GLUT1 is a glucose transporter in the central nervous system. GLUT1 deficiency syndrome (GLUT1-DS) is a congenital metabolic disease caused by mutations in the SLC2A1 gene. It is considered a rare or uncommon disease, due to its low incidence of 1:90,000 in the general population, but more than 500 cases have been reported in the literature.1,2 GLUT1-DS leads to movement disorders with exercise-induced paroxysmal dystonia, seizures and mental retardation. Diagnosis is based on the presence of hypoglycorrhachia, but absence of hypoglycaemia, confirmed by molecular analysis of the SLC2A1 gene. A ketogenic diet is considered the best treatment for GLUT1-DS3–7, providing ketone bodies as the only energy source for the neuron. We present the first case of GLUT1-DS and COVID-19 in the literature. The patient was a 57-year-old woman with a late diagnosis of GLUT1-DS, under follow-up since 2016 in the Neurology and Endocrinology and Nutrition departments of Hospital Universitario Ramón y Cajal in Madrid, Spain. Her symptoms at diagnosis were: cognitive impairment; needing to live in residential care under the guardianship of a sister; and epilepsy and paroxysmal progressive dystonia, requiring treatment with carbamazepine and levodopa/carbidopa. Genetic study confirmed the diagnosis of GLUT1-DS, detecting the mutation p.Arg126Cys (p.R126C) in position 376 (c.376C > T) of exon 4 of the SLC2A1 gene. The patient was being followed up by the Endocrinology and Nutrition Department's Congenital Metabolic Diseases Unit, treated with a 2.5:1 ketogenic diet providing a total intake of 1500 kcal, with foods low in sugar, vegetable and MCT oil, and a supplement of 100 ml every 12 h of Ketocal 4:1 LQ Multi Fibre. This is a nutritionally complete liquid preparation, rich in lipids, low in carbohydrates, with whey proteins supplemented with amino acids and micronutrients. The energy density of the 200 ml carton is 300 kcal (1.5 kcal/mL), and it helps meet the protein and calorie needs.
With this diet the patient showed a notable improvement in her movement disorder, as well as no further epileptic seizures. Her diet and nutritional support were monitored on a monthly basis by email and telephone contact with the care home and her guardian.
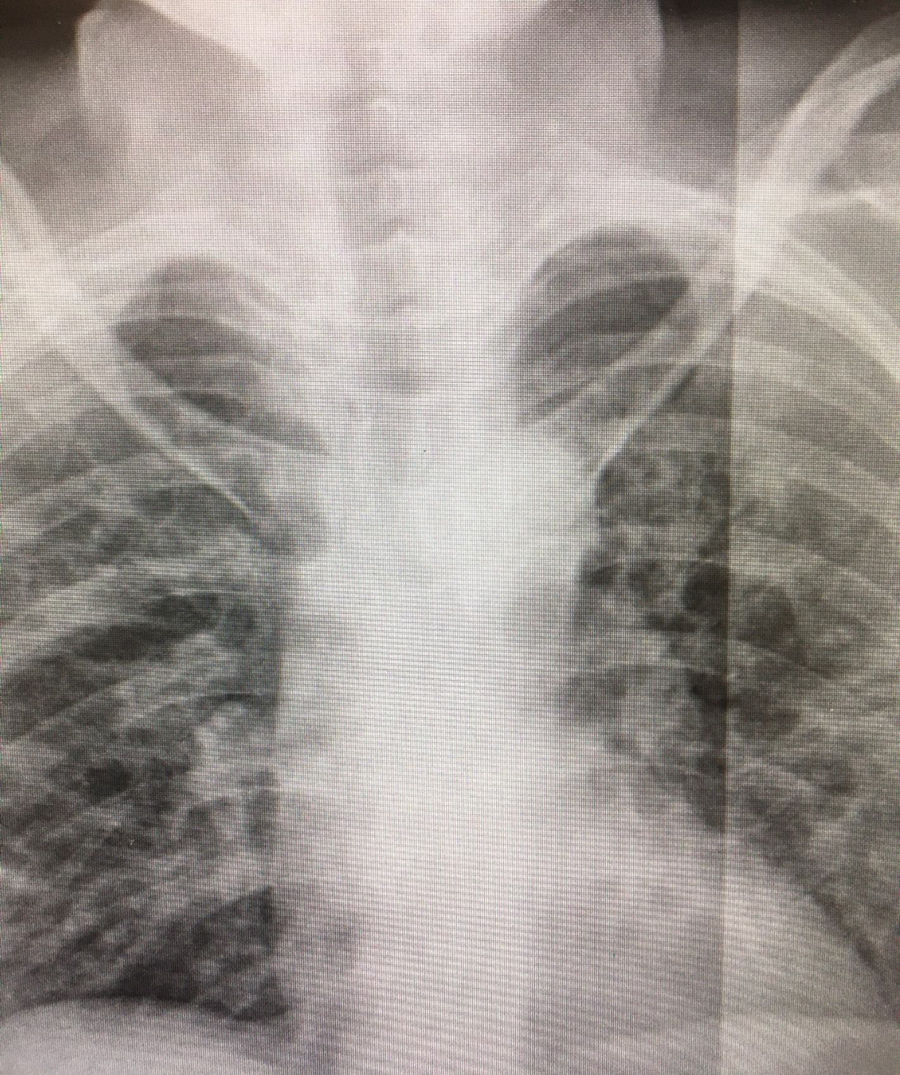
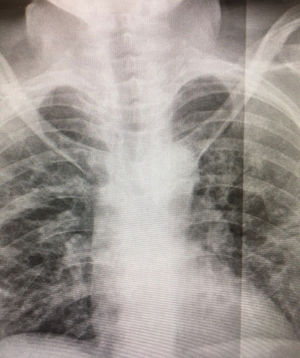
The patient came to the hospital's A&E department with a 48 -h history of fever and dyspnoea, and X-ray showed bilateral pneumonia typical of COVID-19 (Fig. 1). Blood test results on admission were as follows: glucose 104 mg/dl, creatinine 0.62 mg/dl, estimated GFR (MDRD-4 IDMS) 99.93 ml/min, creatine kinase 104 U/l, albumin 3.08 g/dl, total proteins 6.2 g/dl, AST/GOT 57 U/l (4–50), ALT/GPT 30 U/l, GGT 26 U/l, LDH 507 U/l (140–240), alkaline phosphatase 53 U/l, C-reactive protein 129.6 mg/l (0–5), procalcitonin 0.11 ng/mL, troponin I 0.0 ng/mL, pH in gases 7.48, pCO2 31.0 mmHg, pO2 69.0 mmHg, HCO3 23.1 mmol/l, % sO2c 94.8%, sO2m 97.4%, plasma sodium 132.0 mmol /l (135–145), lactate on blood gas 0.90 mmol /l, calcium ion 4.30 mg/dl, Hb on co-oximeter 10.3 g/dl (12–16), D-dimer 540.00 ng/mL (0–500), red blood cells 4.24 106/μl, haemoglobin 13.1 g/dl, haematocrit 35.9%, MCV 84.8 fl, MCH 30.9 pg, MCHC 36.4 g/dl, RDW 12.0%, platelets 312.0 103/μl, MPV 7.12 fl (7.5–11), leucocytes 7.14 103/μl, neutrophils 4.67 103/μl, neutrophils 65.4%, lymphocytes 1.98 103/μl, prothrombin time 12.30 s, prothrombin activity 80.4%, INR 1.11, aPTT 28.50 s, derived fibrinogen 740.00 mg/dl (150–400). The patient was admitted with severe pneumonia (CURB-65 score 2), and her PCR test for COVID-19 was positive. She was started on treatment with Dolquine (hydroxychloroquine) and Kaletra (lopinavir/ritonavir), adjusting the carbamazepine and maintaining fluid therapy with 0.9% saline and 500 ml 5% glucose in 24 h, as well as the 2.5:1 ketogenic diet she was already on. The dystonia worsened within 24 h, so she was changed to an exclusive 4:1 ketogenic diet with Ketocal 4:1 LQ Multi Fibre, four cartons/day, 1200 kcal. The patient progressed favourably after 36 h on this diet and was discharged home with her sister, with clinical, biochemical and radiological improvement, and negative PCR test for COVID-19 three weeks after admission. Since then, the patient has continued with outpatient nutritional support on our part with a 2.5:1 ketogenic diet and is making a good recovery, returning to normal nutritional parameters: glucose 74 mg/dl, total proteins 7.5 g/dl, albumin 4.67 g/dl, prealbumin 22 mg/dl, retinol-binding protein 4.3 mg/dl and C-reactive protein 0.5 mg/dl.
The patient had the symptoms and radiological and laboratory test signs (elevated LDH, C-reactive protein and D-dimer) as markers of the disease, and confirmed with a positive COVID-19 PCR test. Her dystonia worsened, due to the interference in her diet with the 25 g/day (500 cc 5% glucose) increase in her carbohydrate intake, which reduced its ketogenicity. It has been reported that infectious stress can weaken patients with GLUT1-DS.5–8 In our case, the patient had a slight increase in ataxia with no tonic seizures after starting fluid therapy, even although limited in glucose from the time she was admitted. The ataxia resolved within 36 h with an exclusive complete 4:1 ketogenic diet of four cartons/day of Ketocal 4:1 LQ Multi Fibre. As our hospital is a referral centre for congenital metabolic diseases with the means available to treat patients with congenital errors of metabolism,9 the family were able to contact the Endocrinology and Nutrition Department and start specific treatment. Therefore, given the exceptional nature of a case of COVID-19 in an adult patient with GLUT1-DS, we should point out that it was not a risk factor for COVID-19 complication. Finally, we would like to highlight the importance not only of a personalised diet, but also of having specific supplements for metabolic disease in nutrition units where patients with congenital metabolic diseases are treated.
Please cite this article as: Arrieta Blanco F, Bélanger Quintana A, Bengoa Rojano N, Stanescu S, Martinez Pardo M. Síndrome de deficiencia de GLUT1 y COVID-19. Endocrinol Diabetes Nutr. 2021;68:514–515.