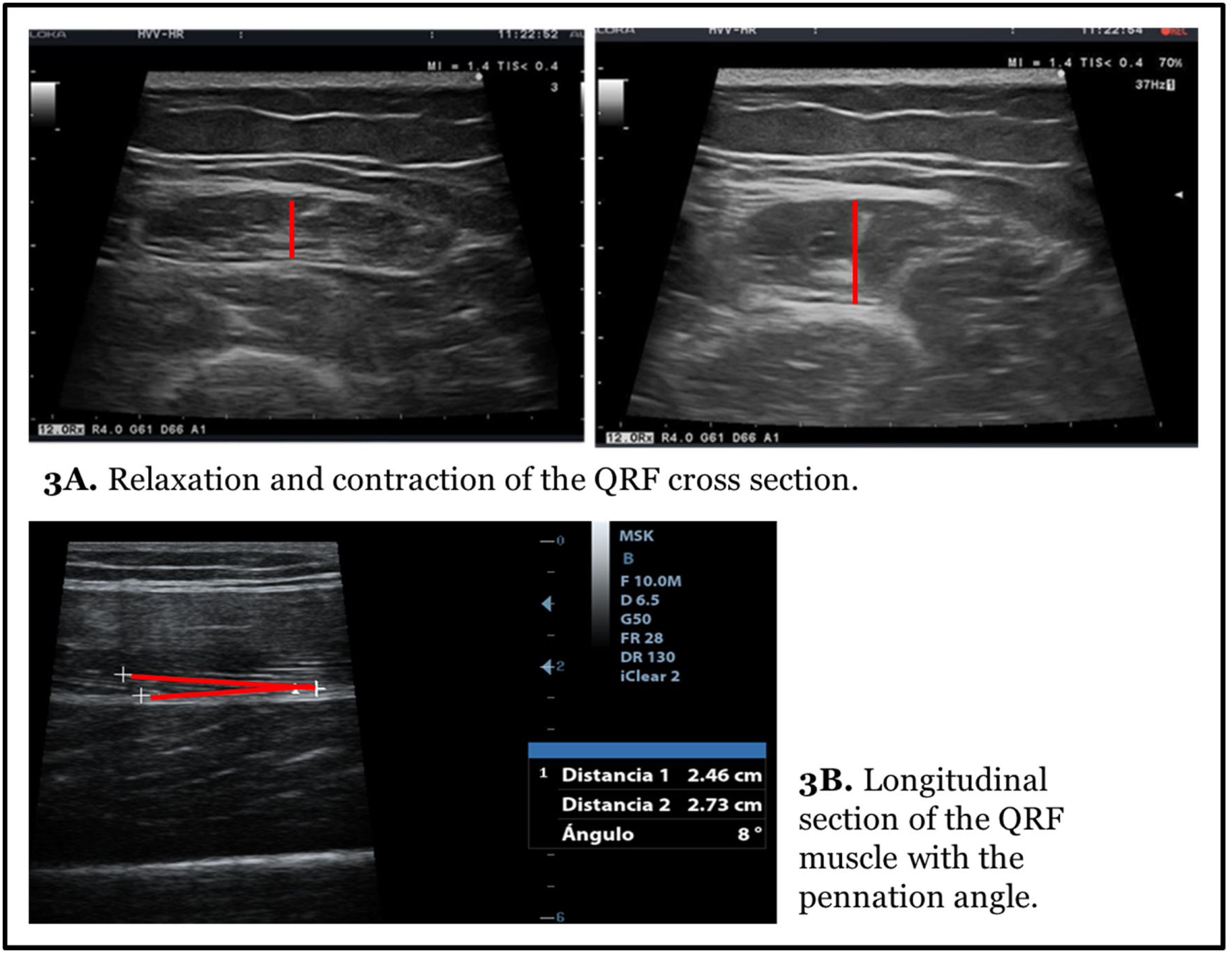
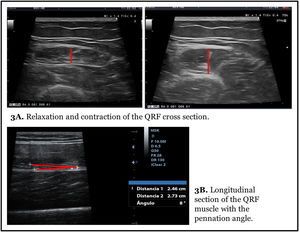
Muscle measurement is done statically and in a relaxation way. The measure in contraction provides different axes and can be useful for evaluating tone and functional changes in muscle mass (Fig. 3A).
Ultrasound is useful for evaluating changes in muscle quality as an increase of echogenicity. This is caused by an alteration of normal muscle architecture due to increased fat content, fibrosis, loss of healthy muscle and inflammation, leading to increased tissue sound reflexes. The loss of bone shadow is the result of increased attenuation of the ultrasound signal, as sound is reflected by increased fat, fibrosis, and other changes. Dynamic changes in the muscle can be evaluated. For example, fasciculations that can provide diagnostic value in conditions such as Amyotrophic Lateral Sclerosis (ALS). These spontaneous contractions can be measured in mode M.18
The surface of the bone is typically hyperechoic with posterior acoustic shadow. A good example is the deeper bright line that is visualised in the axial cut of the leg representing the crown of the femur.
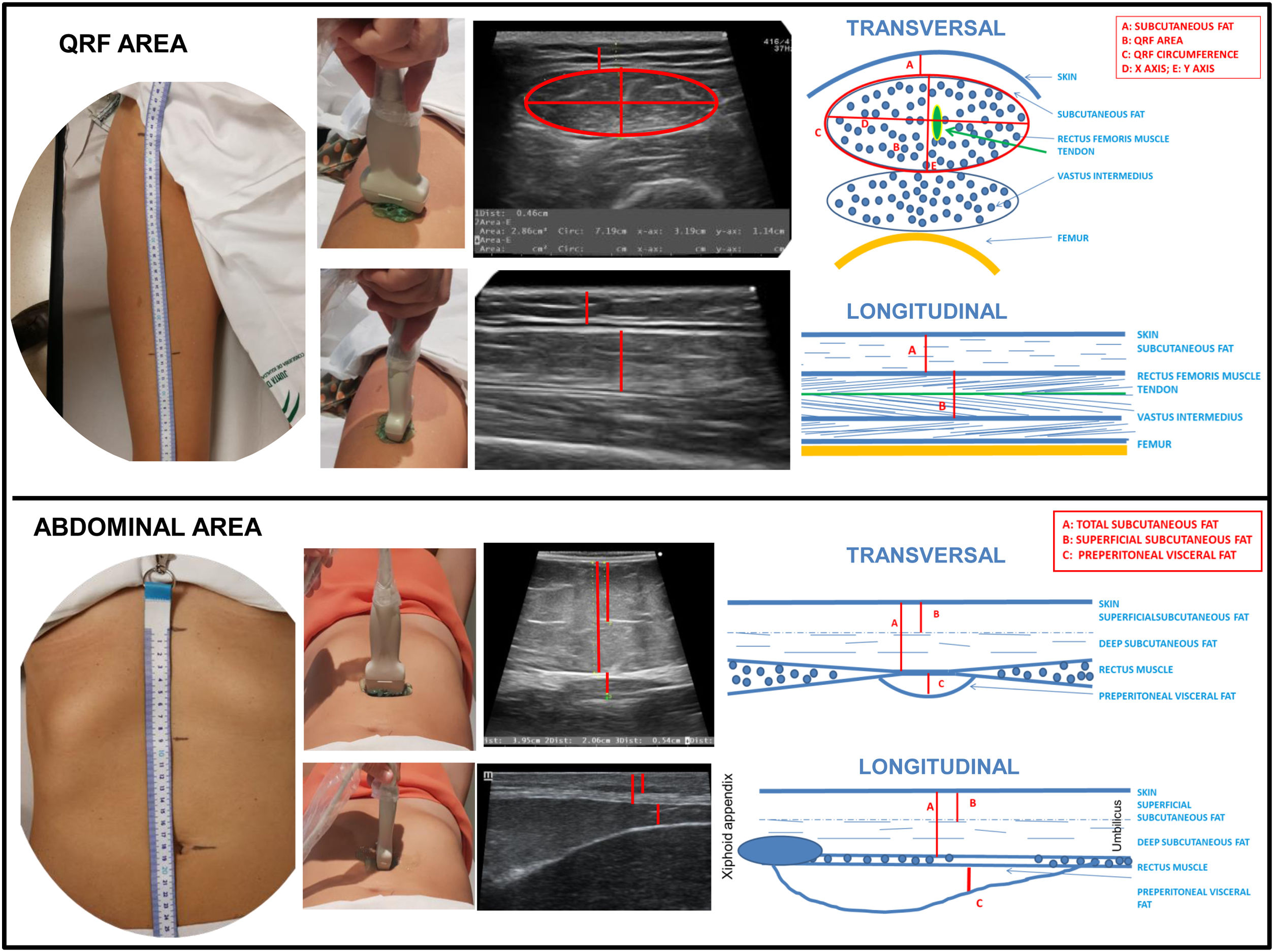
Nutritional ultrasound® measurement techniqueTo conduct the NU® it is important to establish a series of measures of patient position, location of anatomical structures and systematisation of standardised measurement cuts. It is performed with the patient in supine position and in a relaxation position with the determination of the measurement locations in the leg and abdomen.
In the location of the measurement of the leg, the imaginary line between the anterosuperior iliac spine and the upper edge of the patella is established, marking the lower third of that distance. There are other references in different locations, for example, halfway way or in the lower quarter, adaptable to different clinical situations. There are other measurement locations of interest to evaluate muscle mass such as biceps, gastrocnemius muscle, etc., which can be standardised following the published references.19
To facilitate the determination of the measurement point, a standardised reference table can be used to systematically reproduce the measurement in the same location (Appendix 1).
In clinical practice, two measures are standardised: transverse and longitudinal to the axis of the leg (Fig. 2). In the cross section, in the lower third, the crown of the femur is located in the dorsal plane and the epidermis in the ventral plane. Leg angle correction may be necessary to centre the image of the QRF muscle.
In the cross-section, the X and Y axes correspond to the linear measurement of the distance between the muscular limits of the QRF: lateral (X axis) and the anteroposterior (Y axis) is carried out. Muscle area and circumference are assessed through ellipsoidal measurements or by manual tracing around the edge of muscle aponeurosis. The location of this muscle is simple given its morphology and the presence of an easily identifiable hyperechogenic central tendon. The measurement of adipose tissue thickness is established as the linear distance between the epidermis and the aponeurosis of the QRF (Fig. 2).
In the longitudinal section the same structures are identified with the lower limit in the crown of the femur and the upper limit in the epidermis. Linear measurements of adipose tissue and the Y-axis can be made in this image. This section is especially useful for evaluating other morphometric features such as fasciculus length and PA formed by muscle fibres with the contractile major axis or muscle aponeurosis (Fig. 3B).20
The second component of NU® is the evaluation of fat at the level of the abdominal wall. The location of the measurement point is set at the midpoint between the xiphoid appendix and the navel on the midline. The patient remains in a supine position in a situation of relaxation and the image is taken during the unforced expiration, in a transverse plane using the same linear probe perpendicular to the skin (Fig. 2).
In the cross-section, the anatomical structures that are visualised are ordered from the most superficial layer corresponding to the epidermis, followed by the layer of subcutaneous, superficial and deep adipose tissue. Then the two muscles of the anterior rectum of the abdomen that join in the central part in the linea alba are identified.
The ultrasound with linear probe of the abdominal fat allows for the evaluation of the thickest superficial subcutaneous layer that works as a barrier of protection and energy store of the body. Also, on the other side of the fascia superficialis we can measure the deep SAT. This is a very vascularised and innervated tissue that is rich in adipocytes, stem cells and in certain areas of brown adipocytes. Adiponectin, a protective cytokine in terms of cardiovascular risk, is secreted in greater amounts in this area of deep SAT. With the linear probe, deeper layers such as the PAT, omental (intraperitoneal fat) and with a convex probe perirenal and retroperitoneal deposits can also be observed.21
One of the most important aspects is the localisation of the PAT layer which is a component of visceral fat that is located between the lower edge of the linea alba and the parietal peritoneum line. This fat has a triangular shape having its greatest thickness below the xiphoid appendix descending to the navel22 (Fig. 2). In order to be able to locate the PAT more accurately, longitudinal measurement can be carried out from the xiphoid appendix to the navel, visualising the entire longitudinal area of the PAT.
In the case of clinical situations in which the anatomical structures of the reference points cannot be located (e.g., location of iliac spine in extreme obesity or absence of navel in cases of abdominal surgery), the measurement will be carried out on the leg 15cm from the patella, and on the abdomen at 10cm from the xiphoid appendix.
In clinical practice there may be measurement errors in the measurement areas related to anatomical changes in the abdomen or leg, such as for example, postsurgical situations, obesity or achondroplasia due to the dysmorphic situation of the limbs, but this does not imply the impossibility of measurement, but the need to adapt it to the clinical condition.
The measurement of the leg in a conventional way is carried out in the right hemisoma, unless there is anatomical impossibility in this body limb. In this case it is measured on the contralateral side and noted in the medical history. It is recommended that the technique be repeated to obtain three measurements and that they be stored in the computer's memory and the average value of the three measurements recorded to increase the accuracy of the technique.
The evaluation of the qualitative characteristics of NU® includes all the information regarding the morphometric, functional and metabolic assessment.23 Regarding the metabolic properties, it is important to highlight the information on the adipose infiltration of the muscle (myosteatosis) that is characterised by an increase in echogenicity. Degenerative changes with age (senile sclerosis), are also characterised by a hyperechogenic aspect of the muscle. There are also alterations such as myonecrosis in serious patients in a critical care situation. Another image of interest is the visualisation of areas of perimuscular oedema as hypoechoic areas near muscle areas or in adipose tissue.
Quantitative evaluation by 2D texture analysis to evaluate the structure and quality of skeletal muscle is an important aspect with applicability in different pathologies. Analysis systems assisted through programmes such as ImageJ (National Institute of Health, USA) allow the study of echointensity by distributing the built-in histogram tool (0: black, 255: white) by selecting a “region of interest” (ROI) of 50×50 pixels in the medial portion of the muscle on a colour image “Red-Green-Blue” (RGB) 8 bit.24
The future of the application of ultrasound in the assessment of BC will advance towards new areas of research applying new ultrasound modes. It is possible to visualise the vascularisation of the QRF with colour Doppler ultrasound, which allows for better evaluation of the changes in blood flow. Muscle involvement results in a reduction in vascularisation secondary to a decrease in angiogenesis, due to the process of replacing the QRF with adipose and connective tissue during the inflammatory process associated with the critical situation and malnutrition.11
Standardisation and reliability of the techniqueThere are publications on the validity and reliability of ultrasound techniques with moderate to good intraobserver reproducibility and variable agreement among observers, which depends in part, on the muscle mass examined and the characteristics of the disease evaluated.
A systematic review includes 24 articles that shows moderate evidence of the usefulness of ultrasound for the measurement of muscle mass although it highlights the importance of determining cut-off points and their validation.25
Recently the SARCUS group has updated the application of ultrasound to measure sarcopenia focus on the evaluation of muscle mass in various anatomical reference points on at least 39 muscles. The technical characteristics would be the location of the proximal and distal points, the exact demarcation of the area of the exact measurement point, and the position of the patient.19
It is necessary to develop new validated, standardised and reliable techniques that allow an evaluation of normality patterns and cut-off points by each pathology. There are publications that relate the measurement of the rectus femoral by ultrasound with muscle mass in the elderly population with a good correlation with the measurements obtained through DXA (R=0.741, p<0.01).26
Evaluation of muscle steatosis through ultrasound shows a strong correlation with other established imaging techniques such as magnetic resonance imaging (MRI). The percentage of fat from MRI and muscle ultrasound after correcting subcutaneous fat thickness was r=0.91 in QRF.27
Cutting wave elastography is a non-invasive imaging technique that provides estimates of tissue stiffness by measuring the speed of cutting waves that can be used to assess the strength and tone of the QRF.28
The “gold standard” techniques for quantifying abdominal fat are computed tomography (CT) and MRI, however they have several limitations and disadvantages which are their high cost, exposure to high doses of radiation and technical complexity. Several studies have compared the results obtained by CT or MRI with those obtained by ultrasound in the quantification of visceral fat, concluding that there is a strong correlation between both measures.29
The usefulness of ultrasound in the non-invasive prediction of carotid atherosclerosis has been studied due to its correlation in multivariate analyses with the thickness of preperitoneal fat (HR, 4.31; p=0.003).30 On the other hand, there are no published studies that directly correlate the measurement of fat through ultrasound with the analysis of fat by bioelectrical impedance (BIA). Nevertheless, it appears that both methods have a good correlation with liver fat deposits.31
Clinical utility of nutritional ultrasound® in DRMThere is a growing interest in the literature on the evaluation of muscle mass by ultrasound. Recent publications establish the measurement of the muscle area of the QRF as a good measure of correlation of other parameters such as fat free mass (FFM), measured by BIA, hand-holding strength by dynamometry or exercise capacity.32 The clinical utility of NU® focuses on measuring the involvement of muscle mass in the diagnosis of malnutrition.
Diagnostic tool in the detection of DRMIt is important to assess the correlations between the different morpho-functional assessment techniques such as BIA, dynamometry, functional tests, etc., with the results obtained through NU®. In patients hospitalised in the medical area, Ozturk et al. have found a good correlation between the thickness and area of the QRF and the measures of muscle mass by BIA or strength by dynamometry establishing cut-off points of 4cm2 and 7.2cm2 for the area of the rectus femoral for women and men respectively.33
In critically malnourished patients there are QRF measurement protocols where it has been correlated with prognostic factors and hospital stay. Hernández-Socorro et al. have established by ultrasound at the level of the rectus femoral a categorical scale with values from one to four where the main characteristics of the muscle mass of the QRF are represented. Category 1 corresponds to the situation of normality while category 4 includes factors such as atrophy and muscle necrosis that correlate with a poor prognosis and a greater degree of muscle involvement.11 It is necessary to establish a uniform and systematic measurement protocol to obtain results that can be analysed and extrapolated to other populations.
The VALIDUM study of QRF measurement in critically ill patients demonstrates the usefulness of measuring muscle mass in this clinical situation. The most important finding was the diagnosis of low muscle mass in 58% of patients versus only 2.7% of patients with BMI as criteria for malnutrition.25
A recent study in the elderly hospitalised for hip fracture34 has found a strong correlation between quadriceps thickness measured by ultrasound and the risk of sarcopenia, by geriatric criteria and mobility. In this sense, the clinical utility of the technique is to support the diagnosis of malnutrition in situations where it is very difficult to use conventional parameters as BMI, due to the situation of oedema-inflammation or the difficulties of obtaining anthropometric parameters such as height and weight.
One of the important challenges regarding the usefulness of ultrasound is the diagnosis of malnutrition in different clinical situations. Although specific cut-off points are not yet established, there are already publications that try to find the area of the QRF that has sufficient sensitivity and specificity so that it can be used as a criterion for malnutrition. In this sense, there is a multicentre work where it is established that a muscle area of the QRF at the midpoint of the femur in men under 6cm2 or in women of 4.47cm2 have an adequate sensitivity and specificity to diagnose malnutrition related to PEW haemodialysis syndrome (malnutrition, inflammation and muscle wasting syndrome).35
In the future it would be desirable to have these cut-off points to establish levels of normality and to have them adjusted to different pathologies. There are some published reference values in measures of muscle thickness and density of different muscle areas (biceps, upper limbs and quadriceps, lower limbs) adjusted for age and sex, representative of the pattern of muscle normality.36
The evaluation of adipose tissue through ultrasound has shown interest especially in the study of overweight and obesity. However, there are few studies designed with the objective of analysing the decrease in body fat deposits associated with caloric malnutrition. In the pictures of DRM, weight loss associates a decrease in the body's energy reserve in the form of a reduction in body fat that affects the reserves of SAT and PAT measured by morphological techniques (CT, MRI, etc.). Ultrasound offers the opportunity to accurately and standardisedly quantify the body's fat stores through the thickness of SAT.37
Recent studies have shown the potential for predicting risks and complications of preperitoneal fat accumulation in various situations associated with different pathologies. The area of PAT evaluated by MRI in a study of patients with diabetes was the best predictor of steatohepatitis and therefore constitutes a potential new non-invasive marker for use in screening these patients for more aggressive forms of non-alcoholic fatty liver disease (NAFLD).38 Capone et al. demonstrated an association between increased visceral (preperitoneal) fat and the risk of gestational diabetes.39
The evaluation of visceral adipose storage is very important in the nutritional assessment due to the information on the inflammatory component of the malnutrition picture and its relationship with the risk of complications. Visceral adipose tissue is an independent risk marker of cardiovascular and metabolic morbidity and mortality. It is useful to evaluate it in clinical practice for its contribution to adverse health outcomes and to the modulation of response to different treatments. It is necessary to develop simple and clinically applicable tools such as NU® to be able to assess changes in visceral and ectopic fat over time.40
Increased fat storage within skeletal muscle has also been found to be associated with functional impairment and malnutrition in hospitalised elderly patients. An increased risk of malnutrition has been associated with an increase in patients’ quadriceps intramuscular adipose tissue, even after adjusting for factors such as age, sex and inflammation.41
Prognostic value of nutritional ultrasound®In critically ill patients, a prospective unicentric observational study in critically ventilated critical adult patients reported a correlation with prognosis. Every 1% loss of QRF thickness and vast quadriceps intermediate during the first week of disease was associated with a 5% increased 60-day mortality. It is important to establish the prognostic value of muscle ultrasound to plan therapeutic interventions adapted to the risk situation.42
In the area of nutrition, the quadriceps QRF is probably the most representative muscle of the appendicular skeletal muscle mass composition, but in certain clinical situations, it may be interesting to explore other muscle areas. In geriatric patients, the assessment of gait-related functionality can be assessed through the assessment of the gastrocnemius muscle. The decrease in muscle thickness on ultrasound (<1.5cm) can be considered decreased muscle mass.43
Ultrasound evaluation may be associated with clinical and functional outcomes. Quantitative characteristics expressed by area, thickness and volume are associated with risk of mortality, hospital stay, ICU days off and days off from mechanical ventilation. On the other hand, qualitative characteristics are encompassed in biomechanical and physiological properties such as PA, fascicle length, elastography and other metabolic properties such as muscle steatosis or myonecrosis. These are more related to the functional alteration of the muscle and its clinical results.23
Clinical reportIt is important to integrate NU® measures adapted to different clinical scenarios into routine clinical practice. The most relevant information on the evaluation of ultrasound is translated into a series of qualitative and quantitative measures that should reflect the main findings of the exploration in a fast and direct way. The NU® report should follow an orderly structure during the examination that allows comparing the results obtained in different examinations to evaluate the clinical changes of the patient.
In the quantitative aspect, it is important to present the results following the scheme of Table 1. The first contend is the distance at which the measurement is made. It is made followed by the area and circumference of the QRF. Next, the axes are integrated: the X axis (major sagittal axis of the muscle) and the Y axis (minor anteroposterior axis of the same). Finally, the distance of the thickness of the SAT should be noted. In the examination of the abdomen to evaluate the fat, the measurement reference point is noted, also observing the protocol, followed by the distances of the total, superficial and preperitoneal fat thickness.
Nutritional ultrasound® clinical report.
| QRF ultrasound in the leg (…… cm) | |
|---|---|
| Quantitative variables | Qualitative variables |
| QRF muscle: | Metabolic: |
| Area: ……………… cm2 | Myosteatosis (extravisceral adipose tissue): Yes/No |
| Circumference: …… cm | Myonecrosis: Yes/No |
| X axis: …………….. cm | |
| Y axis……………… cm | |
| Y axis (contracted).... cm | Biomechanics: |
| Subcutaneous adipose tissue………… cm | Pennation angle: ……. degrees |
| Fascicle length:……… cm | |
| Elastography: ………... kPa | |
| Ultrasound of the abdominal area (…… cm) | |
|---|---|
| Quantitative variables | |
| Subcutaneous adipose tissue | Others: tissue injury/oedema |
| Total: …………. cm | |
| Superficial: …… cm | |
| Visceral adipose tissue | |
| Preperioneal: ……... cm | |
| Intraperitoneal: …… cm | |
The qualitative aspects of the ultrasound findings can be analysed directly by describing the echogenicity changes found as well as the relevant findings. In the muscular area of the QRF, the increase in echogenicity is associated with myosteatosis due to infiltration of adipose tissue or sclerosis related to the changes of advanced age. The decrease in muscle echogenicity is associated with oedema and myonecrosis. It is important to identify pathological alterations such as muscle injuries, oedema, collections, which can artefact the measurements. If specific measurement protocols for other areas of interest such as biceps, gastrocnemius, interosseous, sternocleidomastoid, diaphragmatic muscles, etc. are added, the references will have to be taken according to the protocols published in the literature.
Of all the mentioned variables, it is necessary to include some that are essential for its clinical application (area of the QRF, thickness of the subcutaneous adipose tissue or preperitoneal fat). On the other hand, other variables are more interesting in the area of research and development such as PA, other biomechanical measures and other specific parameters of adipose tissue such as intravisceral and liver locations, etc.
Limitations and future considerationsThe general limitations of ultrasound are those inherent in the performance of the technique due to the dependence on the ultrasound apparatus, the sonographer, etc. Regarding the equipment, the fundamental determinants of quality are the piezoelectric elements contained in the linear of the probe that directly affect the image quality obtained, as well as the adjustment of the main determinants such as frequency, dynamic range, gain, depth and focus. Experience in handling and configuring the device determines the possibility of determining muscle and adipose tissue structures more easily.
The second aspect of relevance would be measurement techniques. The main limitation is related to the standardisation of the measurement places, as well as the performance of an adequate technique with the placement of the probe, pressure on the surface to be explored, etc. The proper visualisation of the structures is very important in order to make the measurements correctly. These are also a limiting factor since there is inter and intraobserver variability when conducting these measurements. These aspects can be reduced by making several measurements. In research projects the measurement is centralised in one or a few people trained in the measurement of ultrasonographic images. Furthermore, artefacts may appear and may need to be recognised, these include refraction, reverberation, edge and acoustic shadows.
There is little evidence of the value of measuring NU® with respect to its relationship with physical performance, functionality tests, quality of life and overall mortality, as well as the management of nutritional support. The absence of population references adjusted for age and sex is also an important factor in interpreting the images since it is not possible to directly assess whether the measurements are pathological or within the range of normality.
Because ultrasound is not without limitations, it is essential that Endocrinology and Nutrition Specialists have the appropriate training and develop the necessary skills to perform and interpret images through training courses to assess the situation of malnutrition through the evaluation of NU®.
The challenge of NU® is to incorporate it in clinical practice as a support tool in the morpho-functional assessment of DRM by integrating it into the usual tools such as anthropometry, dietary registration, biochemical parameters and in this way, complement emerging parameters such as BIA, functional tests, dynamometry and measures of quality of life and inflammatory parameters (CRP/prealbumin).2 These techniques should be correlated with other established classical and emerging nutritional assessment parameters that provide an established prognostic value, e.g., subjective global assessment, albumin, phase angle, dynamometry, etc. In this area of investigation, an ongoing multicentre study “feasibility of the application of nutritional echography in the diagnosis and follow-up of patients with nutritional risk at the hospital: study and function of body composition” (DRECO, ALM-DRECO-2021-01) could generate knowledge in this area.
Scientific evidence is limited, and future scientific projects should include the design of population studies in different pathologies that permit obtaining reference values and cut-off points to be able to interpret the results of each patient individually. The evaluation of the NU® should enable clinical decisions based on these results that allow the adjustment and individualisation of the nutritional therapeutic and physical exercise plan, along with functional recovery. More prospective studies are needed to assess response times and describe the slightest clinically significant change in all these parameters.
The translation of the results of the evaluation of NU® into diagnostic criteria of malnutrition that can be incorporated into clinical guidelines (GLIM) requires a validation process. The muscle area of the QRF and the other fat-free mass parameters inform us of the protein component of the DRM, just like the fact that the thickness of the adipose tissue and its distribution inform us about the proportion of body fat mass that correlates with the caloric component of the DRM. NU® provides information on morphological characteristics although functional information can be extracted (Y axis of the QRF and PA) that would allow it to be incorporated into the GLIM phenotypic criteria.2
ConclusionsTechnological advances have allowed the real-time use of nutritional ultrasound® to obtain images of muscle mass and adipose tissue in its different subcutaneous, visceral and extravisceral compartments.
It is essential to establish universally accepted standards on the different technical aspects of measurement, patient position and image interpretation to assist in the reading, acquisition, presentation and communication of outcome findings. The storage and export of images must be systematised.
Ultrasound studies in patients with DRM suggest moderate to good intraobserver reproducibility and variable agreement between observers that depends, in part, of the location evaluated and the characteristics of the disease in which it is used (dialysis, critical patients, sarcopenic obesity, etc.).
The clinical utility of nutritional ultrasound® focuses on its ability to diagnose BC changes with fat-free mass involvement focused on the evaluation of the muscle area and the modifications of fat mass and its distribution through the exploration of the different compartments of adipose tissue. Its usefulness as a prognostic factor in different clinical situations should be established, as well as standardisation of the results obtained compared to the population values adjusted for age, sex and probably other determining anthropometric variables such as height and weight.
Ultrasound presents a series of beneficial characteristics including portability, economic cost, non-radioactivity and high versatility of application in different clinical scenarios and can be a very powerful advanced clinical exploration tool in the study of body composition applied to the area of DRM, monitoring the effect of nutritional intervention and physical exercise and in other clinical conditions.
It is necessary to establish a training plan in high-quality standardised musculoskeletal ultrasound to increase the use of these techniques by Endocrinology and Nutrition Specialists, with the aim of improving the diagnosis and treatment of their patients.
FundingThis research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to thank NUTRISEEN, the nutritional area of the Spanish Society of Endocrinology and Nutrition (SEEN), for the support in the dissemination of the contents with training courses in nutritional ultrasound® and research projects in this area.