The oral cavity represents the gateway to the complex digestive system, so the knowledge of the exact mechanisms that link them is vitally important. Recently, oral and dental pathologies have been studied as potential risk factors for pathologies linked to lifestyle habits. Therefore, it could be considered as an interesting preventive way.
We conducted a narrative review with a thorough bibliographic search on MEDLINE and SCOPUS, including international studies related to oral healthcare and gastrointestinal neoplasms, published between 2015 and 2020.
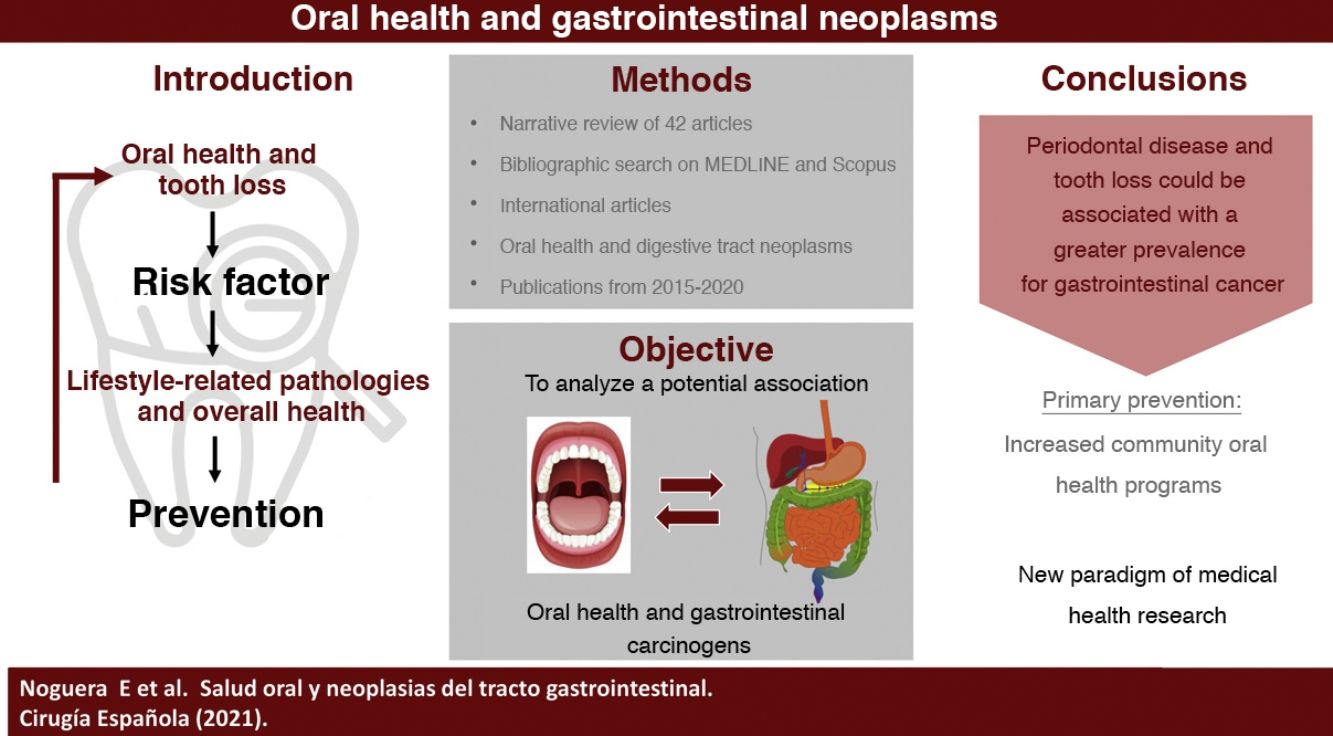
The primary aim of this revision is to analyze the association between oral healthcare and carcinogenic gastrointestinal processes, providing a possible future preventive strategy for dental care. Moreover, we intend to raise awareness about the importance of oral healthcare as a new paradigm and study variable in the global health care system.
La cavidad oral constituye la puerta de entrada al complejo sistema digestivo, por lo que el conocimiento de los mecanismos que los vinculan es de vital importancia. En los últimos años la patología dental y oral ha sido analizada como potencial factor de riesgo de enfermedades vinculadas al estilo de vida y, por tanto, se está considerando como una posible interesante vía de prevención.
Realizamos una revisión narrativa con búsqueda bibliográfica exhaustiva en las bases de datos MEDLINE y SCOPUS, incluyendo artículos internacionales que relacionan la salud oral con neoplasias del tracto digestivo, publicados entre 2015 y 2020.
El objetivo de esta revisión es analizar la evidencia existente sobre la potencial asociación entre salud bucodental y procesos carcinogénicos del tracto gastrointestinal, proporcionando una posible futura vía de prevención a nivel odontológico. Como objetivo secundario, se fomenta concienciar sobre la importancia de la salud oral como nuevo paradigma y variable de estudio en el ámbito de la investigación médico-sanitaria.
It is tremendously important to recognize the impact of oral health, as well as progressive tooth loss, on systemic patient health. Recently, oral pathology has been the subject of numerous studies as a possible risk factor for lifestyle-related pathologies, and oral health is also being considered a new part of prevention.1,2
In recent years, numerous systemic diseases (cardiovascular, neurodegenerative, digestive, certain types of cancer, etc) have been etiologically associated with dental and oral pathologies.1,3–5
Until now, the potential carcinogenic effect of the oral microbiota had not been clearly determined, and, although it was etiologically associated with head-neck cancer (especially oral cancer),6,7 its possible relationship with more distant organs was only a mere possibility.4 Currently, the most widespread and universal method to analyze the different oral microorganisms is genomic sequencing using 16S rRNA (a component of the minor subunit of prokaryotic ribosomes, used for the reconstruction of phylogenies), which has allowed recent studies to begin to explore the different repeated bacterial patterns of beneficial and/or harmful microorganisms.6
In order to make the analysis applicable to more areas, many assays are based mainly on the intestinal microbiota, which is the most extensive community in the body, made up of trillions of resident microorganisms.2,8 It is evident that, during the performance of physiological functions, such as chewing or swallowing, oral microorganisms pass directly to the digestive system.7 Thus, the oral cavity is the entrance to the digestive system. A state of oral dysbiosis due to the deterioration of oral health affects the inflammatory and immunological response, playing a fundamental role in patients with gastrointestinal tract tumors.4 Clinically, this fact allows us to open an evolutionary door towards the identification, prevention and treatment of gastrointestinal neoplasms.2,4,6
The main objective of this review is to analyze the existing evidence on the potential association between oral health and carcinogenic processes of the gastrointestinal tract, establishing a possible way of prevention through the promotion of oral health. As a secondary objective, we discuss the importance of oral health as a new paradigm and study variable in the field of medical health research.
MethodsSearch strategyWe conducted a narrative review with a thorough bibliographic search of articles published in the last 5 years (2015–2020) that related oral health to digestive tract neoplasms.
The systematic search was carried out using the MEDLINE and SCOPUS databases, incorporating the MeSH (Medical Subject Headings) terms or keywords: “digestive system neoplasms”, together with the Boolean operator “AND” and adding the terms “mouth”, “oral health”, “tooth loss”.
Article selection processIn order to filter and screen the articles that best suited the review, we initially included studies focused on the relationship between digestive neoplasms and oral health that had been published in English between 2015 and 2020, which were available in full text or belonging to journals accessible to the Universitat de Barcelona. Studies conducted with animal samples were excluded; only human samples were included, without considering the age variable. Regarding the level of evidence, we evaluated prospective cohort studies, case-control studies, in vitro studies, both systematic and narrative reviews, and meta-analyses.
Subsequently, in a second selection phase, which consisted of reading titles and abstracts, we discarded from our review those articles that did not focus on the topic at hand or presented a low level of impact.
Finally, after a complete and exhaustive reading of the bibliography, 43 articles were included: 13 cohort studies, 14 case-control studies, 8 narrative reviews, 2 systematic reviews, 5 meta-analyses, and 1 in vitro study (Fig. 1).
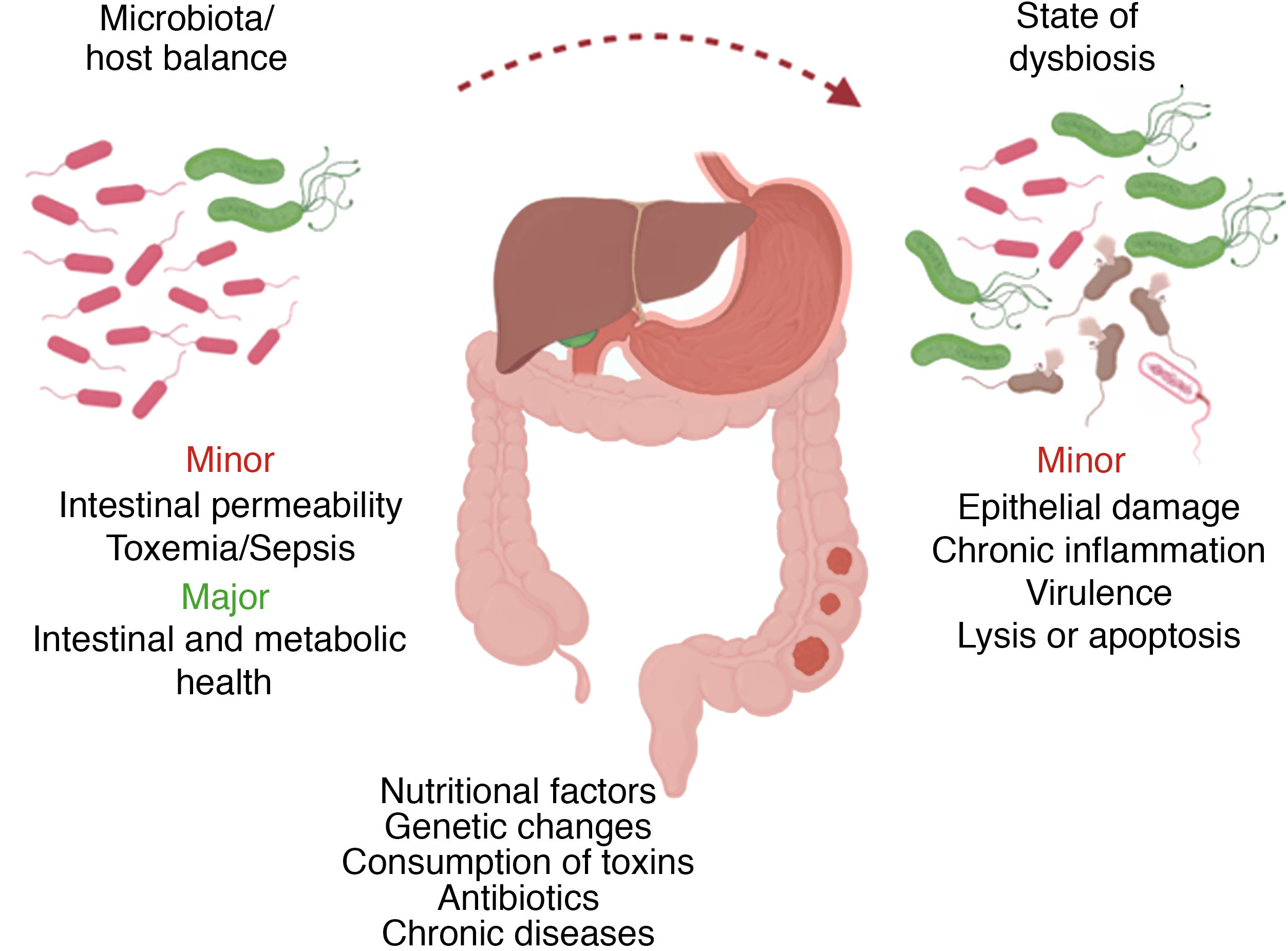
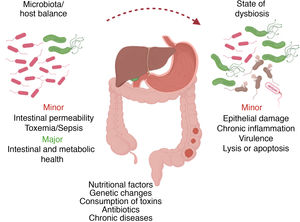
Results and discussionOral microbiota: pathological processesTogether as a whole, the array of oral microorganisms (more than 700 different species) is already recognized as a true organ, whose mechanisms are directly associated with the digestive microbiological ecosystem.6,8–11 In a microbiological state of homeostasis with the host, certain alterations in the oral microbiota can induce susceptibility to pathological changes, both internal (e.g., genetic) and external (e.g., diet, antibiotic therapy, toxins).2
Certain changes in the bacterial composition can induce the appearance of opportunistic pathogens that could have harmful effects on the host, such as direct epithelial damage, inflammatory response and virulence factors, while also influencing cell apoptosis processes2 (Fig. 2). This microbiological dysbiosis is more frequent and accentuated in patients who develop gastrointestinal tumors, specifically esophageal, gastric, pancreatic and colorectal.6
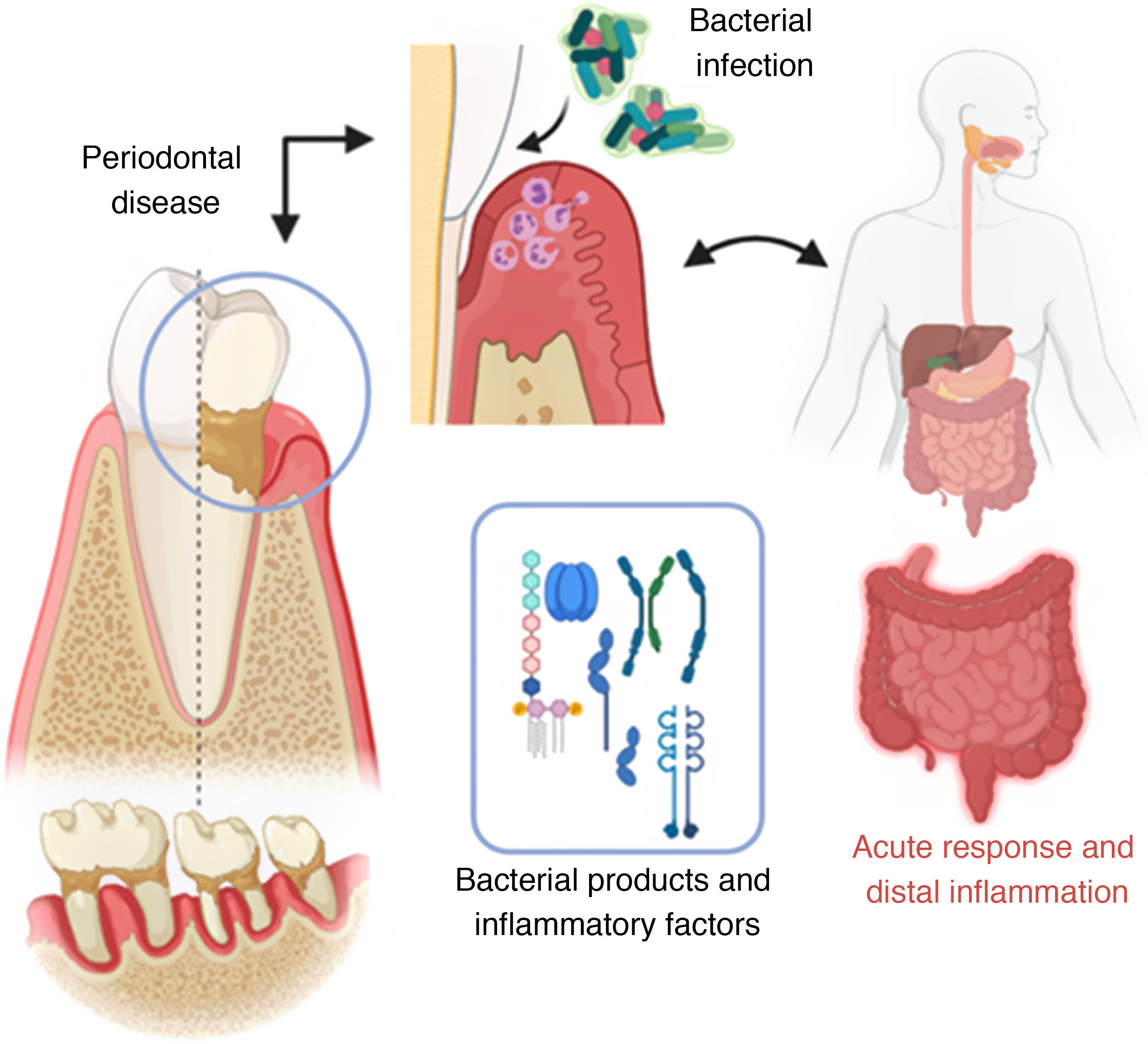
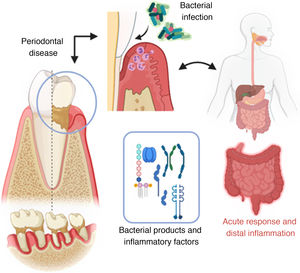
Mechanism of invasion of periodontal pathogenic bacteria and posterior systemic inflammation (created with BioRender; modified from Konkel et al.5).
The analysis by Le Bars et al.8 summarizes that, at the mucosal level, the gastrointestinal and/or oral microbiota could be progressively altered by the processes of mucosal reduction of CD4+, chronic inflammation, altered epithelial enterocytes and, finally, by tissue penetration of microorganisms. From the dental plaque, certain bacteria can directly activate carcinogens, such as nitrosamines and acetaldehyde (from the diet and/or tobacco), establishing this as another possible hypothesis for the initiation of malignant processes.4,12–14 However, differentiating harmless bacterial taxonomies from pathogenic ones continues to be a challenge; the same is true for defining the microbiological mechanism associated with their invasive properties, expression, and virulence factors.6,8,15
Periodontal disease and associated systemic pathologyPeriodontitis begins with ulceration of the gingival epithelium, bacterial invasion and/or alteration, and finally by colonization of immune cells, leading to a state of inflammation that destroys the periodontium (alveolar bone, root cementum, periodontal ligament and gingiva).5 Once pro-inflammatory products derived from periodontal disease are found in the systemic circulation (lipopolysaccharides, interleukins IL-17, IL-6, IL-1β and TNF-α), they can directly (in situ) or indirectly affect in the inflammatory response of distal organs.5,16
Tooth loss and oral healthIn recent years, several studies have been published about different oral health parameters that could favor the appearance of malignant lesions in the digestive tract.3,6,7,12,13,16–19 An example is the study by Sakai et al.,3 which observed that tooth loss (<20 remaining teeth) and/or the lack of prostheses were associated with an increased risk of digestive cancer, being more significant in patients between 60 and 70 years of age. No differences were found in terms of the location of the neoplasm. It should be noted that the authors consider the socioeconomic conditions and the level of education of the included patients as limitations or confounding factors, indicating that they greatly affect individual medical treatment and access to healthcare institutions in general.
Globally, dental diseases are often the costliest for many healthcare systems.1,3 The Japanese study by Saito et al.1 reported that the healthcare cost for several diseases increased for elderly patients who had <28 teeth or were edentulous. In the case of digestive tumors, patients with <19 teeth accounted for a higher public health expenditure, in addition to an increase in days of hospitalization during the one-year follow-up. In this way, the maintenance of as many teeth as possible is described as a probable protective factor.
Epidemiological cofactors associated with digestive cancerThe relationship between dental diseases and the prevalence of gastrointestinal tract neoplasms has not been clearly determined, and it may be due to various factors.3,7,14,18–21 The association between certain nutritional patterns and lifestyle-related parameters with an elevated risk of developing certain types of cancer is widely debated.22–25 Furthermore, a large number of factors are shared with periodontal disease, hence the importance of the estimated risk adjustment for each.3,14
The current studies on the most frequent gastrointestinal neoplasms are analyzed below by sections and related to oral disorders.
Esophageal cancerThree20,26,27 of the 12 articles included on esophageal neoplasms focus on their etiology and modifiable factors, including tooth loss and/or altered oral health status. Tobacco or alcohol consumption alone do not explain the high volume of esophageal squamous carcinoma in the most prevalent regions.18,28
The epidemiological study by Abnet et al. aimed to identify possible risk factors associated with squamous esophageal carcinoma, and the authors argue that its etiology is multifactorial and characteristic of each population. They also suggest the potential role of inflammation associated with periodontal disease and the production of certain metabolites by periodontopathogenic bacteria, such as acetaldehydes and/or nitrites.20,26
In northern Iran (which has the highest incidence rates of this type of neoplasm), the study by Sheikh et al.20 determined that 76% of cases are also attributed to causal factors, one of them being the progressive tooth loss associated with the widespread cultural tendency not to conserve diseased teeth.
Another study carried out in Eldoret (Kenya) concluded that an altered state of oral health, added to or as a result of a high consumption of fluoridated water (concentrations greater than 1.5 mg/L) in areas such as Tanzania and Ethiopia could be one of the major causes of the high number of cases (especially squamous cell esophageal cancer) accumulated in the African Rift Valley. This is an example of the importance of primary and palliative prevention against a pathology that is the third cause of mortality in these specific areas (Menya et al.).27
Four of the analyzed studies7,13,18,21 defend that tooth loss and lack of oral hygiene play important roles in the progression of esophageal cancer, notably in Asian countries. Even so, the groups studied are heterogeneous and complex in order to draw solid conclusions.21 These factors should be considered as markers rather than causal indicators per se.7
Both the study by Tanda et al.29 and the Mizuno et al.30 study advocate the importance of reducing the bacterial load and professional preoperative oral treatment using benzalkonium chloride (antiseptic), interdental brushes and hydrogen peroxide to reduce acetaldehyde levels. Mizuno et al.30 compared two groups of patients treated for esophageal neoplasia and observed that, in the presence of a similar number of postoperative complications, the group with the new oral hygiene regimen significantly reduced the number of oral bacteria and days with high fever compared to the group with routine oral hygiene. However, this study has limitations, such as the small number of patients from a single center and the lack of relationship between high fever and the number of oral bacteria. Therefore, further large-scale studies are needed to corroborate the observed results.
Microbiota and esophageal cancerSaliva samples collected using a 16S rRNA genomic sequence for the case-control study by Chen et al.28 showed less diversity in microbiological composition in patients with squamous esophageal carcinoma. However, further research and characterization of pathogens is needed.
The prospective study by Peters et al.31 of patients diagnosed with esophageal cancer showed different groups of microorganisms for adenocarcinoma compared to squamous cell cancer. The authors propose a meticulous study at the microbiological level to identify the presence of certain characteristic species through the 16S genetic sequence, emphasizing its potential preventive role and individual risk stratification for this type of tumors.
Gastric cancerGastric cancer is the third leading cause of mortality associated with neoplasms in the world. The poor prognosis it presents highlights the importance of its prevention.32
In the study by Ndegwa et al.,12 tooth loss (especially for young patients) and mucosal lesions related with dental prostheses are associated with an increased risk of gastric neoplasm (the results are not consistent for lesions caused by Candida, dental plaque or tongue lesions). In the analysis by Zhang et al.,13 severe tooth loss is related with increased mortality. Although more studies are needed, the review by Yin et al.32 suggests that tooth loss is a potential risk marker for gastric cancer.
Microbiota and gastric cancerThe study by Sun et al.33 conducted in 35 patients with precancerous gastric cancer lesions and 70 control subjects concluded that Treponema forsythia, Treponema denticola, and Actinomyces actinomycetemcomitans were risk microorganisms for gastric cancer. The study also considered factors like the bacterial diversity present in dental plaque and/or lack of hygiene in interproximal dental areas.
Likewise, the lingual mucosa was used to determine the microbiological characteristics (bacterial and fungal) of the 115 patients with gastric cancer in the study by Xu et al.,9 establishing an oncological biomarker through the commensal relationships found there. However, it is necessary to identify the specific microorganisms that recur.
In Japan, Kawano et al.34 described the bacteriological differences found between gastric cancer patients treated by open surgery, comparing them with others who received minimally invasive treatment. Even with daily oral care, the number of bacteria in patients who received open surgery remained high, especially in the anterosuperior gingival area. The study indicates the need for effective preoperative techniques to reduce the number of bacteria, as well as to monitor the presence of gingivitis.
We should also highlight the role of Helicobacter pylori in gastric tumour processes, as it is already considered a potential carcinogen and biomarker, acting in the presence of a specific microbiota and defined luminal pH.9,11,12,14,17,32,33
Colorectal cancerThe study by Lee et al.35 shows that periodontal disease and/or tooth loss is a risk factor for colorectal adenoma, being more accentuated in men, smokers (10–20 pack-years) or frequent drinkers of alcohol. All this suggests the potential application of preventive measures and risk stratification, including strategies such as periodic dental X-rays and check-ups.
Along the same lines, the study by Momen-Heravi et al.16 also wanted to investigate the role of periodontal disease and its possible impact on colorectal cancer. In the 77 443 women studied for 18 years, it was shown that tooth loss (less than 17 remaining teeth) and the presence of periodontitis alter and increase systemic inflammation, immune dysfunction and changes in bacterial composition, thereby increasing the risk of colorectal cancer. The result was an increased probability (48% higher) in women with bone loss (suggestive characteristic) and periodontal disease, regardless of other associated factors.
However, there are also contradictions to the previous results. In the case of the meta-analysis by Ren et al.,36 no association was found between tooth loss, periodontitis, and progression of colorectal cancer. The evidence is not sufficiently conclusive, in addition to possibly being altered by cofactors.
Another meta-analysis by Almeida et al.37 indicates the relationship between familial adenomatous polyposis with some manifestations of oral pathology, mandibular alterations being the most frequent (65.35%). These patients often present with compound odontomas, supernumerary teeth, unerupted teeth, and oral mucosa abnormalities (30.48%). The aforementioned findings could become precursors of colorectal cancer in patients when the disease is not treated or diagnosed, thus establishing another preventive measure for the pathology.
Oral microbiota and colorectal cancerFlemer et al.11 investigated the bacterial taxonomies that are possibly involved in the development of colorectal cancer. The result of their study was a classification of different oral and fecal microorganisms in diagnosed and healthy subjects, detecting a similar composition between oral and colorectal mucosa. The Lachnospiraceae genus showed a protective role, possibly mediated by an adequate diet. This fact could improve the screening and characterization of new diagnostic programs.
Three other studies maintain the same line of research. First is the study by Brennan et al.,38 which investigated the role of Fusobacterium nucleatum in the development of opportunistic infections that can affect colorectal cancer. This is also usually present in the oral cavity; therefore, it is established as a possible promoter of a good state of oral health, as well as an initiator of periodontal processes. More studies are needed on its mechanism of action and possible role in carcinogenesis.
Second, Koliarakis et al.2 support the possible spread of oral bacteria in the colon. This association, according to them, is more evident for the microorganisms Fusobacterium nucleatum and Porphyromonas gingivalis (periodontopathogens), which are able to induce dysbiosis processes that promote inflammatory and immunological changes. Their correlations, however, require knowledge of the exact mechanism of action that governs them.
Finally, Yang et al.39 conducted a study based on African-American subjects (75% of the participants), using 16S rRNA gene sequencing to ultimately differentiate between beneficial microorganisms (Treponema denticola, Prevotella intermedia, Prevotella denticola and Bifidobacteriaceae) and harmful ones (Prevotella melaninogenica, Firmicutes, Carnobacteriaceae, Streptococcaceae, Erysipelotrichaceae, Streptococcus, Solobacterium, Streptococcus sp. and Solobacterium moorei) for colorectal cancer. The conclusion was the need to establish the role of the oral microbiota in the etiology of colon neoplasms.39
Liver cancerStudies by Thistle et al.40 and Yang et al.17 demonstrated an increased risk of liver neoplasm in patients who had lost a large number of teeth. The studies were conducted respectively in a rural, socioeconomically disadvantaged Chinese population (329 cases diagnosed over 30 years), where stronger results were obtained in women with obesity and in a cohort of male smokers from Finland (213 incident cases in the 17-year follow-up).
The Finnish study17 specified that the risk increased in patients who lost between 11 and 31 permanent teeth, or in completely edentulous patients. It would also be necessary to take into account the seropositive presence of H. pylori as a cofactor.
Contrary to the two previous studies, Jordão et al.19 wanted to study the controversial relationship between impaired oral health (gingivitis, periodontitis, tooth loss) and the risk of developing gastrointestinal cancer. After following a UK cohort of 475 766 patients for 6 years, they concluded that there was no definite association, although a trend towards increased risk of hepatobiliary cancer was observed, specifically hepatocellular carcinoma. The study argues that there is significant variation between geographic locations of cancer risk and oral health.
Microbiota and liver cancerThe study by Lu et al.43 characterized the oral microbiota from the lingual mucosa of patients with liver neoplasm, performed by gene sequencing of 16S ribosomal RNA. The study included 35 cases and 25 healthy subjects, and the authors concluded that there was a notable difference in the microbiological composition, with the presence of Oribacterium and Fusobacterium as distinctive microorganisms in patients with liver disease.
Pancreatic cancerThe meta-analysis by Maisonneuve et al.,14 which includes 8 articles, demonstrated an association between two oral pathologies (periodontitis and edentulism) and an increased risk of pancreatic cancer (even when adjusting for other cofactors). However, the mechanisms by which it occurs are not yet determined.
Oral microbiota and pancreatic cancerFour of the studies4,10,41,42 analyzed the microbiological role in pancreatic cancer.
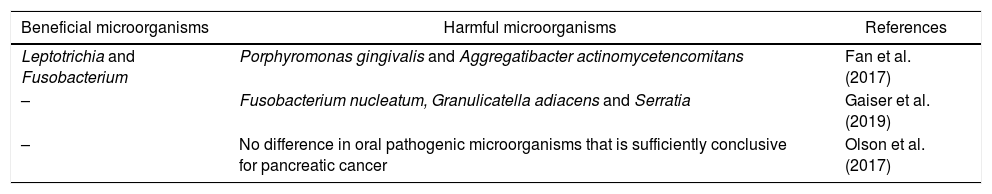
The Fan et al.10 study analyzed new methods to develop faster, non-invasive and accessible mechanisms to identify potential patients at risk. The result was the microbiological genomic characterization of oral samples, showing an association of increased and decreased risk in the taxonomies presented in Table 1.
Summary of bacterial taxonomies for pancreatic cancer.
| Beneficial microorganisms | Harmful microorganisms | References |
|---|---|---|
| Leptotrichia and Fusobacterium | Porphyromonas gingivalis and Aggregatibacter actinomycetencomitans | Fan et al. (2017) |
| – | Fusobacterium nucleatum, Granulicatella adiacens and Serratia | Gaiser et al. (2019) |
| – | No difference in oral pathogenic microorganisms that is sufficiently conclusive for pancreatic cancer | Olson et al. (2017) |
The studies by Gaiser et al.41 and Olson et al.42 related oral bacteria or poor oral hygiene with intraductal papillary mucinous neoplasms present in premalignant cystic precursors suggestive of cellular dysplasia.
The hypothesis of Gaiser et al.41 was that certain genera of oral bacteria could initiate oncogenic processes from immunological modulation and through interleukin-1β, collaborating in synergy with mucinous papillae and thus increasing mucin production. The genomic characterization of intracystic bacteria could open a new etiopathological management of these alterations. Any measure that contributes to reducing pancreatic inflammation is a therapeutic tool to reduce cases of intraductal neoplasms, so a possible route through antibiotic therapy is being studied.
In contrast, the Olson et al.42 study did not obtain any differences strong enough to correlate cases of pancreatic ductal adenocarcinomas with premalignant mucinous lesions and an unfavorable state of oral health, considering the role of oral microbiota. In short, there is not a great difference between the oral bacterial composition of healthy cases and control subjects; only specific taxonomies show such differences. More specific studies are necessary.
LimitationsThe results presented in this review are heterogeneous and specific for each type of cancer, population and/or sample chosen. The lack of agreement between studies may mainly be due to the difference in the sample size, demographics of the populations studied, delimitation of the concept of periodontal disease, follow-up period and proposed inclusion criteria. It is necessary to define a clear etiology with an effective diagnosis, characterize the different digestive neoplasms, and create a good primary prevention mechanism at the community level.
ConclusionsThe high incidence associated with the lack of precise diagnoses and limited therapeutic options for some gastrointestinal neoplasms explains the fundamental need for complete primary prevention. Periodontal disease, tooth loss and the lack of use of dental prostheses could be associated with a higher prevalence of certain types of gastrointestinal cancer. This fact is mainly based on altered inflammatory and immune processes due to a state of microbiological dysbiosis. Consequently, microbiological genomic characterization from oral samples may show promise as a future non-invasive diagnostic route.
It would be necessary to promote oral health at the community level and perform the dental treatments required by each particular patient, while trying to preserve as many teeth as possible. More studies are also needed to verify and try to homogenize the different study parameters of oral health and digestive cancer.
Conflict of interestsThere are no conflicts of interests.
Please cite this article as: Noguera E, Sorribas M, Admella V, Biondo S. Salud oral y neoplasia gastrointestinal. Revisión narrativa. Cir Esp. 2021;99:716–723.
This review is based on a final degree project (Odontology Degree) presented to the School of Medicine and Health Sciences at the Universidad de Barcelona.