The main objective is to establish the overall survival and disease-free survival profiles regarding the patients with retroperitoneal liposarcoma, making a comparison based on the well-differentiated and dedifferentiated histological subtypes. The secondary objectives are to descriptively analyze the clinical characteristics of said patients and to identify and analyze other independent variables that might modify these survival profiles significantly.
MethodsAn observational and analytical study was performed using a retrospective historical cohort that was followed prospectively. The inclusion criteria consisted of: the procedure of liposarcoma located in the retroperitoneum, the well-differentiated and dedifferentiated histological subtypes, between January of 2002 and May of 2019. As a result, 32 patients took part in this study’s sample. Kaplan–Meier estimator was used to summarise the results and log-rank test was used in the comparative analysis.
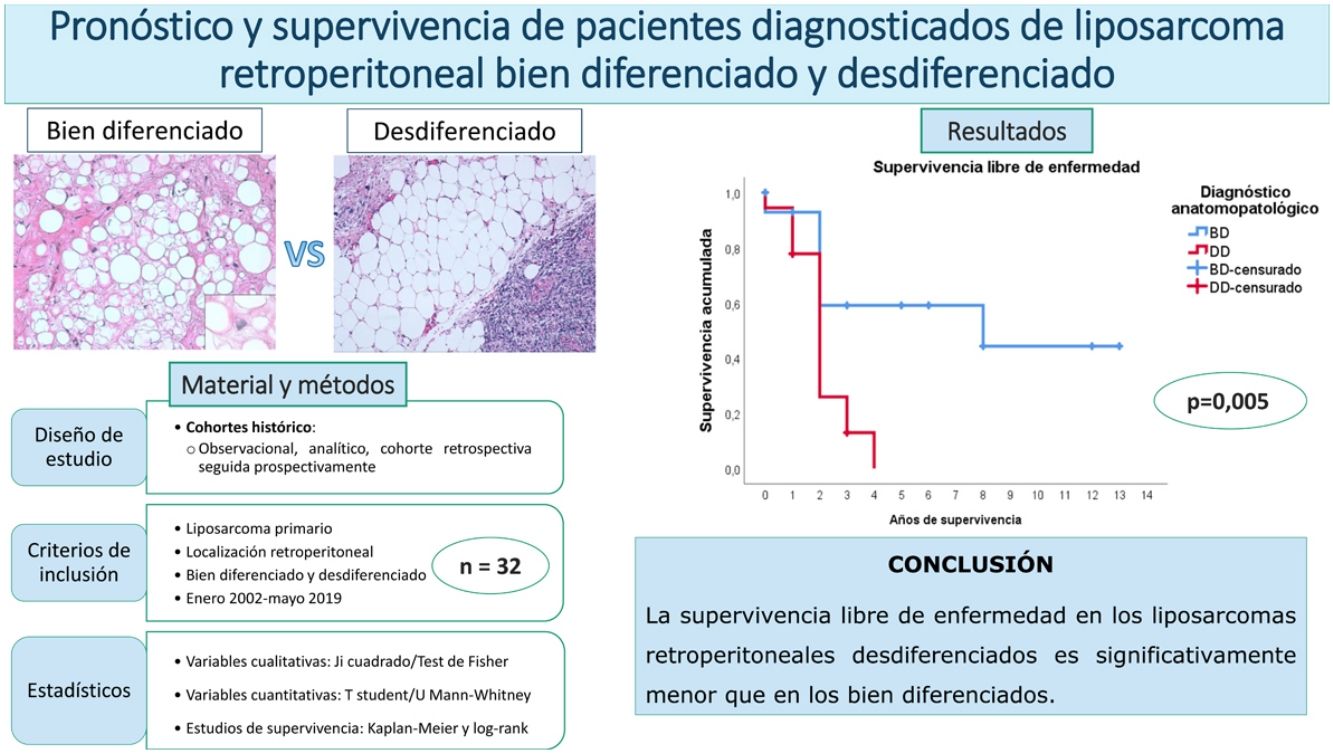
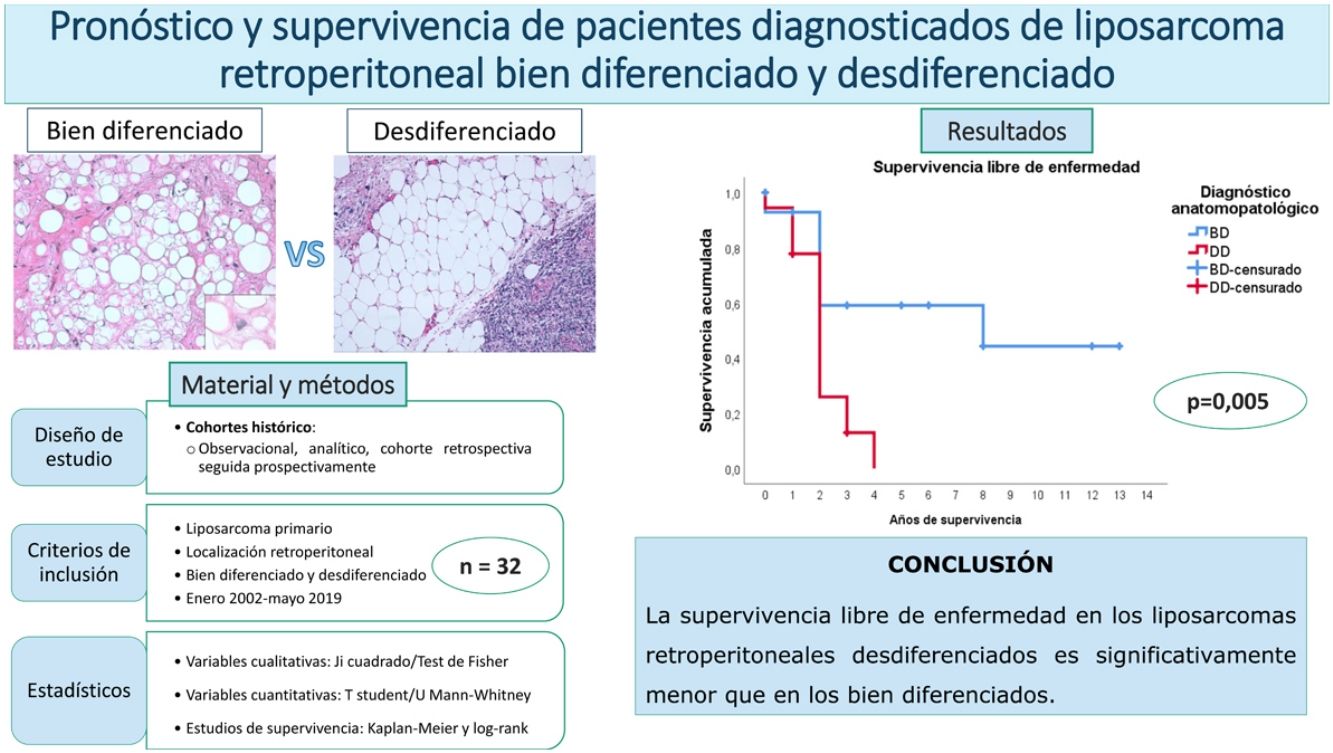
ResultsThe overall survival at 5 years was around 59%. No differences were found between the patients with a well-differentiated subtype compared to the dedifferentiated ones (p = 0.834). The disease-free survival at 2 years was 59% regarding the well-differentiated and 26% regarding the dedifferentiated, with these differences being statistically significant (p = 0.005). None of the other studied variables modified these survival profiles significantly.
ConclusionsDedifferentiated retroperitoneal liposarcomas show less disease-free survival than well-differentiated liposarcomas. However, regarding overall survival no differences can be claimed.
El objetivo principal es determinar la supervivencia global y la supervivencia libre de enfermedad de pacientes intervenidos de liposarcoma retroperitoneal, comparándolos en función de los subtipos histológicos bien diferenciado y desdiferenciado. Los objetivos secundarios son analizar descriptivamente las características clínicas de estos pacientes e identificar otras variables independientes que puedan modificar significativamente estos perfiles de supervivencia.
MétodosSe realiza un estudio observacional y analítico mediante una cohorte histórica retrospectiva, seguida prospectivamente. Los criterios de inclusión fueron: cirugía de liposarcoma de localización retroperitoneal, subtipo histológico bien diferenciado y desdiferenciado, entre enero de 2002 y mayo de 2019. Se incluyeron un total de 32 pacientes. Se utilizó el estimador de Kaplan-Meier para resumir los datos y la prueba log-rank para el análisis comparativo.
ResultadosLa supervivencia global a los 5 años fue del 59%. No se encontraron diferencias entre los pacientes con subtipo bien diferenciado con respecto al desdiferenciado (p = 0,834). La supervivencia libre de enfermedad a los 2 años fue del 59% en los bien diferenciados y del 26% en los desdiferenciados, siendo estas diferencias estadísticamente significativas (p = 0,005). Ninguna de las otras variables estudiadas modificó significativamente estos perfiles de supervivencia.
ConclusionesLa supervivencia libre de enfermedad de los liposarcomas retroperitoneales desdiferenciados es significativamente menor que en los bien diferenciados, pero no puede afirmarse que haya diferencias en la supervivencia global.
Although sarcomas are rare neoplasms,1 approximately one third of malignant tumours of the retroperitoneum are sarcomas,2,3 the most common type (40–50%) being liposarcoma (LPS)4 LPS is classified into 4 histological subtypes according to morphological characteristics and cytogenetic aberrations: well-differentiated (or atypical lipomatous tumour), dedifferentiated, myxoid/round cell and pleomorphic,5, the first two being the most frequently found in the retroperitoneum (90%).6 Due to their location, retroperitoneal liposarcomas (RPLS) are usually diagnosed late, when they have already reached a large size and invaded numerous adjacent visceral structures.7 The rate of distant metastasis, even for high-grade ones, is relatively low (10–15%), so the mortality of RPLS is more related to its local aggressiveness.8
On this basis, it has been stated that the most important prognostic factor in RPLS is complete surgical resection,9–11 with chemotherapy and/or radiotherapy playing a role depending on the type of surgery, recurrence or size of the specimen.12,13 Complete tumour excision is not straightforward and, in many cases, free margins cannot be achieved. This explains the high recurrence rate and morbidity and mortality associated with wide, multivisceral surgical resections in this type of tumour.14 However, even with wide resections, it appears that the course of the disease differs according to the histological subtype of the RPLS, being more benign in well-differentiated RPLS (WDRPLS) than in dedifferentiated RPLS (DDRPLS).15,16 Therefore, it could be suggested that the former, due to their low malignancy potential, could be managed more conservatively, avoiding extensive and mutilating resections and reducing morbidity.17 However, a lax follow-up of the patient may imply a high risk of recurrence, with the consequent added morbidity and mortality, which is why there are contradictory results according to published studies.2,5,10,18,19 Furthermore, there is a not inconsiderable percentage of reoperation due to an increase in histological grade when analysing the complete surgical specimen.3,20,21 This makes it difficult to establish an adequate diagnostic-therapeutic and prognostic protocol for these patients.
Thus, based on the hypothesis that patients with WDRPLS have a better prognosis than those with DDRPLS, the main objectives of this study are to determine overall survival and disease-free survival in patients with retroperitoneal sarcoma, distinguishing between the two histopathological entities and comparing them with each other, as well as analysing the clinical characteristics of the patients in the cohort and identifying other independent variables that may influence their prognosis.
MethodsWe designed an observational and analytical study using a retrospective historical cohort that is followed prospectively. This cohort is composed of 32 patients who underwent surgery for primary RPLS of well-differentiated and dedifferentiated histological subtypes at our centre between January 2002 and May 2019.
All variables were obtained retrospectively from clinical, radiological, surgical, oncological, anatomopathological reports collected from the patients' clinical records, with the formal authorisation of the responsible Biomedical Research Ethics Committee, adopting at all times the due considerations, conditions indicated by this body.
The qualitative variables considered for the analyses were: sex (male/female), occurrence of recurrence during the study (yes/no), vital status (alive/dead), medical history of another malignancy (yes/no), involvement of surgical edges of the primary tumour (R0, free/R1, affected), early recurrence (occurrence of recurrence less than 2 years after removal of the primary tumour; yes/no), presence of metastases (no/ganglionic/distant), visceral resection in the intervention of the primary tumour (simple lumpectomy/nephrectomy/multivisceral with involvement of two or more organs) and treatment of the primary tumour (surgery/surgery plus adjuvant chemotherapy/surgery plus adjuvant radiotherapy). Quantitative variables were: age at diagnosis (years), age at death (years), number of recurrences, tumour size (maximum diameter of the primary tumour in cm), overall survival (years) and disease-free survival (years).
Statistical analysisFor the comparison of proportions between qualitative variables, contingency tables and the Chi-square test were used, or, failing that, Fisher's exact test when the sample size did not allow the former to be applied. For the comparison of means of quantitative variables, Student's t-test was used, after checking for normal distribution using Saphiro–Wilk; in cases where it could not be applied, non-parametric tests were used, specifically the Mann–Whitney U test
For the analysis of overall survival and disease-free period, Kaplan–Meier estimates were used to summarise and represent survival functions and the log-rank test for comparative analysis.
In all cases the hypothesis testing was bilateral and a p-value < .05 was considered statistically significant.
IBM® SPSS Statistics 25 was used for all statistical calculations and estimations in the study.
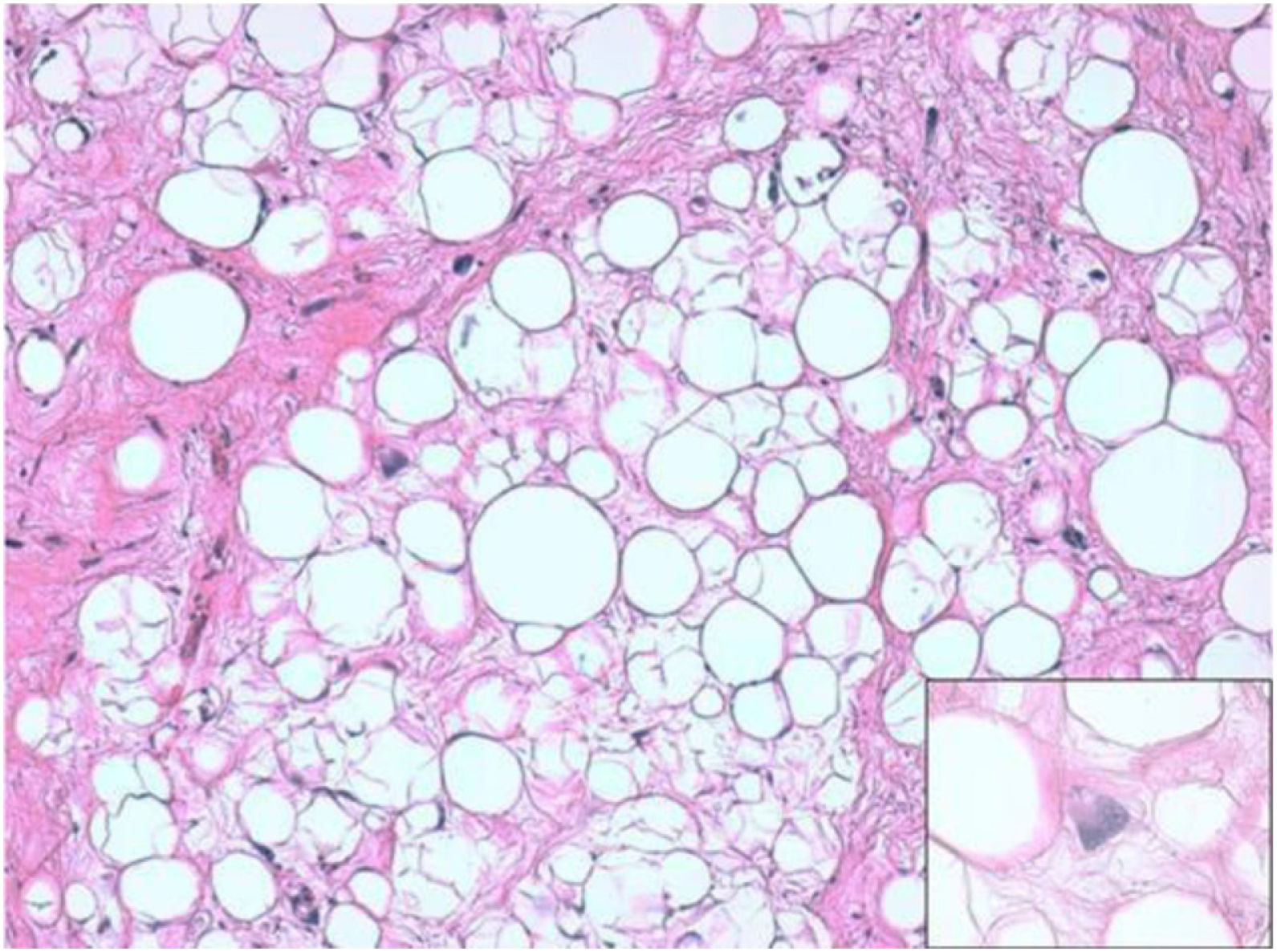
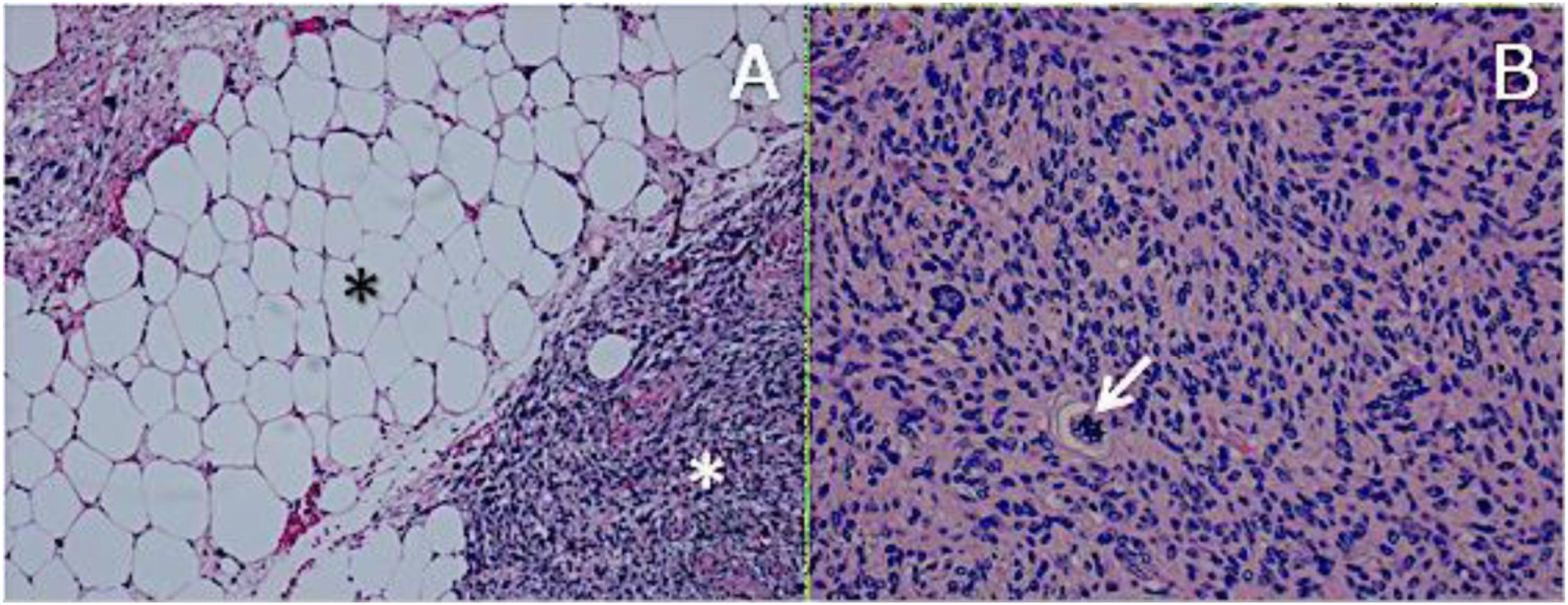
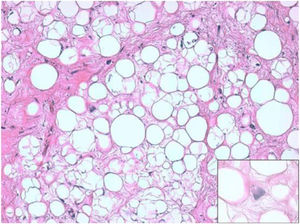
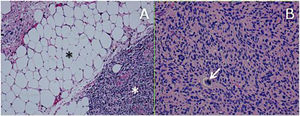
ResultsHistologically, WDRPLS (Fig. 1) is a lipogenic neoplasm of intermediate malignancy consisting of atypical adipocytes (with variability in size, shape and with hyperchromatic nuclei) and atypical stromal cells. In contrast, DDRPLS (Fig. 2) is a malignant lipogenic neoplasm, in which there is an abrupt transition between atypical lipogenic cells and non-lipogenic sarcomatous cells with wide variation in the lipogenic component.
The descriptive data of the sample are shown in Tables 1 and 2. When compared according to histological subtype, the variables generally maintain a homogeneous distribution. However, it is noteworthy that tumour size was larger in the dedifferentiated type, and this difference was statistically significant. In relation to this, it can be seen that tumours of this histological type required more aggressive interventions, especially in terms of nephrectomy, although no significant differences were found.
Descriptive study 1: quantitative variables. Expressed according to median and statistical analysis according to histological diagnosis. In brackets, interquartile range between first and third quartiles.
| Total 32 | WD 14 (43.8%) | DD 18 (56.3%) | p Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 57,50 (50,3–67.8) | 59 (48.8–68.5) | 56 (50.8–66.8) | p = .864 |
| Size (cm) | 23.50 (18.6–31.3) | 20.50 (15.3–25.3) | 28 (19.7–35.5) | p = .013 |
| Age at death (years) | 63 (56–84) | 62.50 (48.5–75.8) | 63 (56.5–86) | p = .634 |
| Number relapses | 1.5 (0–2.8) | 0 (0–2) | 2 (1−3) | * |
WD: well differentiated; dd: de-differentiated.
Statistically significant outcomes are in bold.
Descriptive study 2: qualitative variables and statistical analysis by histological diagnosis.
| Total 32 | WD 14 (43.8%) | DD 18 (56.3%) | p Value | ||
|---|---|---|---|---|---|
| Sex | Man | 11 (34.4%) | 5 (35.7%) | 6 (33.3%) | p = 1.000 |
| Woman | 21 (65.6%) | 9 (64.3%) | 12 (66.7%) | ||
| Surgical margins | Free (R0) | 16 (50%) | 7 (50%) | 9 (50%) | p = 1.000 |
| Affected (R1) | 16 (50%) | 7 (50%) | 9 (50%) | ||
| History of malignant pathology | Yes | 4 (12.5%) | 1 (7.1%) | 3 (16.7%) | p = .613 |
| No | 28 (87.5%) | 13 (92.9%) | 15 (83.3%) | ||
| Early relapse (<2 years) | Yes | 17 (53.1%) | 5 (35.7%) | 12 (66.7%) | p = .121 |
| No | 11 (34.4%) | 7 (50%) | 4 (22.2%) | ||
| Visceral resection | A: No | 6 (18.8%) | 5 (35.7%) | 1 (5.6%) | A vs. B: p = .060 |
| A vs. C: p = .138 | |||||
| B vs. C: p = 1.000 | |||||
| B: Kidney | 18 (56.3%) | 6 (42.9%) | 12 (66.7%) | ||
| C: Multivisceral | 8 (25.0%) | 3 (21.4%) | 5 (27.8%) | ||
| Treatment | Qx | 23 (71.9%) | 11 (78.6%) | 12 (66.7%) | – |
| Qx + QT | 4 (12.5%) | 1 (7.1%) | 3 (16.7%) | ||
| Qx + RT | 5 (15.6%) | 2 (14.3%) | 3 (16.7%) | ||
| Metastasis | No | 30 (93.8%) | 13 (92.9%) | 17 (94.4%) | * |
| Lymph node | 1 (3.1%) | 1 (7.1%) | 0 | ||
| Visceral | 1 (3.1%) | 0 | 1 (5.6%) | ||
| Relapse | Yes | 21 (65.6%) | 6 (42.9%) | 15 (83.3%) | * |
| No | 11 (34.4%) | 8 (57.1%) | 3 (16.7%) | ||
| Status | Alive | 17 (53.1%) | 8 (57.1%) | 9 (50%) | * |
| Dead | 15 (46.9%) | 6 (42.9%) | 9 (50%) |
WD: well differentiated; DD: de-differentiated; QT: chemotherapy; Qx: surgery; RT: radiotherapy.
Although they have been presented in the descriptive study, the variables marked with an asterisk (*) depend on the time factor for development, which is not homogeneous for all patients, so they cannot be analysed using the previous statistics and survival studies will be used for this purpose. It can be intuited, however, that patients with a dedifferentiated phenotype presented a higher percentage of relapses, which were also earlier and more numerous, although these data should be analysed with caution due to the aforementioned bias.
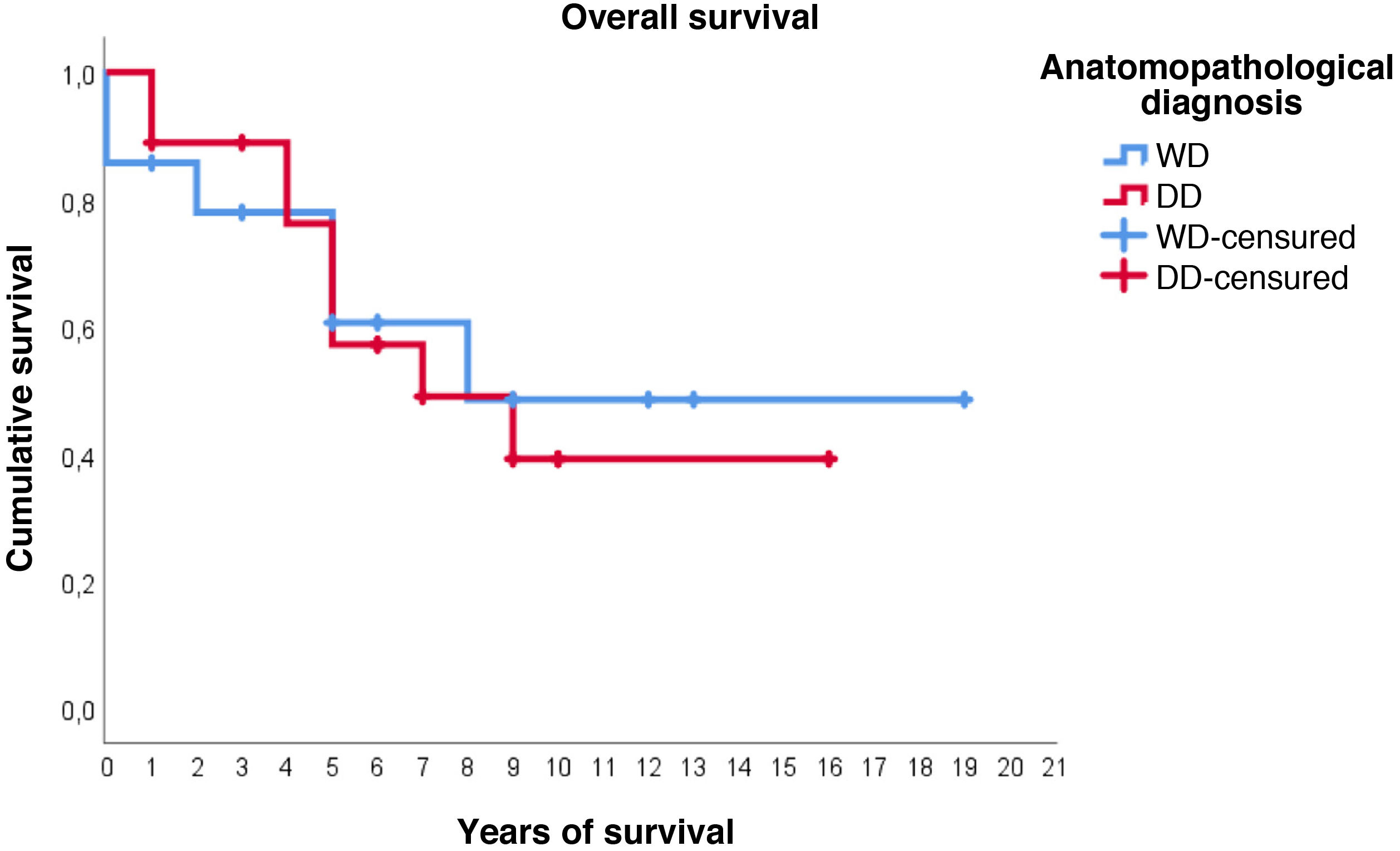
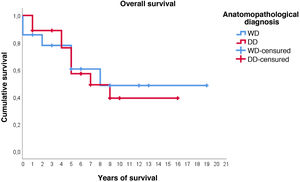
Regarding survival studies, the estimated media overall survival was 10.8 years (95%CI: 7.9–13.6), with a 5-year survival of approximately 59%. Patients diagnosed with WDRPLS had a median estimated survival of 11.2 years (95%CI: 6.7–15.7), while in the dedifferentiated subtype the median was 9.3 years (95%CI: 6.4–12.2). As can be seen in Fig. 3, both survival curves are similar, with no significant differences found in the comparison using the log-rank test (p = .834).
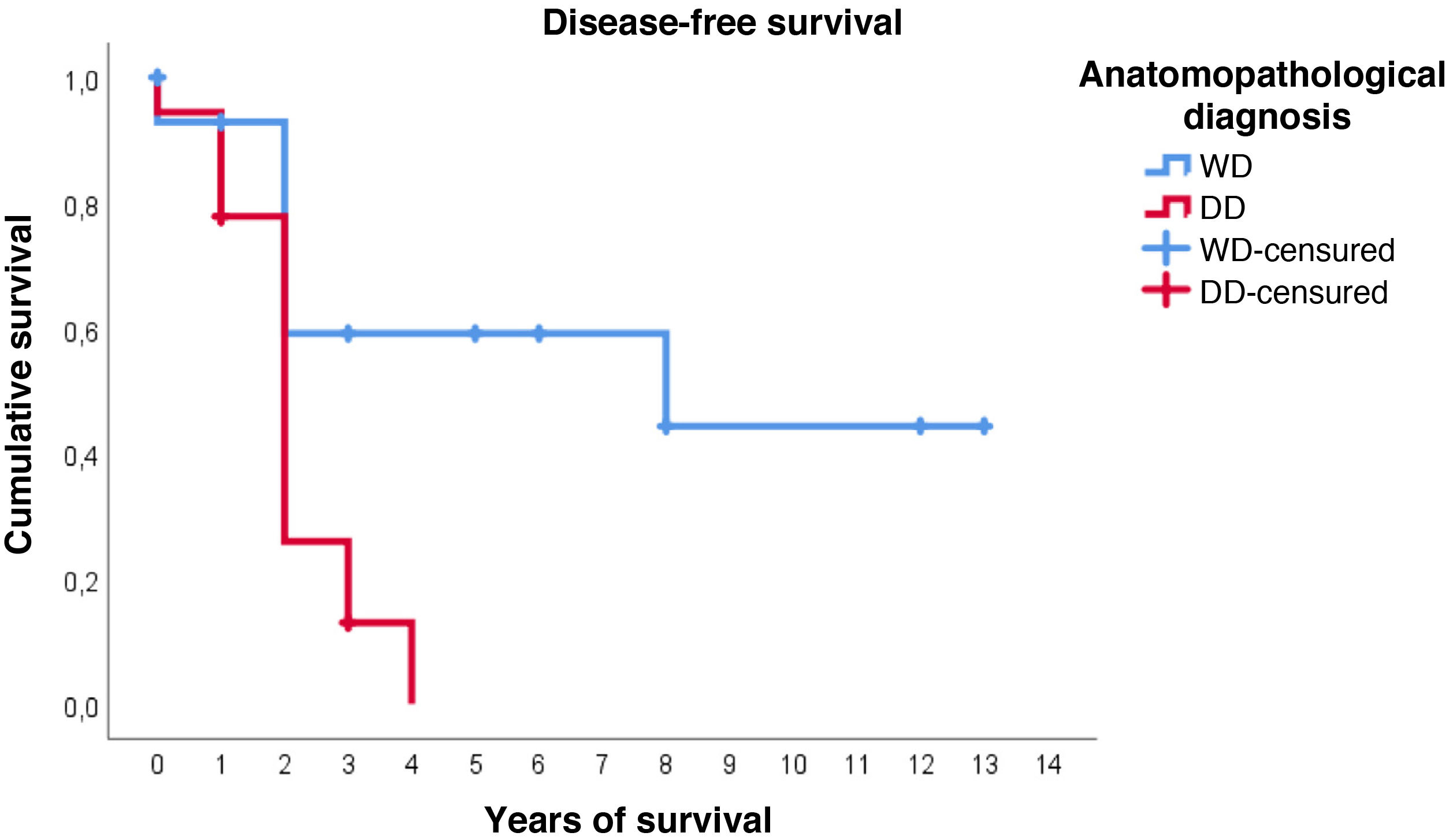
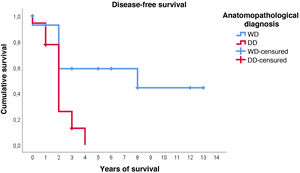
Furthermore, with regard to disease-free survival, overall the estimated media was 4.7 years (95%CI: 2.9–6.5). However, the distribution according to histological type is very different: in WDRPLS the mean disease-free survival was 7.6 years (95%CI: 4.6–10.7), while in DDRPLS the mean was only 2.1 years (95%CI: 1.6–2.6).
In this case, it can be seen (Fig. 4) that the survival curves differ overall in their morphology, and these differences are statistically significant according to the log-rank test (p = .005). In patients with dedifferentiated tumours, in no case did they exceed 4 years of follow-up without recurrence; in fact, only 3 patients in this group completed the study without recurrence. In contrast, of the group of patients with well-differentiated tumours, only one patient had a late recurrence beyond 2 years of follow-up (at 8 years). This means that disease-free survival at 2 years is 59% in well-differentiated tumours, while in dedifferentiated tumours it is only approximately 26%.
With respect to the other variables studied, none of them seem to significantly influence overall survival or disease-free survival. Survival analysis of the variables "metastasis" and "medical history of other malignant processes" was not performed due to the small number of cases.
DiscussionFrom a sociodemographic point of view, our cohort has a median age similar to other published groups, but with a slightly higher prevalence of women.21 Furthermore, the presence of previous malignant pathology was only evident in 4 patients, with no association between the type of previous tumour and the development of RPLS. Although the appearance of sarcomas is described as a complication of radiotherapy, liposarcoma is exceptional in these cases, with other cell lines predominating in locations other than the retroperitoneum.22
Histopathologically, DDRPLS was the most frequent, which contrasts with other groups, mainly English-speaking ones,9,10 and is more consistent with the Spanish series.3,11 Likewise, our data are consistent with a higher recurrence rate and lend statistical power to the assertion that worse differentiated histological subtypes confer a poorer prognosis, since no patient in the DDRPLS group survived 4 years of study without recurrence.
On the other hand, in four of the cases studied there were very early recurrences of the disease, with histological types that had progressed in degree of malignancy and evolved in histological type, and which required subsequent molecular confirmation of the diagnosis made with routine stains. Currently, molecular pathology techniques are an essential tool in the management of these cases8,23, and other published work by Gronchi’s group24 already suggests the need for molecular typing of primary tumours with unfavourable evolution.
Several groups have studied the effect of early recurrence and have related it to lower overall survival.3,5,9 However, this could not be demonstrated in our study; although overall survival at 5 years was 53% in the early recurrence group (less than 2 years) compared to 75% in the non-recurrent group, these differences were not statistically significant.
Given that RPLSs are tumours that may be large in size, surgical intent involves extensive visceral resections to obtain wide tumour-free surgical margins, as this is one of the best prognostic factors in all published series as it increases disease-free survival. enfermedad20,25 In this regard, 25% of the patients included in this study required multivisceral resection, and only 19% underwent simple excision of the tumour without resection of any organ, of which only one was of dedifferentiated lineage. Although these data are interesting, and there is consensus that adequate surgery in a specialised centre is the most effective treatment, there is no defined tumour extension to be removed to guarantee R0.19,20,26 In our experimental development, patients with extensive tumour resections that included kidney or more viscera did not imply statistically significant improvements in survival studies. However, these results should be handled with caution, as more aggressive resections were performed to a greater extent in histologically unfavourable tumours. Also, complications from renal resection should be taken into consideration: 2 of our patients developed end-stage renal failure, although we have not found studies evaluating this in other cohorts.
In addition to these data, microscopic study of the surgical margins revealed tumour involvement in 50% of cases, with the same percentage of 50% between well-differentiated and dedifferentiated tumours. Gronchi et al.25 and Chouliaras et al.26 have shown that free surgical margins are associated with a better prognosis. However, in our study survival estimates show no significant difference between the two curves.
Although there are disparities in the published studies, it seems that radiotherapeutic treatment may be more positive than chemotherapy, as has been shown in studies with large sample sizes.12,13 In our study, few patients were treated with radiotherapy or adjuvant chemotherapy in the treatment of the primary tumour (4 and 5 patients, respectively), with adjuvant therapy being reserved for recurrences in most cases. Furthermore, the decision to apply adjuvant therapy was due to tumours that were difficult to resect and with extensive involvement of the surgical margins, in which the results of surgery were not satisfactory. Therefore, with these data, it is not possible to extrapolate conclusions in this regard, and larger, more adequately designed, targeted trials are needed.
Among the limitations of our study is that the sample size was not very large, which implies a limitation when applying statistical tests to demonstrate significant differences (increased type II error). In addition, retrospective review has a natural tendency to bias. These facts prevent us from establishing certain firm conclusions that can be extrapolated with respect to prognostic and survival factors, so the results should always be taken with caution and as an approximation, and should be checked with other studies with more appropriate methodology.
In short, the only factor studied that significantly modified the prognosis of these patients was the histological subtype of the tumour. This is why we propose that the histopathological factors intrinsic to the tumour are fundamental and largely condition the results of surgery with curative intent. As a result, proper histological diagnosis is of vital importance in the therapeutic management of these patients. However, more studies are needed to unify criteria in the diagnostic and therapeutic management of these tumours in order to increase survival and reduce morbidity and mortality in these patients, because, for the time being, liposarcomas generally continue to have a poor prognosis.
FundingThis research has not received specific support from public sector agencies, commercial or not-for-profit organisations.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to express our most sincere thanks to Prof. Dr. José Peña Amaro, Professor of Histology at the University of Cordoba, for his advice in the writing of this paper and for leading the way without the slightest interference. Also, to Concepción Cabello Laguna, autopsy supervisor of the Anatomical Pathology Department of the Reina Sofía University Hospital in Cordoba, for her help in the search for the cases included in this study.
These authors contributed equally to this paper.
Please cite this article as: Osuna-Soto J, Caro Cuenca T, Sanz-Zorrilla A, Torrecilla-Martínez A, Ortega Salas R, Leiva-Cepas F. Pronóstico y supervivencia de pacientes diagnosticados de liposarcoma retroperitoneal bien diferenciado y desdiferenciado. Cir Esp. 2022;100:622–628.