The cause of death can be attributed to malnutrition in 10–20% of cancer patients. Patients with sarcopenia present more chemotherapy toxicity, less progression-free time, less functional capacity and more surgical complications. Antineoplastic treatments have a high prevalence of adverse effects that compromise nutritional status. The new chemotherapy agents present direct toxicity on the digestive tract (nausea, vomiting, diarrhoea and/or mucositis). We present the frequency of adverse effects with nutritional impact of the most frequent chemotherapy agents used in the treatment of solid tumours, as well as strategies for early diagnosis and nutritional treatment.
Material and methodsReview of commonly used cancer treatments (cytotoxic agents, immunotherapy, targeted therapies) in colorectal, liver, pancreatic; lung, melanoma, bladder, ovary, prostate and kidney cancer. The frequency (%) of gastrointestinal effects, and those of grade ≥3 are recorded. A systematic bibliographic search was carried out in PubMed, Embase, UpToDate, international guides and technical data sheets.
ResultsThey are shown in the form of tables in which the drugs appear together with the probability that they present any digestive adverse effect and the percentage of serious adverse effects (Grade ≥ 3).
DiscussionAntineoplastic drugs are associated with a high frequency of digestive complications with nutritional repercussions, which can reduce QoL and cause death as a result of malnutrition or due to the limiting effect of suboptimal treatments, closing the malnutrition-toxicity loop.
It is necessary to inform the patient about the risks and establish local protocols regarding the use of antidiarrheal drugs, antiemetics and adjuvants in the management of mucositis. We propose action algorithms and dietary advice that can be used directly in clinical practice, to prevent the negative consequences of malnutrition.
Hasta en 10–20% de los pacientes con cancer, la causa de muerte se puede atribuir a desnutrición. Los pacientes con sarcopenia presentan más toxicidad a la quimioterapia, menos tiempo libre de progresión, menor capacidad funcional y más complicaciones quirúrgicas. Los tratamientos antineoplásicos tienen una alta prevalencia de efectos adversos que comprometen al estado nutricional. Los nuevos quimioterápicos presentan toxicidad directa sobre el tubo digestivo (náuseas, vómitos, diarrea y/o mucositis). Presentamos la frecuencia de efectos adversos con impacto nutricional de los quimioterápicos utilizados en el tratamiento de tumores sólidos más frecuentes, así como estrategias para realizar el diagnóstico precoz y el tratamiento nutricional.
Material y métodosRevisión de tratamientos oncológicos de uso habitual (citotóxicos, inmunoterapia, terapias dirigidas) en cáncer colorrectal, hepático, pancreas, pulmón, melanoma, vejiga, ovario, próstata y riñón. Se registra la frecuencia (%) de efectos grastrointestinales y los de grado ≥3. Se realizó una búsqueda bibliográfica sistemática en PubMed, Embase, UpToDate, guías internaciones y fichas técnicas.
ResultadosSe muestran en forma de tablas en las que aparecen los fármacos junto con la probabilidad de que presenten cualquier efecto adverso digestivo y el porcentaje de efectos adversos graves (Grado ≥ 3).
DiscusiónLos antineoplásicos se asocian a alta frecuencia de complicaciones digestivas con repercusión nutricional, lo que pueden disminuir la calidad de vida y provocar la muerte como consecuencia de la desnutrición o por el efecto limitante de tratamientos subóptimos, cerrando el círculo desnutrición-toxicidad.
Es necesario informar al paciente sobre los riesgos y establecer protocolos locales respecto al uso de fármacos antidiarreicos, antieméticos y adyuvantes en el manejo de la mucositis. Proponemos algoritmos de actuación y consejos dietéticos que pueden utilizarse directamente en consulta, para prevenir las consecuencias negativas de la desnutrición.
Cancer is one of the leading global causes of morbidity and mortality. The most recent data available on cancer incidence, prevalence, and mortality in Spain are from the annual report of the Spanish Society of Medical Oncology. The number of cases of cancer diagnosed in Spain in 2021 is estimated at 276,239, according to calculations by the Red Española de Registros de Cáncer [Spanish Network of Cancer Registries] (REDECAN), although this figure may vary, since this estimate does not include the possible effect of the COVID-19 pandemic. The most commonly diagnosed cancers were those of the colon and rectum (43,581 new cases), prostate (35,764), breast (33,375), lung (29,549) and urinary bladder (20,613).
Regarding mortality, cancer continues to be one of the leading causes of global mortality, with approximately 9.9 million tumour-related deaths in 2020. The cancers responsible for the highest number of deaths worldwide were lung cancer (18% of all cancer deaths), colorectal cancer (9.4%), liver cancer (8.3%), stomach cancer (7.7%) and breast cancer (6.9%).1
Malnutrition is a common complication in cancer patients. In the different published series, malnutrition affects 20–70% of patients depending on age, type of cancer and tumour stage, and is more common in older patients, in those with more advanced tumours and in certain locations. Patients with tumours of the gastrointestinal tract, head and neck, liver and lung are most frequently malnourished.2 It is estimated that the cause of death can be attributed to malnutrition in up to 10–20% of cancer patients.3 Furthermore, patients with sarcopenia experience more toxicity to chemotherapy, less tumour progression-free time, less functional capacity and more surgical complications.4,5
Malnutrition in cancer patients is due to the activation of a systemic inflammatory response caused by the molecules produced by the tumour and the consequent activation of different pathways that lead to anorexia, weight loss and sarcopenia. Anorexia is a symptom that is the result of an abnormality of the appetite pathways of the central nervous system caused by the tumour itself or its complications (nausea, diarrhoea, pain).2 It differs from cancer cachexia, which is defined as a syndrome characterised by involuntary weight loss along with decreased lean body mass, with or without loss of fat mass, which cannot be reversed with conventional nutritional therapy and leads to functional impairment.6 It is important to detect malnutrition early in order to establish medical nutritional therapy at the earliest opportunity. For this reason, malnutrition screening programmes should be put in place for these patients, which, alongside the control of pain and other symptoms and a psychosocial approach, are of equal importance in the treatment of cancer patients. Data have been published suggesting increased patient quality of life when a holistic approach to treatment is taken.7
Antineoplastic treatments aimed at reducing or eliminating tumour cells are one of the pillars of cancer patient treatment. However, it must be borne in mind that these treatments have a potentially high prevalence of adverse effects, the most common of which being those that compromise nutritional status by altering gastrointestinal functionality.
There are currently a wide range of antineoplastic drugs available. Classic chemotherapy drugs have been joined by two new drug groups: those directed against molecular targets (biological agents or targeted therapies) and immunotherapy. Targeted therapies block specific molecules involved in the proliferation and growth of cancer cells. Immune checkpoint inhibitors (immunotherapy) act against tumour cells through the immune system itself.
Compared with conventional chemotherapy, these drugs are more specific and cause fewer toxic effects.8,9 Even so, they are not exempt from adverse effects, either because they are administered for long periods of time or because they are capable of inducing a wide variety of direct toxic effects on the gastrointestinal tract, such as nausea, vomiting, diarrhoea and/or mucositis. These side effects are reversible over time but they can contribute to deteriorating nutritional status, which is why actively looking for these effects is recommended in order to detect malnutrition early.
The emetogenic capacity of the cytostatics used in monotherapy is highly variable and the use of drugs in combination increases its incidence and intensity. Despite the widespread use of treatments for the prevention of chemotherapy-induced nausea and vomiting, this adverse effect continues to occur in a high percentage of patients (around 50%) and is more common with classic chemotherapy drugs, such as cisplatin, than in target-specific biological agents, antitarget drugs or targeted therapies. In terms of intestinal conditions, both diarrhoea and constipation can occur, depending on the drug used. Diarrhoea is a very common adverse effect (>10% of patients) of drugs such as capecitabine and irinotecan. This is a dose-limiting toxicity, which is amplified by association with other chemotherapeutic drugs (such as the combination of cetuximab with irinotecan in colorectal cancer). This adverse effect is also common in targeted therapy, especially in epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs). Inflammation of the gastrointestinal mucosa from the mouth to the anus, known as mucositis, is a very common side effect, reported by up to 35–40% of patients on chemotherapy.10
Toxicity must be evaluated in terms of its severity, time of onset (immediate, early, delayed or late), and duration. Common toxicity criteria are framed according to severity and the involvement of the different systems or organs. Each centre may use a toxicity grading system, the most commonly used systems being those of the National Cancer Institute, (Common Terminology Criteria for Adverse Events v5.0)11 (Table 1), the Eastern Cooperative Oncology Group (ECOG)12 or the World Health Organization.13 There are also self-administered scales such as the Patient Reported Outcomes-Common Toxicity Criteria for Adverse Events (PRO-CTCAE).14
Classification of severity of adverse effects of antineoplastics.
| Grade | ||
|---|---|---|
| Grade 1 | Mild | Mild symptoms, no intervention required. |
| Grade 2 | Moderate | Minimal or local non-invasive intervention needed. |
| Grade 3 | Severe or medically significant | Severe but not immediately life-threatening. Requires hospitalisation, disabling |
| Grade 4 | Life-threatening consequences | Urgent intervention indicated |
| Grade 5 | Death | |
Common Terminology Criteria for Adverse Events (CTCAE) v5.0 tool.
This review studies the frequency of adverse effects with a nutritional impact (nausea, vomiting, diarrhoea and mucositis) of the most commonly used chemotherapeutic drugs to treat the most common solid tumours, as well as strategies for early diagnosis and nutritional therapy adapted to each type of toxicity.
Material and methodsA review of all the cancer treatments in routine clinical use in Spain was conducted, including cytotoxic drugs, immunotherapy, targeted therapies and any combination thereof, for the treatment of the most common solid tumours of the gastrointestinal system (colorectal, liver and pancreatic cancer); lung cancer, melanoma and genitourinary tumours (urothelial, ovarian, prostate and kidney). The frequency (%) of gastrointestinal adverse effects, of any grade, such as diarrhoea, nausea/vomiting and mucositis, were recorded, as well as those of grade 3 or higher.
Once gathered, a systematic bibliographic search was conducted. For this purpose, the electronic databases PubMed, Embase and UpToDate were used, with relevant modifications being made according to the requirements of each database, and using the search operators «AND» and «OR». The term «cancer therapy» successively combined with «gastrointestinal toxicity» and «cancer chemotherapy» was used. Meta-analyses, systematic reviews and clinical trials were included, as well as international guidelines that provided good evidence on the toxicity of cancer treatment. In addition to searching electronic databases, all the summaries of product characteristics (SmPC) of the drugs were reviewed, in concert with the studies that support their authorisation.
ResultsThe results are expressed in table form detailing the drugs commonly used in the treatment of each type of tumour, along with the probability of these causing any gastrointestinal adverse effect and, in parentheses, the percentage of serious adverse effects (equal to or greater than grade 3) (Table 2).
Common adverse effects of the different chemotherapy treatments. Drugs for the treatment of colon and rectal cancer.
| Targeted therapies | Immunotherapy | Cytotoxic drugs and combinations | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference |
|---|---|---|---|---|---|---|
| Regorafenib | 80% (33%) | 30% (8%) | 27% (3%) | Grothey et al., 2013 | ||
| Panitumumab | 21% (2%) | 23% (2%) | 20% (1%) | SmPC | ||
| Bevacizumab | 25% (3%) | 21% (2%) | 21% (1%) | Tabernero et al., 2014 | ||
| Aflibercept | 23% (3%) | 19% (2%) | 15% (1%) | SmPC | ||
| Pembrolizumab | 6% (<1%) | 5% (1%) | 3% (1%) | Le et al., 2020 | ||
| 5-FU-oxaliplatin | 62% (11%) | 70% (4%) | 38% (4%) | Guo et al., 2016 | ||
| Capecitabine-Oxaliplatin | 66% (20%) | 71% (8%) | 25% (2%) | Guo et al., 2016 | ||
| 5-FU-Irinotecan | 80% (13%) | 62% (4%) | 30% (3%) | SmPC | ||
| Trifluridine/Tipiracil | 32% (3%) | 28−39% (2%) | 8% (<1%) | Robert et al., 2015 | ||
| 5-FU-Oxaliplatin-Cetuximab | 75% (20%) | 45% (6%) | 50% (10%) | Qin et al., 2018 | ||
| Capecitabine-Oxaliplatin-Cetuximab | 80% (26%) | 60% (7%) | 41% (3%) | Moosmann et al., 2008 | ||
| 5-FU-Irinotecan-Bevacizumab | 80% (21%) | 65% (7%) | 51% (8%) | Heinemann et al., 2014 | ||
| Capecitabine-Oxaliplatin-Bevacizumab | 64% (24%) | 71% (10%) | 30% (3%) | Saltz et al., 2008 | ||
| Capecitabine-Irinotecan-Bevacizumab | 91% (35%) | 78% (15%) | 62% (8%) | Pectasides et al., 2012 | ||
| 5-FU-Oxaliplatin-Panitumumab | 79% (16%) | 61% (10%) | 55% (10%) | Douillard et al., 2014 | ||
| 5-FU-Irinotecan-Panitumumab | 82% (15%) | 65% (6%) | 59% (8%) | Peeters et al., 2010 | ||
| 5-FU-Oxaliplatin-Irinotecan-Bevacizumab | 79% (22%) | 61% (12%) | 52% (9%) | Cremolini et al., 2015 |
| Drugs for the treatment of hepatocellular carcinoma | |||||
|---|---|---|---|---|---|
| Targeted therapies | Cytotoxic drugs | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference |
| Cabozantinib | 54−74% (10%) | 31−50% (2%) | 13−51% (1−5%) | Abou-Alfa et al., 2018 | |
| Lenvatinib | 39% (4%) | 20% (1%) | Kudo et al., 2018 | ||
| Regorafenib | 41% (3%) | 17% (1%) | 13% (1%) | Bruix et al., 2017 | |
| Sorafenib | 46% (4%) | 14% (1%) | Kudo et al., 2018 | ||
| Drugs for the treatment of pancreatic cancer | ||||
|---|---|---|---|---|
| Cytotoxic drugs and combinations | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference |
| Gemcitabine | 9% (<2%) | 21% (%) | 10% (<1%) | Burris et al., 1997 |
| Gemcitabine + Nab-paclitaxel | 30% (6%) | 45% (<1%) | Von Hoff et al., 2013 | |
| FOLFIRINOX* | 45% (12%) | 80% (14%) | Conroy et al., 2011 | |
| Gemcitabine + Capecitabine | 45% (5%) | (<7%) | (2%) | Neoptolemos et al., 2017 |
| Liposomal irinotecan + 5-FU + Leucovorin | 59% (13%) | 52% (11%) | Wang-Gillam et al., 2016 | |
| Oxaliplatin + fluoropyrimidines | 30% (<8%) | 40% (<5%) | Pelzer et al., 2011 | |
| Drugs for the treatment of lung cancer | ||||||
|---|---|---|---|---|---|---|
| Targeted therapies | Immunotherapy | Cytotoxic drugs and combinations | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference |
| Afatinib | 74.7% (9.9%) | 20.7% (1.5%) | 30.1% (4.1%) | SmPC | ||
| Erlotinib | 57% | 33% | SmPC | |||
| Gefitinib | 46.6% (3.3%) | 16% (0.3%) | 6.8% (0.8%) | SmPC | ||
| Osimertinib | 49% (1.2%) | 20% | SmPC | |||
| Alectinib | 16% (0.7%) | 19% (0.5%) | SmPC | |||
| Crizotinib | 54% (8%) | 51% (10.7%) | SmPC | |||
| Brigatinib | 38−53% | 23%–24% | 0.13% | Camidge et al., 2018 | ||
| Pembrolizumab | 21% | 21% | SmPC | |||
| Nivolumab | 16% (1.55%) | 26−14% | 2% | SmPC | ||
| Nivolumab + Ipilimumab | 22% (5%) | 24% | SmPC | |||
| Atezolizumab | 19% | 14−22% | SmPC | |||
| Carboplatin + Pemetrexed | (1.4%) | Socinski et al., 2009 | ||||
| Carboplatin + Gemcitabine | (0.5%) | (1%) | Kosmidis et al., 2008 | |||
| Cisplatin + Pemetrexed | (6.1−7.2%) | Scagliotti et al., 2008 | ||||
| Cisplatin + Gemcitabine | (3.9−6.1%) | Scagliotti et al., 2008 | ||||
| Cisplatin + Etoposide | (3%) | (11%) | Lara et al., 2009 | |||
| Cisplatin + irinotecan | (19%) | (14%) | Lara et al., 2009 | |||
| Vinorelbine + Paclitaxel | 7.40% | 38.90% | Stathopouloset al., 2004 | |||
| Paclitaxel + Gemcitabine | (0.5%) | (1.4%) | Kosmidis et al., 2008 | |||
| Drugs for the treatment of renal cancer | |||||
|---|---|---|---|---|---|
| Targeted therapies and combinations | Immunotherapy and combinations | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference |
| Sunitinib | 57% (8%) | 49% (3%) | Motzer et al., 2014 | ||
| Pazopanib | 46% (0%) | 31% (0%) | 15% (8%) | SmPC | |
| Sorafenib | 46% (0%) | 31% (0%) | 15% (8%) | Khan et al., 2010; | |
| Axitinib | 55% (10%) | 33% (2%) | 15% (1%) | SmPC | |
| Cabozantinib | 74% (11%) | 50% (4%) | 19% (<1%) | Choueiri et al., 2015 | |
| Tivozanib | 23% (2%) | 12% (1%) | 11% (1%) | Motzer et al., 2013 | |
| Afatinib | 75–95% (14%) | 25% (1%) | 30−70% (4−8%) | SmPC | |
| Temsirolimus | 34% (5%) | 34% (1.6%) | 20% (1%) | SmPC | |
| Everolimus | 27% (2%) | 46% (7%) | 23% (3%) | Choueiri et al., 2015 | |
| Axitinib-Pembrolizumab | 54.3% (9.1%) | 27.7% (0.9%) | 13.3% (0.9%) | Rini et al., 2019 | |
| Lenvatinib - Everolimus | 71.4% (0%) | 57.1% (0%) | 42% | Matsubara et al., 2018 | |
| Axitinib - Avelumab | 62.2% (6.7%) | 34.1% (1.4%) | 14.1% (1.2%) | Motzer et al., 2019 | |
| Nivolumab - Ipilimumab | 27% (4%) | 20% (1%) | 2% (0%) | Motzer et al., 2018 | |
| Nivolumab | 12% (1%) | 14% (<1%) | 3% (0%) | Motzer et al., 2015 | |
| Drugs for the treatment of urothelial cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Immunotherapy | Cytotoxic drugs and combinations | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference | ||||
| Pembrolizumab | 9% (1.1%) | 11% (0.4%) | Hussain et al., 2018 | ||||||
| Atezolizumab | 7% (0%) | 11% (0%) | Petrylak et al., 2018 | ||||||
| Avelumab | 16.6% (0.6%) | 15.7% (0.3%) | Powles et al., 2020 | ||||||
| Cisplatin -Gemcitabine | (3%) | (22%) | (1%) | Van der Maaseet al., 2000 | |||||
| MVAC* | (8%) | (21%) | (18%) | Van der Maaseet al., 2000 | |||||
| Docetaxel | (4%) | (3–4%) | (5.3%) | SmPC | |||||
| Vinflunine | 12.9% (1%) | 30.9% (2.9%) | 27.1% (2%) | SmPC | |||||
| Drugs for the treatment of prostate cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Androgen blockade | Cytotoxic drugs | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference | ||||
| Darolutamide | 6.9% (0%) | 5% (0.2%) | Fizaki et al., 2019 | ||||||
| Apalutamide | 16% | SmPC | |||||||
| Abiraterone | 18% (1%) | 21–30% (2%) | Bono et al., 2011 | ||||||
| Enzalutamide | 11–22% | 12–14% | Beer et al., 2014 | ||||||
| Cabazitaxel | 39.7% (3.2%) | 26.2% (0%) | 7.9% (0%) | Wit et al., 2019 | |||||
| Docetaxel | 32% (4%) | 42% (4%) | 20% (5.3%) | Tannock et al., 2004 | |||||
| Drugs for the treatment of melanoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targeted therapies | Immunotherapy | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference | ||||
| Vemurafenib | 28–50% (2%) | 18–26% (1%) | – | McArthuer et al., 2014 | |||||
| Vemurafenib + Cobimetinib | 61% (7%) | 43% (1%) | – | Ascierto et al., 2016 | |||||
| Dabrafenib + Trametinib | 36% (1%) | 37% (1%) | – | Robert et al., 2019 | |||||
| Encorafenib + Binimetinib | 35% (3%) | 42% (2%) | – | Ascierto et al., 2020 | |||||
| Vismodegib | 17% (1–2%) | 32.7% (0%) | – | Basset-Seguin et al., 2015 | |||||
| Sonidegib | 30.4% (1.3%) | 38% (1.3%) | – | Lear et al., 2019 | |||||
| Pembrolizumab | 19% (0.8%) | 11% | – | Robert et al., 2019 | |||||
| Nivolumab | 13% | 12% | – | Robert et al., 2015 | |||||
| Ipilimumab | 38.4% (4.2%) | 33.9% (1.3%) | – | Hodi et al., 2010 | |||||
| Nivolumab + Ipilimumab | 43% | 40% | – | Larkin et al., 2019 | |||||
| Cemiplimab | 13.2% (0.5%) | – | 2.4% (0%) | Migden et al., 2020 | |||||
| Avelumab | 9% (0%) | 9% (0%) | – | Kaufman et al., 2018 | |||||
| Drugs for the treatment of ovarian cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targeted therapies | Cytotoxic drugs and combinations | Diarrhoea: any grade (G ≥ 3) | Nausea/vomiting: any grade (G ≥ 3) | Mucositis: any grade (G ≥ 3) | Reference | ||||
| Niraparib | 19.9% (0.3%) | 73% (3%) | Mirza et al., 2016 | ||||||
| Olaparib | 29% (2%) | 40–70% (2%) | SmPC | ||||||
| Paclitaxel + Carboplatin | 24.4% (2.8%) | 76.3% (5.9%)/45.5% (2.8%) | 20.4% (0.5%) | Andreas et al., 2003 | |||||
| Paclitaxel + Cisplatin | 33.1% (3.3%) | 90.9% (14.3%)/68.4% (10.4%) | 23% (0.3%) | Andreas et al., 2003 | |||||
| Gemcitabine + Carboplatin | 14.8% (1.4%) | 42.3% (2.9%) vomiting | PfistererJ et al., 2006 | ||||||
*FOLFIRINOX: folinic acid or leucovorin, 5-fluorouracil, irinotecan and oxaliplatin.
*MVAC: methotrexate, vinblastine, epirubicin and cisplatin.
In colon and rectal cancer, the percentage of gastrointestinal adverse effects is very high, particularly in combination therapy with cytotoxic drugs, as part of adjuvant chemotherapy. The emetogenic capacity of oxaliplatin is high, and combined with fluoropyrimidines (5-fluorouracil and capecitabine) peaks at 70–71% (4−8% grade ≥3). In the group of targeted therapy drugs, such as bevacizumab (anti-VEGF) or panitumumab (anti-EGFR) in monotherapy, the frequency of nausea and vomiting ranges from 21−23%. However, this group of drugs is usually administered in association with other cytostatics to increase their effectiveness, but this also increases their toxicity up to 45–78% (6–10% grade ≥3). Diarrhoea was found to be the most common and highest grade adverse effect, particularly associated with the use of irinotecan and cetuximab (anti-EGFR). The toxicity of cytotoxic agents and targeted therapy administered in combination can increase from 82% with 5-FU-irinotecan + panitumumab to up to 91% (35% grade ≥3) with the combination of capecitabine-irinotecan-bevacizumab. Mucositis is the least common adverse effect, ranging from 25 to 62% in combination therapy.
In hepatocellular carcinoma, targeted therapy stands out. Cabozantinib in monotherapy is associated with the most adverse effects, causing diarrhoea in up to 74% of patients (10% grade ≥3), nausea/vomiting in 50% and mucositis in up to 51%.
Regarding pancreatic cancer, the most widely used treatment is cytotoxic drugs in combination. The FOLFIRINOX regimen is associated with a high rate of adverse effects, causing nausea/vomiting in 80% of patients (14% grade ≥3), and diarrhoea in 45% (12% grade ≥3).
In non-small cell lung cancer, the widely used anti-EGFRs and TKIs are characterised by their high gastrointestinal toxicity, with diarrhoea occurring in 46–74.4% of treated patients, especially with afatinib (almost 10% grade ≥3). The lowest rate of diarrhoea, at around 16% (with only 0.7% grade ≥3), is associated with alectinib. The frequency of nausea/vomiting is lower, occurring in 16–51% of patients, while mucositis continues to be the least common toxicity (<20%), except for treatment with afatinib, in which it can increase up to 30%. Gastrointestinal toxicity is common, especially diarrhoea, with the use of immune checkpoint inhibitors (ICIs). Programmed cell death protein 1 (PD-1) inhibitors cause diarrhoea in 16–21% (1.5% grade ≥3). This severe toxicity increases (5%) in combination therapy such as nivolumab plus ipilimumab (anti-CTLA4). Antibodies against programmed cell death-ligand 1 (PD-L1), such as atezolizumab, can also cause diarrhoea (19%), although it is usually mild.
Treatment with cytotoxic drugs, most commonly used to treat small cell lung cancer, is characterised by its emetogenic power, and it is capable of causing grade ≥3 nausea and vomiting in more than 7% of patients with cisplatin- and pemetrexed-based treatment. The highest percentage of grade ≥3 diarrhoea (19%), nausea and vomiting (14%) is associated with treatment based on cisplatin and irinotecan. Regarding mucositis, its incidence increases in up to 38.9% of patients when treated with vinorelbine and paclitaxel.
In renal cancer, the most commonly used treatment is targeted therapy. Anti-VEGFRs are characterised by a high incidence of diarrhoea (23–74%). Top of the list is cabozantinib, with 74% of patients experiencing diarrhoea (11% grade ≥3), followed by sunitinib with 57% (8% grade ≥3). Regarding mucositis, its frequency increases to 15–19% with cabozantinib. Treatment with afatinib is associated with a high incidence of diarrhoea (75−95%) and mucositis (30−70%). Everolimus and temsirolimus are mTOR inhibitors and their most common adverse effects are diarrhoea (27–34%) and mucositis (23−20%). Regarding treatment with immunomodulators, the incidence of both diarrhoea (12%) and nausea (14%) is lower than in combination. As such, in treatment with axitinib plus avelumab, the incidence of diarrhoea is 62.2% (6.7% grade ≥3), nausea up to 34% and mucositis 14%.
In relation to urothelial cancer, the most common treatment is adjuvant chemotherapy with cytotoxic drugs. Platinum-based regimens cause nausea/vomiting in up to 22% of patients. Vinflunine can increase this toxicity up to 30.9%, in addition to mucositis in 27.1%. Immunotherapy, which is increasingly used, reduces emetogenic toxicity in up to 11–15% of patients. Diarrhoea remains an effect of immunotherapy, occurring in 16% of patients treated with avelumab, although less than 1% of cases were grade ≥3.
In persistent or recurrent prostate cancer, androgen receptor blockers are used. Tolerability in this group of drugs is quite good, with the exception of abiraterone, which causes diarrhoea in 18% of patients and nausea/vomiting in 21−30% (<2% grade ≥3). Treatment with taxanes increases toxicity, especially docetaxel, with diarrhoea in 32% (4% grade ≥3); nausea/vomiting in 42% (4% grade ≥3) and mucositis in 20% (5% grade ≥3).
Immunotherapy is an important systemic treatment modality for metastatic melanoma. It is characterised by high gastrointestinal toxicity, particularly diarrhoea. CTLA-4 blockade by ipilimumab causes diarrhoea and colitis in 38% of patients (4.2% grade ≥3). Treatment in combination with nivolumab (anti-PD1) can increase its toxicity up to 43%. Its emetogenicity is also high, with a frequency of 33.9–40%. Toxicity related to the other anti-PD1 drugs (pembrolizumab and cemiplimab) is less common (diarrhoea: 19% and nausea/vomiting: 11%). Regarding treatment with BRAF-targeted therapy plus MEK inhibitors, vemurafenib monotherapy is associated with diarrhoea in 28−50% of patients and nausea/vomiting in 18−26%. The combination associated with the greatest toxicity is treatment based on vemurafenib + cobimetinib, causing diarrhoea in 61% (7% grade ≥3) of patients and nausea/vomiting in 43%. Mucositis is not a common or serious adverse reaction in this group of drugs.
In ovarian cancer, platinum-based chemotherapy is the most widely used. Paclitaxel in combination with cisplatin is the treatment with the highest associated toxicity, causing nausea in more than 90% (14.3% grade ≥3) and vomiting in 68.4% (14.3% grade ≥3). Diarrhoea occurs in 33% of patients and mucositis in 23%. When combined with carboplatin, toxicity is somewhat lower but still high (nausea: 76.3% and vomiting: 45.5%; diarrhoea: 24.4%; mucositis: 20.4%). A gemcitabine plus carboplatin regimen reduces gastrointestinal toxicity (vomiting: 42.3%, diarrhoea: 14.8%).
Regarding targeted therapy, PARP inhibitors, used in maintenance treatment, are associated with very high emetogenic toxicity, with 73% of patients for niraparib and 40−70% for olaparib. Diarrhoea is also a very common effect, experienced by up to 29%.
DiscussionGastrointestinal adverse effects caused by antineoplastic drugs are a very common problem in cancer patients. Oncology departments are increasingly aware of the risk of malnutrition associated with these treatments. However, patients referred to endocrinology and nutrition departments often arrive with marked malnutrition; or, the first contact occurs during an admission due to the toxicity of the treatment, associated with a high frequency of intestinal complications with nutritional repercussions: diarrhoea, constipation, colitis, intestinal perforation, mucositis, nausea and vomiting. They can also significantly reduce patient quality of life and even cause death as a direct consequence of associated malnutrition or due to the limiting effect that results in the need for cancer treatments that are suboptimal.
Gastrointestinal toxicity due to antineoplastic drugs can affect patients through three mechanisms: the adverse effect itself and its complications (discomfort, pain, dehydration, bleeding, etc.), decreased treatment effectiveness (dose delays and suspensions) and the onset, or worsening of malnutrition and sarcopenia. This last mechanism can in turn increase toxicity, creating a vicious circle (malnutrition-toxicity) by limiting the administration of the initially calculated dose.15 For example, 93% of patients with colon cancer and sarcopenia have been reported as having adverse effects from the administration of fluorouracil, compared with 52% of patients without sarcopenia.16
In the case of targeted therapies, TKIs have been associated with the appearance or exacerbation of a specific malnutrition condition, sarcopenia or loss of muscle mass, and in some cases it is related to decreased survival. The underlying mechanism of this toxicity is not well understood, but it is believed to be related to the inhibition of the PI3K/AKT/mTOR pathway, which would inhibit muscle protein synthesis.17
DiarrhoeaDiarrhoea is a very common side effect of drugs used to treat solid tumours. As shown in the tables, diarrhoea is most closely related to fluoropyrimidines and irinotecan of all the conventional chemotherapies. The underlying mechanism in the case of chemotherapy is usually acute damage to the intestinal mucosa, with the onset of secretory diarrhoea five to seven days following the administration of the cytostatic agent. In the case of irinotecan, clinical signs and symptoms of acute diarrhoea may appear in the first 24 h after administration due to an acute cholinergic syndrome (which is why a prior dose of atropine is usually administered). In the case of fluoropyrimidines, this diarrhoeal condition can be very severe in those patients with dihydropyrimidine dehydrogenase (DPD) deficiency, so its determination prior to the administration of cytostatics is recommended.18
Regarding molecularly targeted therapies (antitarget agents), some TKIs cause a high incidence of diarrhoea (75−45%) through different mechanisms, direct damage to the colonic mucosa, increased motility, dysbiosis and even hyperchlorhydria caused by abnormality of the EGFR signalling pathway.
On the other hand, anti-EGFR monoclonal antibodies, such as cetuximab and panitumumab, or anti-VEGFR monoclonal antibodies, such as bevacizumab, do not usually cause diarrhoea in monotherapy, but do cause diarrhoea when combined with conventional chemotherapy. The same is true of aflibercept, a vascular endothelial growth factor A (VEGF-A) antagonist.
A relevant aspect is the onset of immune-mediated colitis, the main gastrointestinal adverse effect caused by the administration of ICIs, derived from the production of autoreactive T lymphocytes against different tissues. These symptoms usually appear at the start of treatment. However, diarrhoea and/or colitis may recur months after treatment interruption or completion, mimicking chronic inflammatory bowel disease.
Regarding immune checkpoint inhibitors, ipilimumab causes diarrhoea in 23–33% of patients and programmed cell death protein 1 (PD-1) inhibitors in 19%. The incidence is higher among patients taking a combination of both (44%). Antibodies against programmed cell death-ligand 1 (PD-L1) can also cause diarrhoea, although it is usually mild. BRAF inhibitors (vemurafenib, dabrafenib and encorafenib) have a very acceptable profile in terms of diarrhoea risk, with figures of less than 5–6% for grade 2–3 diarrhoea. However, when associated with MEK inhibitors, unlike with skin toxicity, they slightly increase the risk of diarrhoea and nausea/vomiting.19
Although gastrointestinal toxicity is relatively common with poly (ADP ribose) polymerase (PARP) enzyme inhibitors (niraparib and olaparib), such toxicity is usually mild and, in the case of diarrhoea, the risk is low and not usually a reason for dose adjustment or discontinuation. Regarding mTOR receptor inhibitors (temsirolimus and everolimus), severe gastrointestinal toxicity is rare.
Finally, neutropenic enterocolitis, a clinical syndrome that mainly develops in patients undergoing treatment with high doses of chemotherapy, is worthy of special mention. Although the actual incidence of this condition is unknown, most studies place it at 5.3%, although this figure is probably an underestimate. It is characterised by abdominal pain and fever with neutropenia (<0.5 × 109/l) and increased thickness of the colonic intestinal wall. The related chemotherapeutic agents are those used for the treatment of leukaemia and solid tumours such as etoposide, taxanes, platinum, gemcitabine and 5-fluorouracil.20,21
Nausea/vomitingChemotherapy-induced nausea and vomiting was such a common side effect of classical chemotherapy that a division of cytostatic agents was established based on their emetogenicity: highly emetogenic chemotherapy (>90% probability of vomiting, such as cisplatin, high-dose cyclophosphamide or dacarbazine), moderately emetogenic chemotherapy (30–90%, such as carboplatin, oxaliplatin and anthracyclines), low-emetogenic risk chemotherapy (10–30%, such as taxanes, gemcitabine, capecitabine or topotecan) and minimally emetogenic chemotherapy (<10%, such as bleomycin, vinca alkaloids or oral methotrexate). Similarly, chemotherapy-induced nausea/vomiting can be divided into three categories based on the time elapsed following administration, with different aetiopathogenic mechanisms: acute emesis (<24 h), related to the action of serotonin; delayed emesis (>24 h), of less well-known aetiology, probably related to substance P; and anticipatory emesis, as a conditioned response to previous episodes. Predisposing factors are the female gender, age <50 years, as well as a history of motion sickness or emesis gravidarum. However, chronic excessive alcohol consumption reduced the risk.5
Improved knowledge about its pathophysiology, the development of different antiemetic drugs (5-HT3 receptor antagonists, NK1 receptor antagonists) and the dissemination of prevention guidelines have achieved better control, especially in the case of acute emesis. Despite this, more than a third of patients continue to experience chemotherapy-induced nausea and vomiting.22–24 This elevated incidence, alongside other common effects of chemotherapy such as hyporexia, dysgeusia and cacosmia, pose a high risk of malnutrition.
In the case of antitarget therapies or immunotherapy, they are always included within the categories of low emetogenic risk. However, both the mechanisms underlying the emesis induced by these drugs and the efficacy of the usual anti-emetic drugs are unknown, which entails a limited but poorly understood risk of malnutrition.
MucositisOral mucositis is a common process in conventional chemotherapy and can entail a serious risk of malnutrition in severe or prolonged cases. The risk of mucositis is especially significant in chemoradiotherapy treatments in the oropharyngeal area, as well as in treatments with high doses of myeloablative chemotherapy, where figures reach 75% for grade 3−4 mucositis.25 Chemotherapeutic agents that affect DNA synthesis (alkylating agents, anthracyclines, antimetabolites) more frequently cause mucositis.
Of particular importance, as for diarrhoea, is DPD deficiency in patients who are administered fluoropyrimidines.
Chemotherapy-induced mucositis usually begins as painful erythematous lesions, which can progress to mucosal ulceration, and which in severe cases may prevent oral intake. Onset is usually five to seven days following the administration of cytostatic treatment and frequently coincides with other toxicities such as diarrhoea or haematological toxicity, which can aggravate nutritional stress. Poor oral hygiene and a prior state of malnutrition are predisposing factors.
The risk of mucositis is relatively low with both immunotherapy and most molecularly targeted cancer therapies, with the exception of mammalian target of rapamycin (mTOR) inhibitors (temsirolimus and everolimus), where rates of grade ≥3 mucositis range from 33 to 53%.26
Medical and dietary-nutritional therapy against gastrointestinal toxicity induced by antineoplastic drugsFirst of all, the patient must be informed about the risks of the appearance of the different toxicities so they can be addressed early. In addition, it would be very useful to establish local protocols regarding the escalating use of antidiarrhoeal drugs (loperamide, codeine, octreotide, etc.), antiemetics (dopamine antagonists, serotonin receptor antagonists, corticosteroids) and adjuvants in the prevention and management of mucositis (topical drugs, palifermin).
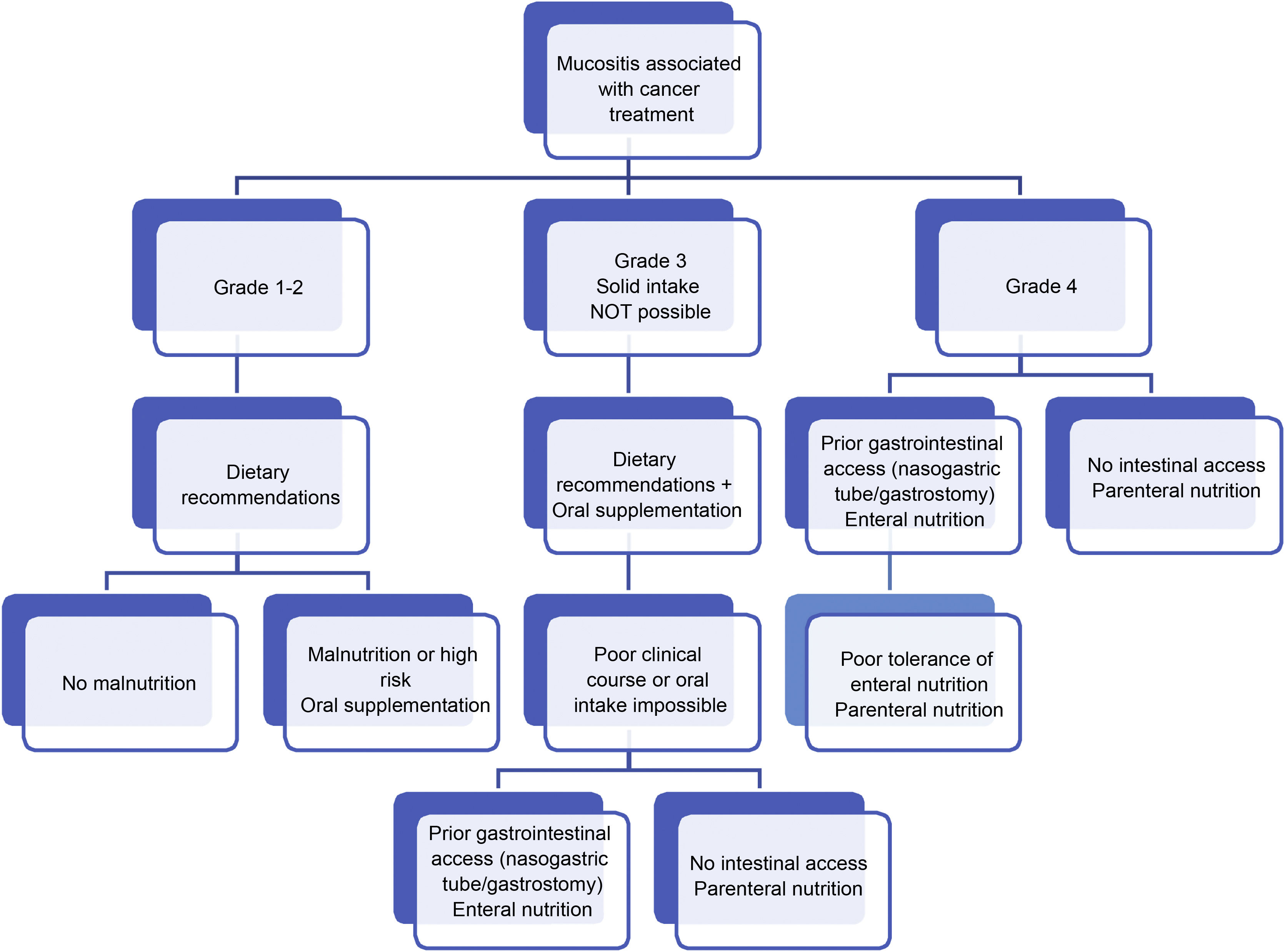
Regarding dietary-nutritional therapy, depending on the grade and existence of risk factors or warning signs of complications, we propose the following action algorithms (Figs. 1–3).27 The dietary advice for each of the gastrointestinal toxicities detailed in the algorithm is set out in Table 3.28–30 It must be pointed out that the dietary-nutritional therapy of the different toxicities should be started early to reduce their progression and associated malnutrition, and they will sometimes be maintained in the long term and even chronically.
Dietary recommendations for patients.
| 1) Diarrhoea caused by cancer treatment |
Tips when choosing what to eat:
|
Tips when cooking:
|
General tips:
|
| 2) Dietary recommendations for patients with nausea/vomiting due to cancer treatment |
Tips when choosing what to eat:
|
Tips when cooking:
|
General tips:
|
| 3) Recommendations for patients with mucositis |
Tips when choosing what to eat:
|
Tips when cooking:
|
General tips:
|
It is important to understand the gastrointestinal adverse effects associated with each type of chemotherapy drug in order to prevent their onset and reduce the risk of malnutrition. Professionals involved in the treatment of cancer patients must adapt the nutritional medical therapy to the needs required at each point of the disease process. Furthermore, the importance of establishing a diagnosis of malnutrition risk and implementing nutritional medical therapy early must be borne in mind, ideally following diagnosis in the different medical specialties, at the same time that the patient is referred to the specialist, to prevent the negative consequences of associated malnutrition.
Conflicts of interestThe authors declare that they have no conflicts of interest.