Pylorus-preserving pancreatoduodenectomy with gastric partition (PPPD-GP) seems to be associated to a better postoperative outcome than conventional pancreaticojejunostomy in the setting of a prospective-randomised study. The aim of this study is to further evaluate the surgical outcome in a series of 129 consecutive patients.
MethodsBetween 2007 and June 2013, 129 patients with periampullary tumours surgically treated with PPPD-GP were retrospectively analysed. Surgical complications (Clavien–Dindo score), as well as pancreatic and non-pancreas related complications were analysed.
ResultsOverall postoperative complication rate was 77%, although 50% of complications were graded I–II by the Clavien–Dindo classification. Incidence of clinically relevant pancreatic fistula was 18%: ISGFP type B: 12%, and type C: 6%. Other pancreas specific complications such as delayed gastric emptying and pospancreatectomy haemorrhage were 27% and 15%, respectively, similar to results published in the literature. Overall perioperative mortality rate was 4.6%.
ConclusionPPPD-GP results show that it is a technique with an acceptable morbidity, low mortality and pancreatic fistula rate similar to other techniques currently described of pancreaticoenteric reconstruction.
La técnica de la reconstrucción pancreática tras duodenopancreatectomía cefálica con conservación del píloro mediante bipartición gástrica (DPC-BG) parece asociarse a una mejor evolución postoperatoria en comparación con la pancreaticoyeyunostomía convencional en el marco de un estudio aleatorizado prospectivo. El objetivo de este estudio es evaluar aún más el resultado quirúrgico en una serie de 129 pacientes consecutivos.
MétodosEntre 2007 y junio de 2013, se analizaron retrospectivamente un total de 129 pacientes con tumores periampulares tratados quirúrgicamente con DPC-BG. Se analizaron los resultados a partir de las complicaciones precoces quirúrgicas (escala de Clavien-Dindo), así como las complicaciones relacionadas y no relacionadas con el páncreas.
ResultadosLa tasa de complicación postoperatoria global fue del 77%, aunque el 50% de las complicaciones se clasificaron I–II en la clasificación Clavien-Dindo. La incidencia de la fístula pancreática clínicamente relevante fue del 18% (tipo ISGFP B: 12%, tipo ISGFP C: 6%). Otras complicaciones específicas del páncreas tales como retraso del vaciamiento gástrico y hemorragia pospancreatectomía fueron del 27 y del 15%, respectivamente, similares a los resultados publicados en la literatura. La tasa de mortalidad perioperatoria global fue del 4,6%.
ConclusiónLos resultados de la DPC-BG muestran que es una técnica segura, con una morbilidad aceptable, baja mortalidad y tasa de fístula pancreática similar a otras técnicas actualmente descritas de reconstrucción pancreaticoentérica.
Pancreatic neoplasms have shown a growing prevalence in recent years. In 2011, pancreatic cancer was diagnosed in 8773 patients, and was responsible for 8320 deaths in the United Kingdom, with an overall survival rate after 5 years of 3.7% in 2005–2009.1 In the US, estimates for newly diagnosed cases and deaths for 2014 were 46,420 and 39,590, respectively.2
The surgical treatment of pancreatic neoplasms has traditionally been considered a challenge, mainly due to 2 factors: on the one hand, the survival rate of patients with a pancreatic ductal adenocarcinoma after suitable surgical and cancer treatment is poor, with a survival rate of approximately 20% after 5 years, in most studies. On the other hand, complications after pancreatic surgery are frequent. In all the samples of patients with pancreatic resection, specific complications of the pancreas, such as delayed gastric emptying (DGE), pancreatic haemorrhage or fistula (PF), constitute a major source of morbidity which may ultimately affect the results. With the aim of avoiding or minimising these complications, efforts have been focused on amending this scenario. Since one of the main sources of morbidity is the appearance of PF, various pancreaticoenteric reconstruction techniques have been described. Currently, the use of different types of pancreatic anastomosis used in each centre shows the absence of a consistent and reliable technique. Traditionally, the preferred site of pancreatic anastomosis after a pancreaticoduodenectomy (PD) has been the jejunum (pancreaticojejunal anastomosis [PJA]), but there is a growing trend in recent years towards pancreatico-gastric anastomosis (PGA), due to the publication of several randomised, controlled studies as well as of meta-analysis supporting the superiority of a PGA.
In 2007, we described our pancreatic reconstruction technique with a PGA after a pylorus-preserving PD with gastric partition (PPPD-GP), in the setting of a randomised, controlled trial that showed its superiority compared to a conventional PJA.
In this study, our aim is to describe the outcome for the period between 2007 and 2013 for 129 patients treated with PPPD-GP.
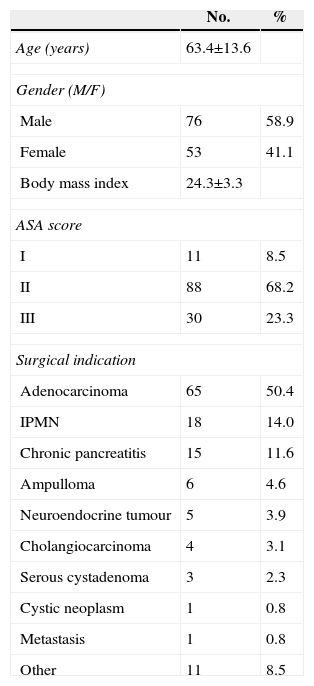
MethodsPatientsThis article is a retrospective analysis of a database built prospectively. From 2007 to 2013, 129 patients were treated with PPPD-GP due to pathological findings in the head of the pancreas. Surgical indications for these patients were based on the presence of lesions located to the right of the mesenteric vessels, symptomatic or not, and were confirmed by a multidisciplinary committee. Demographic data of these patients is shown in Table 1.
Demographic Data of the Patients Included (n=129).
| No. | % | |
|---|---|---|
| Age (years) | 63.4±13.6 | |
| Gender (M/F) | ||
| Male | 76 | 58.9 |
| Female | 53 | 41.1 |
| Body mass index | 24.3±3.3 | |
| ASA score | ||
| I | 11 | 8.5 |
| II | 88 | 68.2 |
| III | 30 | 23.3 |
| Surgical indication | ||
| Adenocarcinoma | 65 | 50.4 |
| IPMN | 18 | 14.0 |
| Chronic pancreatitis | 15 | 11.6 |
| Ampulloma | 6 | 4.6 |
| Neuroendocrine tumour | 5 | 3.9 |
| Cholangiocarcinoma | 4 | 3.1 |
| Serous cystadenoma | 3 | 2.3 |
| Cystic neoplasm | 1 | 0.8 |
| Metastasis | 1 | 0.8 |
| Other | 11 | 8.5 |
During the time period between 2007 and 2013, surgical interventions were conducted by 5 surgeons (LFC, SSC, MLB, JF, DC), all of them with the same surgical technique previously described.3 Briefly, the dissection stage of the intervention is a standard procedure with pylorus preservation. The pancreatic section is performed with an electric scalpel until the main pancreatic duct is identified and severed with scissors. A standard lymphadenectomy4 is conducted in the region of the common hepatic artery, the hepatic pedicle, the right area of the superior mesenteric artery, and the celiac trunk. The main bile duct is sectioned right above the cystic duct, and temporarily blocked with a clamp to avoid bile leaking into the peritoneal cavity during the intervention.
The reconstruction stage begins with the preparation of the pancreatic anastomosis. To ensure the best possible anastomosis and optimal vascularisation of the pancreatic stump, the remaining pancreas is only separated by 2–3cm from the splenic vein. Then, the greater curvature of the stomach is prepared for anastomosis. Starting with the superior end, and having carefully identified and preserved the gastroepiploic arcade, a gastric partition is prepared with 3 sections of a 60mm endostapler. Once haemostasis is confirmed, a continuous 5/0 polypropylene suture is applied to the stomach to avoid postoperative haemorrhage. Then, a 2-layer Wirsung-gastric anastomosis is performed by means of a non-reabsorbable suture, with the routine placement of an internal stent. Internal drainage is only omitted in patients with a pancreatic duct larger than 3mm; additionally, during this period, an external pancreatic drainage was applied to 63 patients (48.8%) due to a prospective study aimed at clarifying the effect of postoperative octreotide on pancreatic secretion.5 Once the pancreatic anastomosis is complete, a terminolateral duodenojejunostomy with a continuous 2-layer, non-absorbable suture and, finally, a hepaticojejunostomy at approximately 40cm from the duodenal anastomosis are conducted with a 5/0 polydioxanone suture. Two Jackson-Pratt drainages are placed anterior and posterior to the pancreatic anastomosis, and another one is placed close to the bile anastomosis and the enteric reconstruction.
After surgery, all the patients were transferred to ICU, where they spent the first few days after surgery. Early mobilisation of the patients was attempted from postoperative day 1. The routine nasogastric tube was maintained until normal peristalsis was restarted, as well as a daily aspiration of under 400ml, with an average of 4.2±3.3 days. Full parenteral nutrition was used for the 1st week following surgery. Oral feeding was re-introduced after removing the nasogastric tube.
Amylase and lipase detection in the drainage was conducted routinely on the 1st, 3rd and 5th day after the intervention. Drains were removed on the 5th day, in the absence of laboratory or clinical evidence of PF or biliary fistula.
DefinitionsPatient complications were prospectively recorded after clinical discharge. Specific pancreatic complications such as PF, DGE and postpancreatectomy haemorrhage (PH) were classified using the definitions published by the International Study Group on Pancreatic Fistula (ISGPF)6 and the International Study Group of Pancreatic Surgery (ISGPS).7,8
General complications after surgery were classified according to a modified Clavien–Dindo score.9
Statistical AnalysisValues are expressed as mean±standard deviation. A Chi Square test was used for the comparison of data between groups. A measurement of P<.05 was considered statistically significant. The statistical analysis of the data was conducted with the SPSS Statistics 18.0 software (SPSS, Chicago, IL, US).
ResultsPatient Clinical DataTable 1 shows the main demographic aspects of the patients. The median age was 63.4±13.6 years, and the gender ratio was 1.4/1 (M/F). The main indication for conducting a PPPD-GP was a pancreatic duct adenocarcinoma in 65 of the 129 patients (50.4%). The other 64 patients had various diagnoses, as shown in Table 1. The most common were an IPMN of the head of the pancreas (18 patients, 14%), chronic pancreatitis (15 patients; 11.6%), neoplasia of the ampulla of Vater (6 patients; 4.6%) and distal cholangiocarcinoma (4 patients; 3.1%).
The main clinical manifestations in the patients were jaundice (71 patients; 55%), abdominal pain (67 patients; 51.9%), weight loss (41 patients; 31.8%), diarrhoea (11 patients; 8.5%) and the onset of diabetes (2 patients; 1.6%). Before surgery, 26 patients with jaundice had endoscopic treatment for the placement of a biliary stent, in most cases, made of plastic.
The median stay after PPPD-GP was 18 days (range 3–197) (median of 23.7±22.1 days), and the stay in ICU was less than 3 days in 92% of cases, with a median stay of 1.9±0.8 days, and up to 8% of patients went directly to regular hospitalisation after surgery.
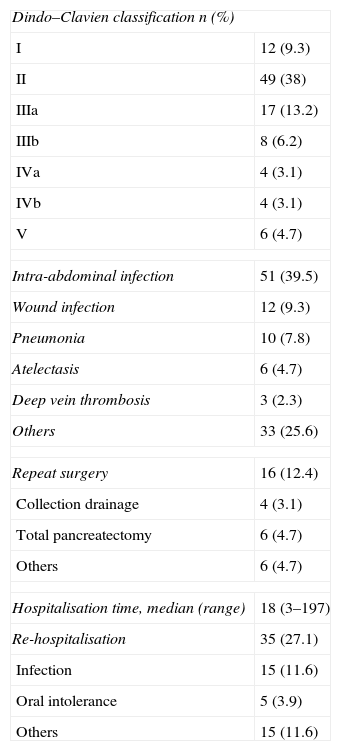
Overall Complications After SurgeryStrictly applying the Clavien–Dindo classification, 77.5% of patients presented at least 1 complication after surgery. However, in spite of this high ratio of complications, most of them (86%) were classified between groups I and III, with almost 50% of the complications of type II. Even so, overall mortality after 30 days was 4.7%.
The most frequent complications were intra-abdominal infections (51 patients; 39.5%), followed by pulmonary complications (16 patients; 12.5%), wound infections (12 patients; 9.3%) and deep vein thrombosis (3 patients; 2.3%).
A total of 16 patients required re-intervention due to complications. Among them, the commonest re-intervention was a pancreatectomy, which was finally conducted in 7 cases (5.4%), followed by the drainage of peripancreatic fluid collection in 4 patients (3.1%). The main causes of re-intervention in the remaining patients were bile leakage (2 patients), gastric puncture independent of the partition (1 patient), subdural haematoma as a consequence of the epidural catheter which required a laminectomy (1 patient), and vesical haemorrhage which was treated transurethrally (1 patient). Finally, 35 patients (27.1%) were re-hospitalised, due to infectious complications in 15 patients, and due to oral diet intolerance in 5 cases. Table 2 shows the overall complications for the 129 patients included in this study and for each group.
Overall Surgical Complications After a PPPD-GP.
| Dindo–Clavien classification n (%) | |
| I | 12 (9.3) |
| II | 49 (38) |
| IIIa | 17 (13.2) |
| IIIb | 8 (6.2) |
| IVa | 4 (3.1) |
| IVb | 4 (3.1) |
| V | 6 (4.7) |
| Intra-abdominal infection | 51 (39.5) |
| Wound infection | 12 (9.3) |
| Pneumonia | 10 (7.8) |
| Atelectasis | 6 (4.7) |
| Deep vein thrombosis | 3 (2.3) |
| Others | 33 (25.6) |
| Repeat surgery | 16 (12.4) |
| Collection drainage | 4 (3.1) |
| Total pancreatectomy | 6 (4.7) |
| Others | 6 (4.7) |
| Hospitalisation time, median (range) | 18 (3–197) |
| Re-hospitalisation | 35 (27.1) |
| Infection | 15 (11.6) |
| Oral intolerance | 5 (3.9) |
| Others | 15 (11.6) |
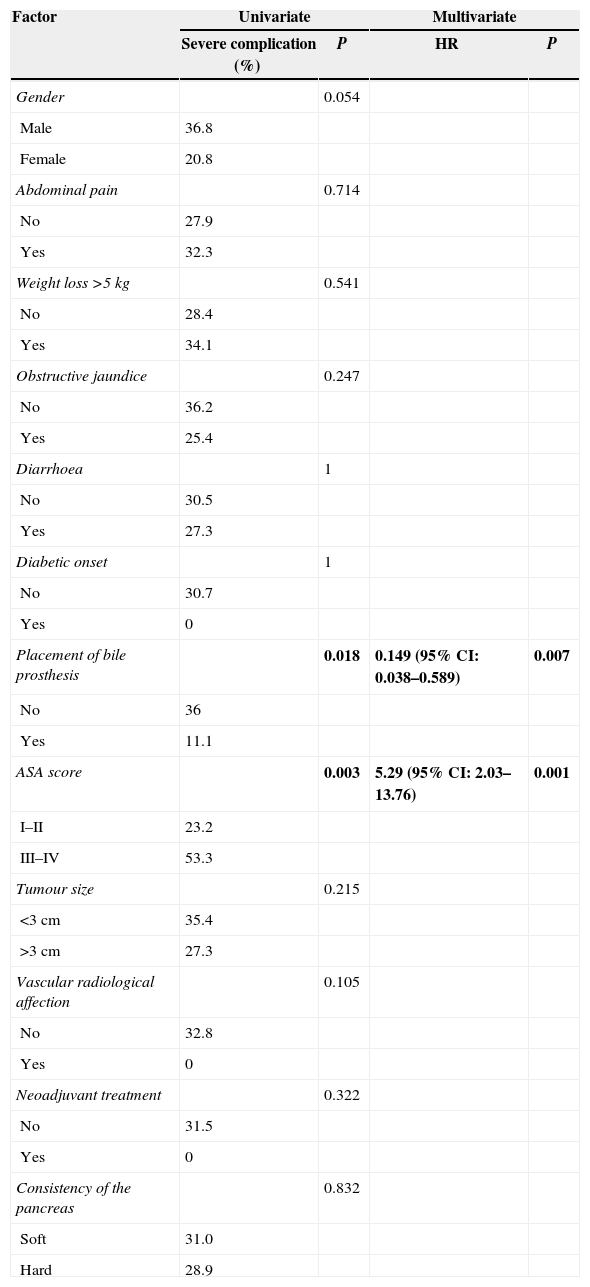
We conducted a univariate and multivariate analysis of pre-operative factors related to the appearance of major postoperative complications (classified as Clavien–Dindo III or higher), which is shown in Table 3. Amongst them, 2 factors are noteworthy: in the first place, in our experience a preoperative application of biliary prosthesis is associated with reduced major postoperative complications, in the univariate (36% vs 11.1%; P=.018) as in the multivariate (0.149; 95% CI: 0.038–0.589) analysis. In second place, classifying patients as ASA III–IV involves a significant risk factor for the appearance of major complications, both in the univariate (23.2% vs 53.3%; P=.003) and in the multivariate (5.29; 95% CI: 2.03–13.76) analysis.
Univariate and Multivariate Analysis of Preoperative Factors With the Presence of Major Complications in the Post-Operative Period (Dindo–Clavien>III).
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| Severe complication (%) | P | HR | P | |
| Gender | 0.054 | |||
| Male | 36.8 | |||
| Female | 20.8 | |||
| Abdominal pain | 0.714 | |||
| No | 27.9 | |||
| Yes | 32.3 | |||
| Weight loss >5kg | 0.541 | |||
| No | 28.4 | |||
| Yes | 34.1 | |||
| Obstructive jaundice | 0.247 | |||
| No | 36.2 | |||
| Yes | 25.4 | |||
| Diarrhoea | 1 | |||
| No | 30.5 | |||
| Yes | 27.3 | |||
| Diabetic onset | 1 | |||
| No | 30.7 | |||
| Yes | 0 | |||
| Placement of bile prosthesis | 0.018 | 0.149 (95% CI: 0.038–0.589) | 0.007 | |
| No | 36 | |||
| Yes | 11.1 | |||
| ASA score | 0.003 | 5.29 (95% CI: 2.03–13.76) | 0.001 | |
| I–II | 23.2 | |||
| III–IV | 53.3 | |||
| Tumour size | 0.215 | |||
| <3cm | 35.4 | |||
| >3cm | 27.3 | |||
| Vascular radiological affection | 0.105 | |||
| No | 32.8 | |||
| Yes | 0 | |||
| Neoadjuvant treatment | 0.322 | |||
| No | 31.5 | |||
| Yes | 0 | |||
| Consistency of the pancreas | 0.832 | |||
| Soft | 31.0 | |||
| Hard | 28.9 | |||
Bold means P<.05.
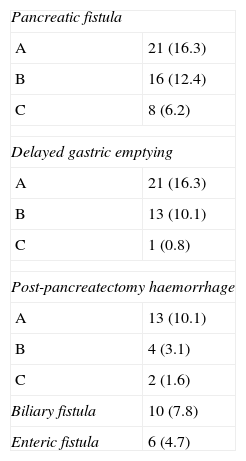
A total of 45 patients presented some type of PF, with an overall rate of 34.8%. However, nearly half of cases were a type A fistula and, therefore, had no clinical consequences. A total of 24 patients (18.6%) presented clinically significant fistulae (grades B–C). Patients with a PF grade C had a Clavien–Dindo IV–V complication in about 50% of the cases, a significant difference in relation to patients without PF or with PF type A/B (P=.024). Additionally, the presence of clinically significant PF was associated with the need for surgical re-intervention at a higher rate than the rest of the patients (41.7% compared to 5.7%; P<.001). Specifically, patients with PF grade C required re-intervention in order to perform a pancreatectomy in 6 cases (75%), whereas all the patients with PF type B were treated conservatively. Curiously, and in contrast with current published data, there was no statistical association between the apparition of PF and the diameter of the pancreatic duct (<3: 34.7% vs ≥3mm: 34.5%; P=1), pancreas consistency (soft: 31.6% vs hard: 37.7%; P=.64), the use of an internal/external transanastomotic pancreatic stent (internal: 32.1% vs external: 41.3%; P=.34), nor based on the source of the lesion (pancreatic adenocarcinoma 33.8% vs other diagnoses: 35.9%, P=.86). Interestingly, we could not find any association between these factors and a B/C grade of PF in patients with PF.
DGE was diagnosed in 35 (27.1%) of the patients, grade A in most cases, with just 1 case (0.8% of patients) of grade C DGE. The median number of days using a nasogastric tube was 4.2±3.3 days. Conversely to PF, the appearance of DGE was not related to a worse classification on the Clavien–Dindo scale, but rather to a trend of increased hospital stay, although it was not significant (no DGE: 21.2±21.0 days; DGE A: 31.2±28.6 days; DGE B: 27.8±13.7 days; DGE C: 51 days; P=ns).
PH was present in 14.7% of patients, again, with a high ratio of grade A. Probably due to the relatively low number of cases, there are no differences in the PH groups and the Clavien classification, although grade C is associated with 50% of Clavien–Dindo IV–V complications.
The presence of other anastomotic disruptions, although less common, was worth noting: there was bile leakage in 7.8% of the patients and 6 patients (4.7%) were diagnosed with leakage of the duodenojejunal anastomosis. No patient presented leaks in relation to the transection line of the stomach with this specific technique. Table 4 shows the specific pancreatic complications.
DiscussionSurgical treatment of diseases of the head of the pancreas poses a challenge to surgeons, for many reasons. They include, among others, the intrinsic complexity of the surgical procedure (even today, with a preoperative morbidity and mortality rate far higher than other abdominal procedures), a high rate of surgical complications, and, for most patients, a grim prognosis due to pancreatic adenocarcinoma.
However, morbidity after PD, particularly PF, has become one of the complications that surgeons could theoretically modify. Many factors have been suggested in the genesis of this specific complication: optimal vascularisation of the pancreatic stump, the diameter of the pancreatic duct, or pancreatic consistency. However, surgical technique remains one of the most important factors, and the debate today is how to conduct an anastomosis of the pancreatic remnant: use of the jejunum or the stomach?
Many studies have tried to address this particular issue. Specifically, 6 randomised controlled trials (RCT) of good design have been conducted during the last 20 years.10–15 Their main objective was to assess the non-inferiority of PGA compared to PJA. However, and in spite of relevant differences found in some studies, others have shown that PGA was associated with a lower rate of PF and of complications in general. In 2008, our group published the results of a RCT comparing PJA with a modified PGA, the PPPD-GP, which showed clear benefits for the latter in terms of PF.
Our aim in this study has been to describe the results of PPPD-GP outside of the limits of a RCT, and its general applicability. There are 3 main differences in this study with regard to the conditions of the RCT: the procedure was conducted by 5 different surgeons, unlike the first results, where just 1 surgeon performed all the interventions; in 63 cases, 1 external transanastomotic pancreatic duct was applied to obtain pancreatic juice for a later examination; finally, in the technique originally described, the pancreatic duct is anastomosed to an opening in the gastric mucosa of a diameter similar to that of the pancreatic duct. This technique was later modified, and the gastric mucosa opening was larger in this study, approximately 2–3cm, therefore a terminolateral pancreatogastrostomy (and not a Wirsung-gastrostomy) was conducted.
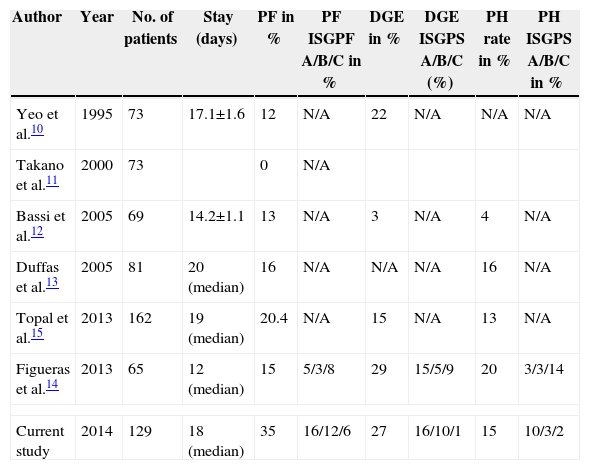
Therefore, are the PPPD-GP results comparable to other published series of PGA, which show overall better results than a PJA? A direct comparison of these series is hindered by the different diagnostic criteria applied for the classification of complications. Many of these studies were published before the adoption of the classifications by ISGPF and ISGPS, which were universally adopted, and even a recent RCT did not publish all the data relating to complications. However, the post-operative hospital stay and the rates of DGE-PH were entirely comparable to other published research samples (Table 5). As for PF, our rate is 34.8%, with a rate of clinically significant fistula of 18.6%. Analysing this data, the only 2 studies that classify PF according to the ISGFP criteria show that post-operative clinical PF is 8% (in the research by Topal et al.), and 11% in the research by Figueras et al., values close to our own. Additionally, in our analysis we have not found a correlation between this high PF rate and other published potential PF factors, such as the diameter of the Wirsung duct, a soft pancreas, the placement of an internal/external stent in the pancreatic duct, or the source of the pancreatic lesion.
Comparison Between PPPD-GP and Other Published PGA Results.
| Author | Year | No. of patients | Stay (days) | PF in % | PF ISGPF A/B/C in % | DGE in % | DGE ISGPS A/B/C (%) | PH rate in % | PH ISGPS A/B/C in % |
|---|---|---|---|---|---|---|---|---|---|
| Yeo et al.10 | 1995 | 73 | 17.1±1.6 | 12 | N/A | 22 | N/A | N/A | N/A |
| Takano et al.11 | 2000 | 73 | 0 | N/A | |||||
| Bassi et al.12 | 2005 | 69 | 14.2±1.1 | 13 | N/A | 3 | N/A | 4 | N/A |
| Duffas et al.13 | 2005 | 81 | 20 (median) | 16 | N/A | N/A | N/A | 16 | N/A |
| Topal et al.15 | 2013 | 162 | 19 (median) | 20.4 | N/A | 15 | N/A | 13 | N/A |
| Figueras et al.14 | 2013 | 65 | 12 (median) | 15 | 5/3/8 | 29 | 15/5/9 | 20 | 3/3/14 |
| Current study | 2014 | 129 | 18 (median) | 35 | 16/12/6 | 27 | 16/10/1 | 15 | 10/3/2 |
Since the publication of the positive results of the RCT conducted in our institution, PPPD-GP has been adopted as the surgical procedure of choice. This study shows the results obtained by the application of this surgical technique, particularly on a day-to-day basis. Since some factors were not present at the time that the RCT was conducted, and the setting was not so controlled, we present them here as the actual data for pancreatic surgery in our institution. In our opinion, PPPD-GP offers other advantages over PJA, mainly since pancreatic anastomosis is not in direct contact with the intestinal tract, with a much lower degree of bacterial contamination in the event of disruption, and is easily manageable in the event of surgical re-intervention due to a clinically relevant PF. This allows for a review of a technically easy pancreatic anastomosis and, if necessary, a relatively simple application of a pancreatectomy. Additionally, compared to a conventional PGA, the advantage of a PPPD-GP is that the gastric partition area is relatively removed from the food transit, and is therefore easier to manage in the event of severe complications.
In conclusion, the results of PPPD-GP in a real-world scenario are essentially comparable to those published in research and obtained within the framework of a RCT, and may constitute a valuable resource for pancreatic surgeons.
Conflict of InterestThe authors state that there are no conflicts of interest.
Please cite this article as: Sánchez Cabús S, Saavedra D, Sampson J, Cubel M, López-Boado MÁ, Ferrer J, et al. Resultados de la pancreatogastroanastomosis con bipartición gástrica después de duodenopancreatectomía con preservación pilórica. Cir Esp. 2015;93:502–508.