Obesity is the new global pandemic of this century, associated with a higher risk of de novo tumors and cardiovascular disease. Due to its diffusion, obesity may even reduce global life expectancy, with no effective pharmacological treatment to date.1
The new GLP-1 and GIP analogues are drugs designed to improve the control of type II diabetes, which have the effect of weight loss.2,3 However, experimental studies have already related these with decreased steatosis in rats,4,5 and they seem to have a treatment potential in steatosis associated with MAFLD, including a reduction in the fibrosis associated with this disease.6
In patients with hepatic steatosis, major liver surgery is burdened with higher rates of liver failure of the remnant and higher morbidity and mortality.7,8
We present the case of a 59-year-old patient with resection of adenocarcinoma of the rectum (pT3N1b) in 2020, subsequently treated with CRT (8 cycles of XELOX and capecitabine). He required resection of one liver metastasis in S8 in January 2022, confirming 30% macrosteatosis. In February 2022, he presented 3 new SOL, for which he restarted CTx with FOLFIRI+ bevacizumab. The lesions continued to be observed on subsequent imaging tests, so SBRT and capecitabine were administered, ending in December 2023 with the disappearance of lesion uptake. In April 2024, radiotherapy was given again for a new metastasis in S8 on the right suprahepatic vein.
Recurrence was observed in October 2024, with 2 lesions in S8 and S4, compatible with metastases.
We decided to perform right trisegmentectomy. However, due to insufficient left remnant, we opted for a right portal associated with RSHV embolization. At this time, the patient weighed 136 kg (175 cm tall; BMI 44.4 kg/m2), so it was decided in agreement with the nutritionist to start Tirzepatide (Mounjaro®), which was initiated at 2.5 mg the first month and increased to 5 mg in the second month, in addition to a 1000 kcal diet.
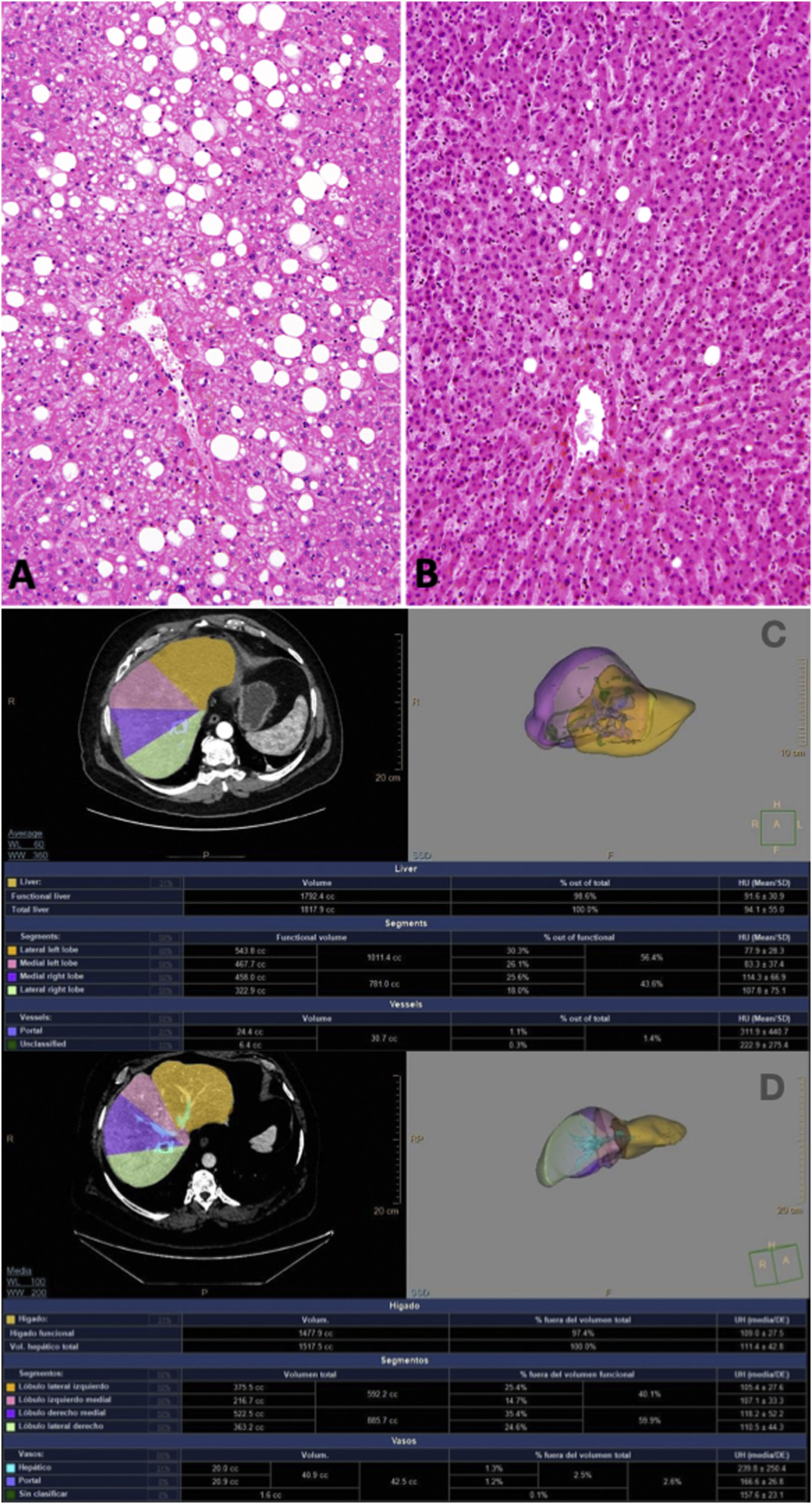
Embolization was unsuccessful due to difficulty in closing the right portal vein, so it was decided to evaluate for ALPPS. After 3 months with Tirzepatide and prior to the first ALPPS stage, the patient was at 106 kg (BMI 34.6 kg/m2), which implies a loss of 30 kg, 50.4% excess weight loss and 22% total weight loss. In the biopsy from this surgery, the ME was 5% (Fig. 1), so ALPPS was finally completed the following week, resulting in a growth of the SLI from 375 cc (24.7% of the total volume) to 545 cc (31% of the total volume; 45% increase).
A) Prior to embolization. At this time, the patient weighed 136 kg (height 175 cm; BMI 44.4 kg/m2) with 30% macrosteatosis; B) After the 1st stage of ALPPS, and after three months with Tirzepatide, macrosteatosis was 0%–5%; C) Growth of the SLI from 375 cc (24.7% of the total volume) to D) 545 cc (31% of the total volume; 45% increase).
Surgery brought about no complications, and the patient was discharged on the 3rd day of the second stage. Follow-up CT scan showed no evidence of disease.
This case of extreme liver resection is within the limits of safe resectability using ALPPS.9,10 The possibility of reducing steatosis in a liver subjected to high doses of chemotherapy or radiotherapy significantly reduces the possibility of surgical complications after major hepatectomy.8 The possibilities with GLP-1 agonists should be explored in cases that allow for a minimum treatment time of at least 2 months, such as those subjected to portal embolization or preoperative biliary drainage. The tolerability of these medications remains a major concern due to their gastrointestinal side effects. Future research should focus on optimizing drug regimens, identifying patients most likely to benefit from them, and balancing efficacy with tolerability.6
CRediT authorship contribution statementIago Justo: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. María Calatayud: Data curation, Resources. Javier Salamanca: Formal analysis, Methodology. María Camara: Resources, Data curation, Methodology. Alvaro García-Sesma: Methodology, Resources. Carmelo Loinaz: Formal analysis, Conceptualization.