Gastrointestinal stromal tumors (GIST) are the most frequent mesenchymal neoplasms of the digestive tract. They are usually seen in patients over the age of 50, with equal sex distribution, with an annual incidence of approximately 5000 in the U.S.1 They originate from cells of Cajal and the most frequent location is the stomach (60%), followed by the jejunum and ileum (30%). Although they frequently extend to the liver and peritoneum, GIST rarely metastasize to the lymph nodes, lungs or extraintestinal regions.
Their main characteristic is that they present mutations in the c-KIT proto-oncogene or in the platelet-derived growth factor receptor alpha (PDGFR-α). Anti-tumor therapies have been developed against these targets based on tyrosine-kinase inhibitors such as imatinib and sunitinib, which have become the paradigm of molecular therapy against cancer. Around 80% of patients respond to imatinib, versus 5% to conventional chemotherapy, a fact that has increased the mean survival with metastatic disease from 15 months to almost 5 years.1,2
In the management of primary GIST, imatinib can be used as neoadjuvant therapy in cases that are unresectable from the onset, as well as adjuvant therapy after surgical treatment. In cases of recurrence, locally advanced or metastatic GIST, the association of imatinib is indicated with more aggressive cytoreductive surgery. Meanwhile, pharmacological therapies such as sunitinib are used as a second line of treatment in cases that are resistant to imatinib.2
In locally-advanced tumors, this radical debulking could require the peritonectomy procedures described by Sugarbaker3 in 1995 (with or without associated intraperitoneal perioperative chemotherapy), given the excellent results with their use for treating malignant peritoneal disease of different origins.4 Nonetheless, in the case of GIST with peritoneal dissemination (called “peritoneal GISTosis” by Sugarbaker), the indication of these procedures is currently under dispute.4
Because of a case of these characteristics, we have assessed the role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in these patients.
The patient is a 45-year-old woman who had been diagnosed with high-risk jejunal GIST (c-KIT+) that had been treated surgically. She then received adjuvant therapy with imatinib for one year. Three months after finalizing the treatment, she presented with recurrence and once again underwent surgery. Given the age of the patient, type of disease, surgery performed and relapse after one year, it was proposed to reinitiate imatinib 400mg/day, continuously. Nevertheless, the patient presented progression of the disease in the peritoneum, and the therapeutic regime was modified to sunitinib 37.5mg/24h. Partial response was achieved during the first few months, and the disease became stabilized after one year of treatment with this drug. In this situation, and given the potential resectability of the tumor using peritonectomy procedures, the patient was sent to our unit for surgical intervention.
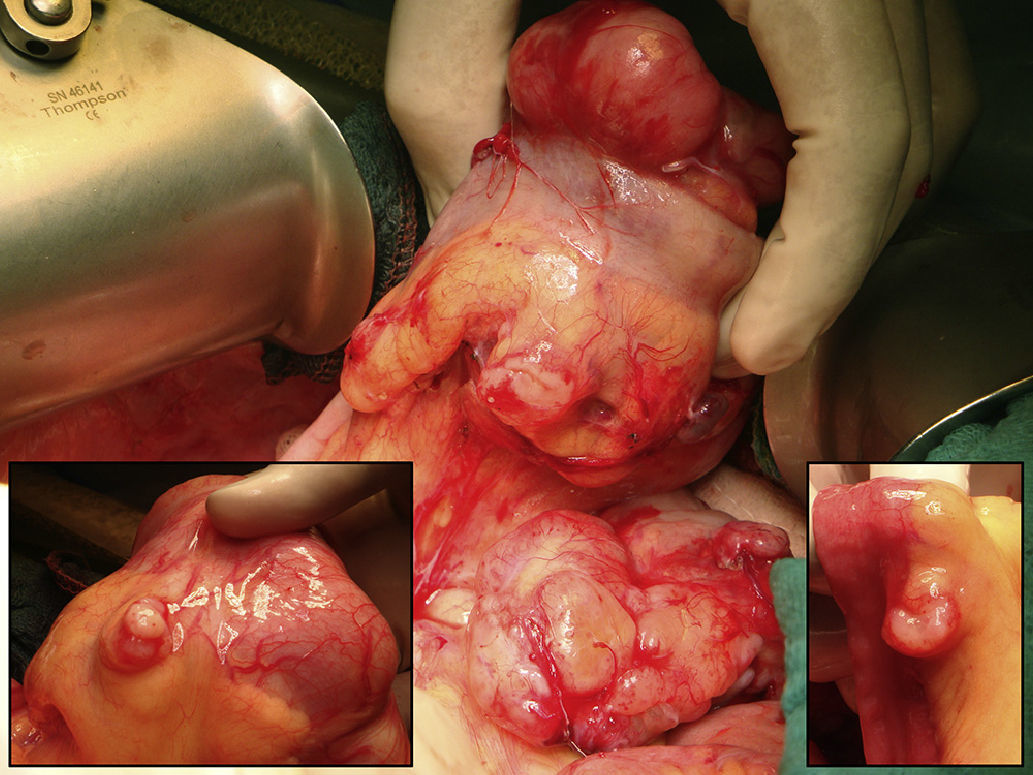
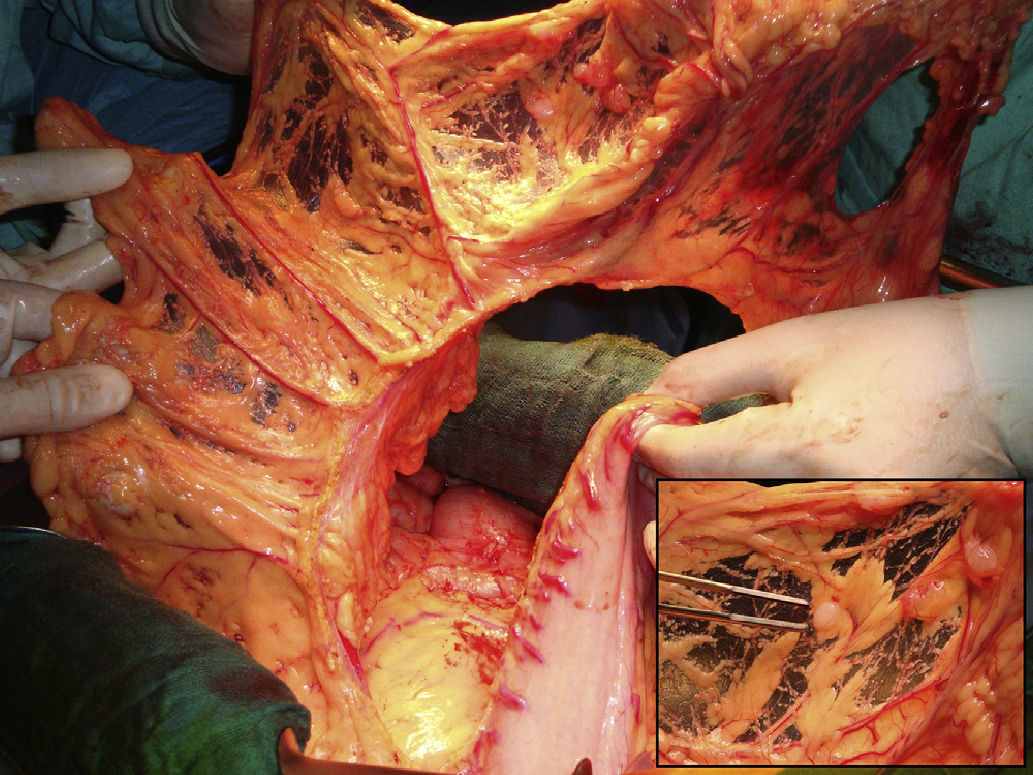
The operative findings demonstrated several tumor masses larger than 5cm, as well as several centimeter and sub-centimeter lesions disseminated in the abdominal cavity (peritoneal cancer index [PCI] 23/39) (Fig. 1). Optimal cytoreduction (CC-0) was achieved with right diaphragmatic, bilateral parietal and complete pelvic peritonectomy and resection of the rectum-sigmoid colon, uterus, fallopian tubes, ovaries, gallbladder and greater omentum (Fig. 2). In addition, resection with fulguration was performed on the isolated mesenteric lesions. Prior to colorectal anastomosis, the patient received an hour of HIPEC with cisplatin and doxorubicin. The post-operative period did not present incident and, at the writing of this article 6 months after surgery, the patient is asymptomatic and disease-free.
This is a case of locally advanced GIST that was resistant to treatment with imatinib and partially responsive to sunitinib. Faced with the option of exclusively palliative chemotherapy, we proposed the possibility of potentially-curative radical surgery with the procedures recommended by Sugarbaker.3 The systemization of peritonectomy surgical techniques has provided low morbidity and mortality rates in experienced hospitals, thus expanding its possible indications. In the context of GIST, just as in the intensive treatment of hepatic metastatic disease,5,6 achieving optimal cytoreduction (CC-0) can improve survival,7–10 especially in those patients who respond to chemotherapy.
On the other hand, the use of HIPEC with cisplatin and doxorubicin in this type of tumor is more controversial due to the low sensitivity of GIST for these cytostatic agents. Clinical trials would enable us to determine the actual influence of HIPEC in the evolution of this neoplasm. In our opinion, and in accordance with Sugarbaker's recommendations,4 peritonectomy procedures with multiple visceral resections would be indicated in those patients in whom complete cytoreduction is achievable, while the option of HIPEC should be left for those cases in which there is also a low risk of anastomotic dehiscence and fistula. Likewise, it is recommended that these procedures should be done at experienced hospitals and always under the criteria of clinical studies.
Please cite this article as: Medina Fernández FJ, Muñoz-Casares FC, Arjona-Sánchez Á, Casado-Adam Á, Rufián Peña S. Gistosis peritoneal: Papel de la cirugía de citorreducción y quimioterapia intraperitoneal perioperatoria. Cir Esp. 2014;92:289–290.