The development of Cushing’s syndrome produces a range of signs and symptoms secondary to chronic exposure to excess glucocorticoids. The syndrome may be due to the abnormal endogenous production of glucocorticoids (induced by ACTH-secreting pituitary tumours, adrenal tumors) or to the exogenous administration of glucocorticoids.1 Cushing’s syndrome is more common in women than in men, with an incidence of 0.7–2.4 cases per million inhabitants per year.2,3 The typical clinical picture consists of a series of morphological and anthropometric, metabolic, cardiovascular, musculoskeletal, reproductive, dermatological, neuropsychiatric and infectious disorders.4–6 The involvement of the gastrointestinal system is exceptional, however.4
We report a case of Cushing’s syndrome due to bilateral adrenal nodular hyperplasia with long-standing hypopotassemia and persistent constipation, with the development of a megacolon, that improved after hypercortisolism was brought under control.
The case corresponded to a 66-year-old male with arterial hypertension (AHT) known since 2008 and associated with hypopotassemia since 2015, two years before evaluation at our centre. The patient history included persistent constipation with chronic laxative use in recent years. He had been admitted twice in recent months due to paralytic ileus attributed to hypopotassemia. The patient had received multiple antihypertensive drugs over time, and had been treated in recent months with furosemide, spironolactone, olmesartan/hydrochlorothiazide, manidipine, doxazosin and atenolol. Assessment of the patient prior to our evaluation revealed marked bilateral adrenal hyperplasia on the CAT scan, and the hormone study showed a normal aldosterone/plasma renin activity ratio (aldosterone 3 ng/dl, [normal range (NR) 1.1–50.6]; plasma renin activity 0.88 ng/ml/h [NR 0.35–1.8]), elevated 24-h urinary cortisol levels <2 times the normal value on a single occasion, and isolated elevated plasma cortisol.
At initial evaluation, the patient presented hypotension and symptomatic bradycardia in the context of atrial flutter with variable ventricular conduction at 45 bpm, in addition to evidence of intestinal pseudo-obstruction with a globose abdomen, generalized pain in response to palpation, tympanism and metallic sounds. Severe proximal muscle atrophy of the lower extremities was also noted. In view of this situation, the patient was admitted to a monitored unit, and positive chronotropic drugs were administered. The placement of a pacemaker was avoided following heart rate stabilization within 48−72 h. Transthoracic echocardiography revealed evidence of hypertensive heart disease with severe left ventricular hypertrophy and left atrial dilatation. Bradycardia was attributed to basal beta-blocker treatment. Following suspension of the latter, the cardiological course proved favourable.
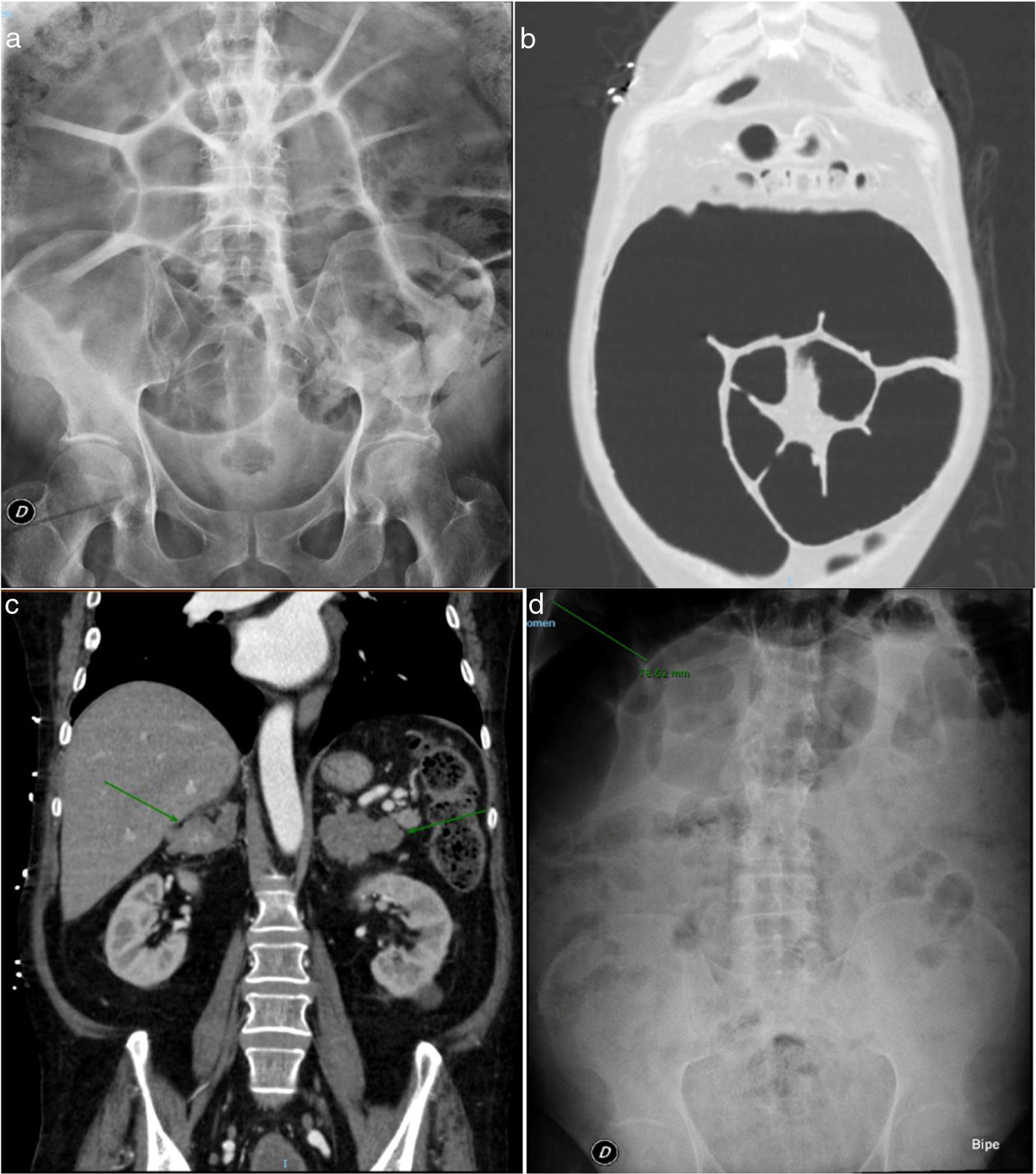
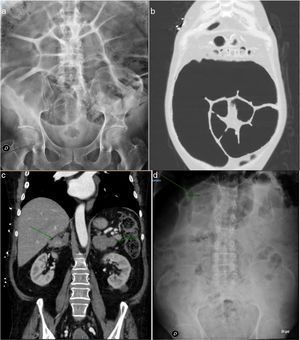
However, the abdominal symptoms persisted, and the plain abdominal X-ray study showed important left colon distension, with a maximum diameter of 11.2 cm (Fig. 1a), requiring intermittent rectal catheterization and daily enemas to relieve the abdominal bloating.
a) Plain abdominal X-ray view. Large left colon distension with a maximum diameter of 11.2 cm. b) CAT scan of the chest and abdomen. Large sigmoid colon dilatation. c) CAT scan of the chest and abdomen. Greatly enlarged adrenal glands of hyperplastic appearance. d) Plain abdominal X-ray view. Colon calibre reduction to 7.8 cm following bilateral adrenalectomy.
The study was completed with the suspicion of Cushing’s syndrome based on the patient phenotype and elevated urinary free cortisol levels. The arterial hypertension and hypopotassemia were considered to be an expression of the mineralocorticoid activity inherent to hypercortisolism. From the hormonal perspective, we confirmed hypercortisolism with suppressed ACTH (2 pg/ml [NR 3.5–60.5]): elevated urinary free cortisol on 3 occasions (1100, 1750 and 2633 nmol/24 h [NR 100–379]), elevated plasma cortisol after dexamethasone 1 mg (721 nmol/l [NR 172–497]), and basal 281 nmol/l (NR < 21) and nocturnal salivary cortisol 117 nmol/l (NR < 5.7). The serum TSH and free T4 levels were normal. A CAT scan of the chest and abdomen revealed large sigmoid colon dilatation together with greatly enlarged adrenal glands, of hyperplastic appearance (Fig. 1b and c). Colonoscopy revealed no cause of obstruction.
Based on the suspicion of Cushing’s syndrome due to bilateral adrenal gland hyperplasia, and considering the severe systemic condition (hypertensive heart disease, megacolon, amyotrophy with functional impairment), bilateral adrenalectomy was planned after treatment with ketoconazole for 21 days in an attempt to mitigate the systemic effects of hypercortisolism. The diagnosis of macronodular adrenocortical hyperplasia in both adrenal glands was confirmed, and hormone replacement therapy was started.
Initial postoperative management was complicated by the persistence of hypopotassemia despite oral and intravenous supplementing, together with a new episode of intestinal pseudo-obstruction, though after the first week the patient started to progress favourably. Over follow-up after admission, the patient showed very good blood pressure control (systolic blood pressure 110−130 mmHg and diastolic blood pressure 70−80 mmHg), sinus rhythm was maintained at a good frequency without the need for beta-blockers, the constipation had been significantly reduced, without the need for laxatives or the administration of enemas (which had previously proved essential), and the colon diameter was reduced to 7.8 cm (Fig. 1d). The Coloproctology Unit was consulted regarding the need for surgery, but the decision was made to continue patient monitoring given the continued gradual improvement.
Gastrointestinal symptoms in Cushing’s syndrome are very uncommon, and mainly consist of abdominal pain.4 However, in the context of exogenous glucocorticoid use there have been reports of gastritis, ulcers, gastrointestinal bleeding (1.5%),7 pancreatitis,8 fatty liver, and hollow organ perforation.9 Specifically, 6 cases of diverticular perforation have been described in patients with clinical signs analogous to those of our patient, though with no evidence of a megacolon. These conditions have been related to persistent hypopotassemia, severe hypercortisolism, advanced age, malnutrition, uremia or immune deficiencies.10
In our case, we wish to underscore two aspects: severe hypopotassemia (minimum value: 1.8 mEq/l [NR 3.5–5.1]) and bowel involvement with a megacolon.
Hypopotassemia can be explained by the mineralocorticoid action of high glucocorticoid levels, as well as by the possible overproduction of steroids with mineralocorticoid activity occurring in macronodular hyperplasia, resulting in hyperactivity not only in the fascicular zone but also in the glomerular zone of the adrenal cortex.
In turn, although the megacolon might be explained by the chronic use of laxatives and, indeed, has been reported as a side effect of drugs such as diuretics (which favour hypopotassemia) or calcium antagonists (due to their effect upon smooth muscle), we have found no reported cases of an iatrogenic megacolon of this calibre. We therefore consider the main cause of the megacolon and hypotonia to have been Cushing’s syndrome (due to the catabolic proteolytic effect of glucocorticoids upon the intestinal smooth muscle), together with chronic hypopotassemia, no other similar cases having been found in the literature. The partial response after bilateral adrenalectomy supports the hypothesis of chronic damage established upon the colon wall.
In sum, we have reported a case of hypercortisolism associated with a megacolon, the cause of which may be related to the structural damage induced by cortisol overproduction and chronic hypopotassemia.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Márquez López L, Beltrán Romero LM, Mangas Cruz MÁ, Pumar López A, Acosta Delgado D. Megacolon en el contexto de síndrome de Cushing de larga evolución. Endocrinol Diabetes Nutr. 2021;68:137–139.