Postoperative small bowel or colic anastomotic bleeding (PSCAB) is often a mild complication and is generally treated by a conservative approach. Other therapeutic options are surgery, endoscopic management and angiographic embolization. Our aim is to review our cases of postoperative anastomotic bleeding in patients with small bowel or colic anastomosis, with special attention to their treatment and complications.
Patients and methodsObservational retrospective study including patients with PSCAB in the department of General and Digestive Surgery in Vall d’Hebron University Hospital, between 2007 and 2012. Demographic and bleeding characteristics as well as therapeutic management were reviewed, including complications derived from the different therapeutic options.
ResultsThere were 44 cases of bleeding after performing small bowel or colic anastomosis, 25 patients were men (56.8%), with a mean age of 68.2 years (R: 28–92). The mean hematocrit decrease was 8 points (R: 0–17), and hemodynamic instability was detected in 13 patients (29.5%). A conservative management was undertaken in 27 patients (61.3%), surgery in 6 (13.6%), endoscopic treatment in 2 (4.5%) and embolization in 9 (20.5%). Four patients of cases treated with embolization presented anastomotic leak (44.5%). Mortality was 13.6% (6 patients). A total of 4 of 6 deaths were in the group of patients treated with embolization.
ConclusionsMost patients with PSCAB have a good response to conservative management. When there is failure of this approach, there are different therapeutic options, including angiographic embolization. In our series, we have seen a high incidence of post embolization anastomotic leak; further trials will be necessary to provide valuable evidence of the risk of this therapeutic option.
La hemorragia postoperatoria de una anastomosis intestinal o cólica (HPAIC) suele ser una complicación leve, manejada generalmente de forma conservadora. Otras opciones terapéuticas son la cirugía, la endoscopia y la embolización angiográfica. Nuestro objetivo es realizar un análisis descriptivo de las hemorragias anastomóticas postoperatorias en pacientes con anastomosis intestinales o cólicas, el tratamiento realizado y las complicaciones derivadas.
Pacientes y métodosEstudio observacional retrospectivo, que incluye pacientes con HPAIC en el Servicio de Cirugía General y del Aparato Digestivo del Hospital Universitario Vall d’Hebron entre 2007 y 2012. Se han recogido las características de los pacientes, del tratamiento y las complicaciones según la opción terapéutica.
ResultadosHallamos 44 casos de hemorragia anastomótica, siendo varones 25 (56,8%), con una media de edad de 68,2 años (R: 28-92). La caída media de hematocrito fue de 8 puntos (R: 0-17), presentando inestabilidad hemodinámica 13 pacientes (29,5%). Se realizó manejo conservador en 27 pacientes (61,4%), cirugía en 6 (13,6%), manejo endoscópico en 2 (4,5%) y embolización en 9 (20,5%). De los casos embolizados, 4 pacientes presentaron dehiscencia anastomótica (44,5%). La mortalidad fue de 13,6% (6 pacientes). Un total de 4 de las 6 muertes pertenecen al grupo embolizado.
ConclusionesLa mayoría de pacientes con HPAIC responden al tratamiento conservador. Cuando fracasa, existen diferentes opciones terapéuticas que incluyen la embolización angiográfica. En nuestra serie observamos una elevada incidencia de dehiscencia anastomótica postembolización, siendo necesario reevaluar el tipo de embolización así como sus indicaciones y contraindicaciones.
Lower gastrointestinal bleeding from bowel or colonic anastomosis is a relatively common clinical problem; the percentage of patients experiencing this complication is between 1% and 5.4% of those treated with this type of surgery. These patients will require systematic diagnosis and a defined treatment algorithm.1–3
This type of bleeding appears in a manner similar to gastrointestinal bleeding resulting from other causes; the two are usually treated with conventional methods, as severe bleeding is a rare occurrence.4 In any case, surgical treatment is required when conventional treatment fails.
Different treatment options include endoscopic therapy, angiographic embolization and resurgery treatment. Currently, however, these treatment options continue to be discussed in terms of their success rate and associated risks.3
This paper aims to describe the frequency of postoperative bleeding in patients treated at our center who underwent bowel or colonic anastomosis, the treatment performed, and complications that appeared after treating this disease with interventional radiology.
Patients and MethodWe conducted a retrospective study involving patients who had postoperative gastrointestinal bleeding that originated from bowel or colonic anastomosis during the period from January 1, 2007 and December 31, 2012 in the General Surgery and Gastrointestinal Diseases Department of the Hospital Universitario Vall d’Hebron [Vall d’Hebron University Hospital].
We included patients undergoing both scheduled and emergency surgery, by open or laparoscopic approach. Anastomoses were performed manually or mechanically, and the surgeon performing the procedure selected the technique in each case.
The concept of postoperative anastomotic bleeding was defined as the presence of direct (hematochezia) or indirect (anemia or hemodynamic) signs of bleeding after surgery in which this type of anastomosis was performed. When we refer to patients who had hemodynamic instability, we have included those with systolic pressure of less than 90mmHg and heart rate over 100 beats per minute.
Demographic data and characteristics concerning bleeding and therapeutic care were reviewed, including the incidence of suture dehiscence and mortality. Likewise, data was collected on complications with angiographic treatment.
Conventional treatment has been defined as one that included the stabilization by fluid therapy, correction of coagulopathy, if applicable, and the administration of blood products. Vital signs were monitored in order to identify patient destabilization.
In some cases, a computed tomography (CT) with intravenous contrast was performed to confirm active bleeding and to exclude other diseases.
Anastomosis-related post-embolization ischemia was defined as having sepsis after completing the anastomosis, which was accompanied by clinical signs of peritonitis, with or without confirmation by CT with intravenous contrast or by direct visual verification during surgery.
Statistical AnalysisContinuous variables have been presented as means, ranges and medians. Categorical variables have been presented as absolute numbers and percentages.
ResultsDuring the study period, 2069 patients underwent surgery with a small bowel or colonic anastomosis. A total of 44 patients (3.17%) had postoperative anastomotic bleeding, 25 were male (56.8%), and 19 female (43.2%), with a mean age of 68.2 (28–92) years.
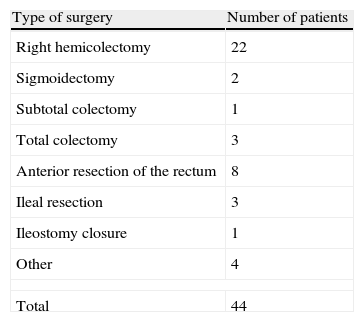
A total of 34 patients (72.7%) underwent surgery for malignant disease, the second most common cause was inflammatory bowel disease (5 patients, 11.4%), and the remaining percentage attributed to other minor causes. The type of surgery performed in each case is shown in Table 1, with 20 surgeries (45.5%) by open approach and 24 (54.5%) by laparoscopic approach. A total of 11 (25%) anastomoses were performed manually and 33 (75%) mechanically.
The mean interval between surgery and the bleeding episode was 7.6 (0–46) days, with a median of 5.5 days. The most common clinical event was hematochezia in 42 patients (95.5%), while 2 (4.5%) had initial hypotension without other symptoms. Thirteen patients (29.5%) experienced hemodynamic instability during the episode. The average drop in hematocrit was 8 (0–17) points. A total of 35 patients (79.5%) required blood transfusion; 3.7 (1–11) was the average of packed red blood cells transfused per patient.
Of the 44 cases reviewed, 16 (36.4%) had an abdominal CT with intravenous contrast. In the 9 embolized patients, angiography confirmed the previously active bleeding diagnosed by CT.
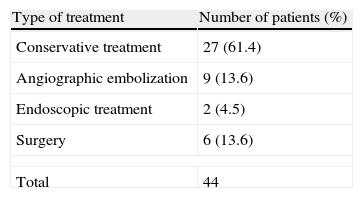
Table 2 shows the various treatment options performed in our department to treat bleeding. Conventional treatment was the most frequent choice, 27 (61.4%), followed by angiographic embolization, which was performed in 9 patients (20.4%). Bleeding stopped in all cases within the latter group.
For this series, 7 patients experienced anastomosis dehiscence (20.4%), 4 of them after completing angiographic embolization.
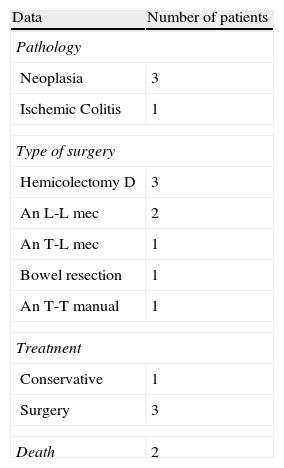
Table 3 lists the data regarding anastomotic dehiscence after completing an angiographic procedure. Most cases with post-embolization dehiscence were patients who had undergone a right hemicolectomy with stapled anastomosis. The mean time interval from embolization to onset of dehiscence was 3.8 (2–8) days; surgical treatment remains the option of choice in this clinical situation.
Data on Cases of Post-Angiographic Embolization Suture Dehiscence.
| Data | Number of patients |
| Pathology | |
| Neoplasia | 3 |
| Ischemic Colitis | 1 |
| Type of surgery | |
| Hemicolectomy D | 3 |
| An L-L mec | 2 |
| An T-L mec | 1 |
| Bowel resection | 1 |
| An T-T manual | 1 |
| Treatment | |
| Conservative | 1 |
| Surgery | 3 |
| Death | 2 |
An L-L mec: latero-lateral mechanical anastomosis; An T-L mec: termino-lateral mechanical anastomosis; An T-T manual: termino-terminal manual anastomosis.
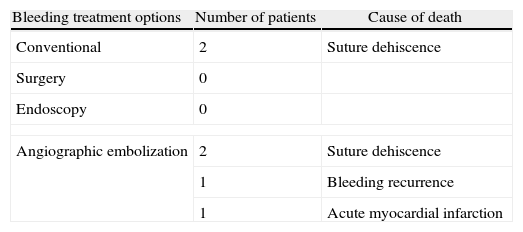
Of the 44 patients with anastomotic bleeding, 6 died (13.64%), 4 of them after angiographic embolization. The causes of death were acute myocardial infarction in one case, recurrent bleeding in another, and anastomotic dehiscence in 2 patients (Table 4). In these last 2 cases, we chose conservative treatment due to the advanced age and comorbidity of the two patients.
Mortality After an Episode of Postoperative Bowel or Colonic Anastomotic Bleeding.
| Bleeding treatment options | Number of patients | Cause of death |
| Conventional | 2 | Suture dehiscence |
| Surgery | 0 | |
| Endoscopy | 0 | |
| Angiographic embolization | 2 | Suture dehiscence |
| 1 | Bleeding recurrence | |
| 1 | Acute myocardial infarction | |
The presence of bleeding at an anastomosis after resection of the small bowel or colon usually occurs as hematochezia, especially in the early stages of recovery of bowel transit. Generally, it is self-limiting bleeding without other clinical impact.3 However, there is a small percentage of patients having significant anemia, hemodynamic instability, and occasionally, shock and death.4
Postoperative anastomotic bleeding in colorectal surgery is a rarely discussed topic in the literature.5 According to published data, it is placed between 1% and 5.4%,1,2,5,6 values similar to those observed in our series (3.2%).
Although there are publications on the increased risk of this complication with respect to certain treatments or previous illnesses, as well as the place or type of anastomosis, not all studies support these results.1,3,5,7,8
The mean bleeding interval in our series from the day of surgery was one week, consistent with previously published data.3,9
In these patients, the initial treatment must be conservative, based on blood volume recovery and strict control of hemodynamic parameters.10–12 In our study, 61.4% of patients were treated conservatively; the bleeding stopped spontaneously in all cases. When conservative treatment fails other therapeutic options should be considered.7,9,10,13
Formerly, if conventional treatment was unsuccessful, the option was to perform resurgery, with a nearly double increase in mortality compared to those patients not requiring resurgery. Currently, there are other methods to avoid the morbidity and mortality associated with resurgery.12,14 The above includes endoscopic treatment and angiographic embolization, initially considered less aggressive than resurgery, however, proven over time to also pose some risks.3,12,14–16 Currently, there is still debate on which method provides higher success rates and lower risks, the most important of which is anastomotic suture dehiscence.9,17
As part of the diagnostic–therapeutic treatment algorithm for mild gastrointestinal bleeding, many authors advocate conducting a fibrocolonoscopy17,18; however, evidence shows the success rate will be lower than in upper gastrointestinal bleeding.19 The advantage of this procedure is the precise location of the source point of the bleeding by direct visualization, excluding other causes, besides being a safe and effective method. However, a noted disadvantage is poor visualization in an unprepared colon or with massive bleeding,19 and the risk of anastomotic dehiscence, especially if endoscopy is performed in the first 4 or 5 days after surgery, when the integrity of the anastomosis still depends on the suture.9 However, Chardavoyne et al. concluded in a paper published in 1991 that, in an emergency of this kind, colonoscopy was safe even on the first postoperative day.20 Few papers have been published on endoscopy-based anastomotic bleeding treatment, but those available prescribe it as the first option for colonic anastomosis, preferably with sclerotherapy, clip placement or electrocautery3; however, due to the limitations of this technique, its application in the bleeding of small bowel anastomoses is not possible. In our series, we only have 2 cases in which fibrocolonoscopy was used as a therapeutic option, placing clips, with bleeding cessation and good outcome for the two cases.
Most authors advocate treatment of lower gastrointestinal bleeding using angiography as a second therapeutic option, when endoscopic treatment is contraindicated or has failed. However, there is no consensus, as there are other groups that use this technique as a first option, depending on hospital policy and the availability of means.3,4,14,16,18,19,21
Although the first transcatheter embolization was performed in 1967 by Newton and Adams,22 the first therapeutic vascular embolization for gastrointestinal bleeding was described by Bookstein et al. in 1974.23 Subsequently, this group and other authors reported the risk of colonic ischemia after the procedure. In 1982, Rosenkrantz described the occurrence of 3 cases of colonic ischemia following embolization of 23 bleeding cases; this made the procedure be considered unsafe.24
However, angiographic embolization remains an attractive alternative to surgical or endoscopic control of the bleeding. In most cases, this can be done after locating the bleeding site by angiography, with immediate control of bleeding in a percentage of patients from 76% to 100%.14,25
Early publications placed the percentage of post-embolization ischemia at 13%–20%,23,26,27 but currently, this percentage is estimated to be below 10%.27 This could be explained by the increased experience of the team performing the procedure and by the advent of supraselective embolization.
Published literature on this topic is scarce and no study shows conclusive data.3–5 There are fewer than 20 published cases of anastomotic bleeding that required surgery, with no consensus on treatment.5 In our series, conservative treatment failed in 17 patients, and 9 patients underwent angiographic embolization. Currently at our center, we have no defined anastomotic bleeding diagnostic–therapeutic algorithm; thus, the choice of method used was defined according to the treating physician and the availability of other specialists.
Almost half of the embolized patients had anastomotic dehiscence; 75% required resurgery, with 50% mortality. Although the series submitted is small, the results should provide a foundation for further studies, ideally prospective and multicenter, in order to define the actual figures on angiographic embolization safety.
Similarly, and following the results obtained, the creation of a clinical guide for diagnosis and therapeutic care of this disease in our hospital seems to be a priority.
In conclusion, when conservative treatment has failed, angiographic embolization appears to be a technical alternative to surgery when bleeding occurs after performing small bowel or colonic anastomosis. However, in our experience, we have found a high rate of anastomotic dehiscence after performing this procedure; further studies are still needed to assess the evidence of risk with this treatment option.
Conflict of InterestThe authors declare having no conflict of interest.
Please cite this article as: Fernández de Sevilla Gómez E, Vallribera Valls F, Espin Basany E, Valverde Lahuerta S, Pérez Lafuente M, Segarra Medrano A, et al. Hemorragia en anastomosis intestinales y cólicas. Manejo terapéutico y sus complicaciones. Cir Esp. 2014;92:463–467.
Presented as an oral communication in the XVII National Meeting of the Foundation of the Spanish Association of Coloproctología. Palma, May 2013.