Multimodal rehabilitation programs are perioperative standardized strategies with the objective of improving patient recovery, and decreasing morbidity, hospital stay and health cost.
The nutritional aspect is an essential component of multimodal rehabilitation programs and therefore nutritional screening is recommended prior to hospital admission, avoiding pre-surgical fasting, with oral carbohydrate overload and early initiation of oral intake after surgery. However, there are no standardized protocols of diet progression after pancreatic surgery.
A systematic review was been performed of papers published between 2006 and 2016, describing different nutritional strategies after pancreatic surgery and its possible implications in postoperative outcome.
The studies evaluated are very heterogeneous, so conclusive results could not be drawn on the diet protocol to be implemented, its influence on clinical variables, or the need for concomitant artificial nutrition.
Los programas de rehabilitación multimodal precoz son estrategias estandarizadas perioperatorias con el objetivo de mejorar la recuperación del paciente, disminuir las complicaciones, la estancia hospitalaria y el coste sanitario.
El aspecto nutricional es un componente esencial de la rehabilitación multimodal precoz, recomendándose realizar un cribado nutricional previo al ingreso hospitalario, evitar el ayuno prequirúrgico mediante una sobrecarga oral de hidratos de carbono, e iniciar de manera precoz la ingesta oral posquirúrgica. Sin embargo, no existen protocolos estandarizados de progresión de dieta en cirugía pancreática.
Se realiza una revisión de las diferentes estrategias nutricionales publicadas desde 2006 hasta 2016 en la rehabilitación multimodal precoz de este tipo de cirugía y sus posibles implicaciones en la evolución postoperatoria.
Los estudios evaluados son muy heterogéneos por lo que no se pueden extraer resultados concluyentes sobre el protocolo de dieta a implementar, su influencia en variables clínicas ni la necesidad o no de nutrición artificial concomitante.
Recent research in surgery is focused on lowering perioperative surgical stress in order to reduce postoperative complications and achieve better and faster patient recovery, shorter hospitalization stay and lower healthcare costs.1 In this context, “enhanced recovery after surgery” (ERAS) programs have been created. These multimodal postoperative accelerated recovery programs, also known as “fast track programs”, include standardized care strategies and multidisciplinary approaches, such as nutritional evaluation and therapy.2 These nutritional strategies include the elimination of preoperative fasting, preoperative oral carbohydrate overload and the early postoperative establishment of oral intake.3 The application of these protocols in pancreatic surgery is more complex because of the greater difficulty of the surgical techniques and the elevated postoperative morbidity.4
Among the most frequent complications after pancreatic surgery are delayed gastric emptying (25%–50%), the appearance of fistulae5 (pancreatic, biliary or gastrointestinal) and postoperative infections, which can influence the nutritional status of patients by increasing nutritional requirements and complicating oral nutrition; total or complementary artificial nutrition is frequently needed. In addition, the possible appearance of multifactorial diabetes mellitus (insulin resistance due to postoperative stress, insulinopenia, use of somatostatin analogs, etc.) makes the management of these patients even more complex.
The prevalence of malnutrition in oncologic patients before undergoing surgical procedures is high. In pancreatic surgery, the percentage can be even higher,6 so preoperative nutritional screening is recommended in these patients. The ESPEN7 guidelines recommend (with the highest grade of evidence) nutritional support for 10–14 days before surgery in severely malnourished patients (at least one of the following parameters: weight loss of 10%–15% in 6 months; BMI<18.5kg/m2; serum albumin <3g/dL, subjective overall assessment grade C), and even delayed surgery if necessary.8
Another determining aspect is preoperative overloading with carbohydrates. It is not necessary to fast before surgery, so solid foods may be eaten up to 6h before surgery, and liquids up to 2h before. This elimination of fasting does not increase the residual gastric volume, and in general more episodes of vomiting or pulmonary aspirations have not been observed. As advantages, patient anxiety is reduced with decreased thirst and hunger, improving the sensation of wellbeing and also improving the immune function. This reduces insulin resistance and the loss of nitrogen and muscle mass, thereby enabling patients to recover more quickly.9,10
An essential pillar in the ERAS protocol is the early initiation of oral intake within the first 24h. Conventionally, it was recommended to maintain nil per os until the appearance of intestinal sounds and elimination of gases and/or feces to avoid nausea, vomiting, ileus or anastomotic leak. Nonetheless, it has been observed that the initiation of early intake and its progression is feasible and safe within a multimodal protocol favoring it.11 However, the high incidence of gastroparesis in pancreatic surgery means that oral intake should progress with caution; it may even need to be suspended and a nasogastric tube placed in certain patients. Another factor that could delay or create difficulties for oral tolerance is the appearance of pancreatic fistulae, and artificial nutritional support would also need to be assessed in these cases.2
The particularities of pancreatic surgical procedures and complications mean that the ERAS nutritional protocols described in other surgical procedures, such as colorectal surgery, should be adapted and specific strategies are required. As there are no standardized protocols about the nutritional aspects of ERAS after pancreatic surgery, we completed a review of the literature.
MethodologySeveral medical and scientific databases were consulted, including Pubmed, The Cochrane Library and Medline. The search was designed to identify material published between 2006 and 2016 in English and in Spanish. The keywords used, in all possible combinations, were: pancreas, pancreatic surgery, pancreaticoduodenectomy, enhanced recovery after surgery, fast track, nutrition, nutritional status, enteral nutrition, parenteral nutrition, malnutrition and clinical pathway, combining them with connectors and/or using synonyms of these terms. Furthermore, we reviewed the bibliography of other systematic reviews or meta-analyses12–14 found after the initial search.
The studies chosen were those that met the following criteria: studies carried out in adults who had undergone pancreatic resection; those that included description of an ERAS protocol, as well as the progression of postoperative oral intake; and evaluation of at least one of the following results: compliance with the nutritional protocol, mean hospital stay, morbidity, mortality and rate of re-hospitalization. Excluded from the review were studies about urgent surgery, descriptive studies and systematic reviews.
The primary response studied was the evaluation of oral intake progression. The secondary responses were: adherence to the protocol for postoperative oral diet progression, morbidity, mortality, rate of re-hospitalization, incidence of delayed gastric emptying and mean hospital stay.
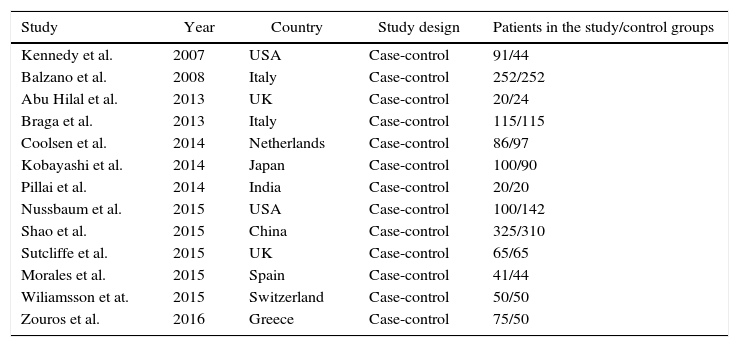
ResultsA total of 1315–27 studies were obtained that met the previously described criteria and whose main characteristics can be observed in Table 1. No studies designed as clinical trials were found. All the studies included had a case-control design and the type of surgery that was completed was pancreaticoduodenectomy.
Study Characteristics.
| Study | Year | Country | Study design | Patients in the study/control groups |
|---|---|---|---|---|
| Kennedy et al. | 2007 | USA | Case-control | 91/44 |
| Balzano et al. | 2008 | Italy | Case-control | 252/252 |
| Abu Hilal et al. | 2013 | UK | Case-control | 20/24 |
| Braga et al. | 2013 | Italy | Case-control | 115/115 |
| Coolsen et al. | 2014 | Netherlands | Case-control | 86/97 |
| Kobayashi et al. | 2014 | Japan | Case-control | 100/90 |
| Pillai et al. | 2014 | India | Case-control | 20/20 |
| Nussbaum et al. | 2015 | USA | Case-control | 100/142 |
| Shao et al. | 2015 | China | Case-control | 325/310 |
| Sutcliffe et al. | 2015 | UK | Case-control | 65/65 |
| Morales et al. | 2015 | Spain | Case-control | 41/44 |
| Wiliamsson et at. | 2015 | Switzerland | Case-control | 50/50 |
| Zouros et al. | 2016 | Greece | Case-control | 75/50 |
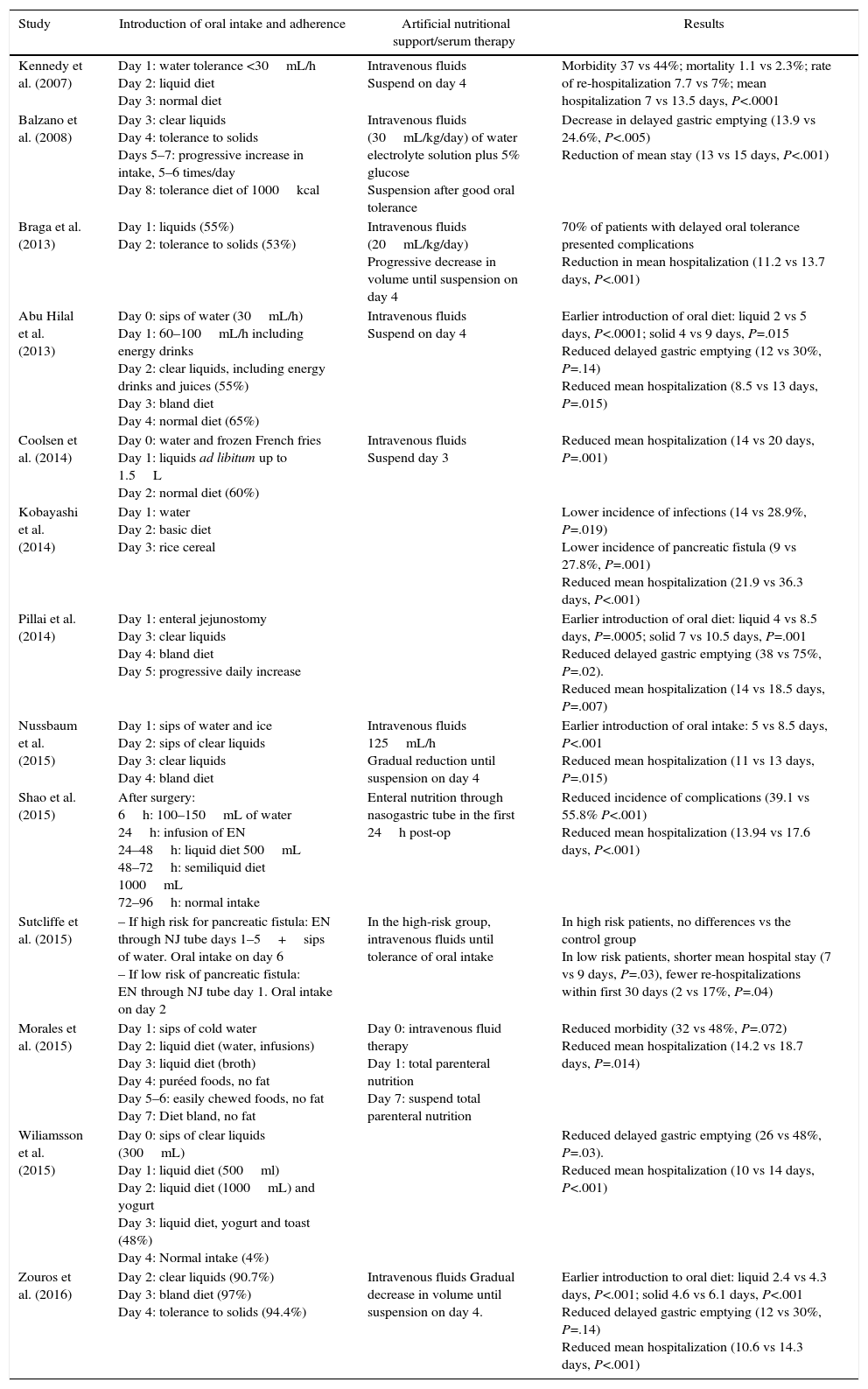
From the studies analyzed, we observed that there was no standard protocol for the progression of oral intake, as shown in Table 2. Braga et al.18 carried out rapid progression of intake by initiating oral tolerance to liquids on the first day post-op and to solids on the second day post-op. Following a similar protocol, Coolsen et al.19 proposed initiating tolerance to fluids the same day of surgery and ingesting normal diet on the second day post-op. However, other authors used a slower progression of oral intake. Balzano et al.16 did not initiate oral tolerance until the third day post-op and to solids on the on the fourth day after surgery, reaching an intake of 1000 calories on the eighth day. Other authors proposed the progression of oral intake together with the administration of artificial nutrition: Morales et al.25 used total parenteral nutrition until the seventh day post-op; Sutcliffe et al.24 and Shao et al.23 used enteral nutrition. In addition, Sutcliffe et al.24 presented a protocol of oral intake progression that was different for patients at high risk for developing a pancreatic fistula. In this group, they proposed a more conservative protocol in which enteral nutrition is prolonged until the fifth day after surgery, combined with water intake alone. Nonetheless, for patients at low risk, they proposed maintaining enteral nutrition only the first 24h post-op and initiating oral diet the second day. The remaining studies proposed intermediate regimens between those reported in which intake is initiated between days 2 and 3, progressing to complete oral intake on days 4 and 5.17,21,22,26,27
Models of Oral Intake Progression in Each Study and Main Results Obtained.
| Study | Introduction of oral intake and adherence | Artificial nutritional support/serum therapy | Results |
|---|---|---|---|
| Kennedy et al. (2007) | Day 1: water tolerance <30mL/h Day 2: liquid diet Day 3: normal diet | Intravenous fluids Suspend on day 4 | Morbidity 37 vs 44%; mortality 1.1 vs 2.3%; rate of re-hospitalization 7.7 vs 7%; mean hospitalization 7 vs 13.5 days, P<.0001 |
| Balzano et al. (2008) | Day 3: clear liquids Day 4: tolerance to solids Days 5–7: progressive increase in intake, 5–6 times/day Day 8: tolerance diet of 1000kcal | Intravenous fluids (30mL/kg/day) of water electrolyte solution plus 5% glucose Suspension after good oral tolerance | Decrease in delayed gastric emptying (13.9 vs 24.6%, P<.005) Reduction of mean stay (13 vs 15 days, P<.001) |
| Braga et al. (2013) | Day 1: liquids (55%) Day 2: tolerance to solids (53%) | Intravenous fluids (20mL/kg/day) Progressive decrease in volume until suspension on day 4 | 70% of patients with delayed oral tolerance presented complications Reduction in mean hospitalization (11.2 vs 13.7 days, P<.001) |
| Abu Hilal et al. (2013) | Day 0: sips of water (30mL/h) Day 1: 60–100mL/h including energy drinks Day 2: clear liquids, including energy drinks and juices (55%) Day 3: bland diet Day 4: normal diet (65%) | Intravenous fluids Suspend on day 4 | Earlier introduction of oral diet: liquid 2 vs 5 days, P<.0001; solid 4 vs 9 days, P=.015 Reduced delayed gastric emptying (12 vs 30%, P=.14) Reduced mean hospitalization (8.5 vs 13 days, P=.015) |
| Coolsen et al. (2014) | Day 0: water and frozen French fries Day 1: liquids ad libitum up to 1.5L Day 2: normal diet (60%) | Intravenous fluids Suspend day 3 | Reduced mean hospitalization (14 vs 20 days, P=.001) |
| Kobayashi et al. (2014) | Day 1: water Day 2: basic diet Day 3: rice cereal | Lower incidence of infections (14 vs 28.9%, P=.019) Lower incidence of pancreatic fistula (9 vs 27.8%, P=.001) Reduced mean hospitalization (21.9 vs 36.3 days, P<.001) | |
| Pillai et al. (2014) | Day 1: enteral jejunostomy Day 3: clear liquids Day 4: bland diet Day 5: progressive daily increase | Earlier introduction of oral diet: liquid 4 vs 8.5 days, P=.0005; solid 7 vs 10.5 days, P=.001 Reduced delayed gastric emptying (38 vs 75%, P=.02). Reduced mean hospitalization (14 vs 18.5 days, P=.007) | |
| Nussbaum et al. (2015) | Day 1: sips of water and ice Day 2: sips of clear liquids Day 3: clear liquids Day 4: bland diet | Intravenous fluids 125mL/h Gradual reduction until suspension on day 4 | Earlier introduction of oral intake: 5 vs 8.5 days, P<.001 Reduced mean hospitalization (11 vs 13 days, P=.015) |
| Shao et al. (2015) | After surgery: 6h: 100–150mL of water 24h: infusion of EN 24–48h: liquid diet 500mL 48–72h: semiliquid diet 1000mL 72–96h: normal intake | Enteral nutrition through nasogastric tube in the first 24h post-op | Reduced incidence of complications (39.1 vs 55.8% P<.001) Reduced mean hospitalization (13.94 vs 17.6 days, P<.001) |
| Sutcliffe et al. (2015) | – If high risk for pancreatic fistula: EN through NJ tube days 1–5+sips of water. Oral intake on day 6 – If low risk of pancreatic fistula: EN through NJ tube day 1. Oral intake on day 2 | In the high-risk group, intravenous fluids until tolerance of oral intake | In high risk patients, no differences vs the control group In low risk patients, shorter mean hospital stay (7 vs 9 days, P=.03), fewer re-hospitalizations within first 30 days (2 vs 17%, P=.04) |
| Morales et al. (2015) | Day 1: sips of cold water Day 2: liquid diet (water, infusions) Day 3: liquid diet (broth) Day 4: puréed foods, no fat Day 5–6: easily chewed foods, no fat Day 7: Diet bland, no fat | Day 0: intravenous fluid therapy Day 1: total parenteral nutrition Day 7: suspend total parenteral nutrition | Reduced morbidity (32 vs 48%, P=.072) Reduced mean hospitalization (14.2 vs 18.7 days, P=.014) |
| Wiliamsson et al. (2015) | Day 0: sips of clear liquids (300mL) Day 1: liquid diet (500ml) Day 2: liquid diet (1000mL) and yogurt Day 3: liquid diet, yogurt and toast (48%) Day 4: Normal intake (4%) | Reduced delayed gastric emptying (26 vs 48%, P=.03). Reduced mean hospitalization (10 vs 14 days, P<.001) | |
| Zouros et al. (2016) | Day 2: clear liquids (90.7%) Day 3: bland diet (97%) Day 4: tolerance to solids (94.4%) | Intravenous fluids Gradual decrease in volume until suspension on day 4. | Earlier introduction to oral diet: liquid 2.4 vs 4.3 days, P<.001; solid 4.6 vs 6.1 days, P<.001 Reduced delayed gastric emptying (12 vs 30%, P=.14) Reduced mean hospitalization (10.6 vs 14.3 days, P<.001) |
As for compliance with these oral intake progression protocols, few studies report this, and there is wide variability in the results obtained.17–19,26,27 Braga et al.18 reported that 55% of patients tolerated liquid intake on the first day post-op and 53% intake of solids on the second day. Furthermore, they observed an inverse relationship between oral tolerance and the appearance of postoperative complications (70% of patients with delayed oral tolerance presented postoperative complications). Abu Hilal et al.17 reported that tolerance to a liquid diet was achieved on the second day in 55% of cases and 65% initiated normal intake on the fourth day. The most heterogeneous data are those from Wiliamsson et al.,26 who described that 48% completed a liquid diet on the third day and only 4% normal diet on the fourth day, and Zouros et al.,27 who reported tolerance to liquids on the second day of 90.7%, day 3 soft foods of 97%, and a solid diet in the fourth day of 94.4%.
No significant differences were observed between the groups in mortality or re-hospitalization rates. A significant decrease was detected in mean hospital stay in the ERAS group in all the studies, with rates ranging between 7–21 vs 13.5–36 days.15–27
Several studies reported a statistically significant decrease in the incidence of delayed gastric emptying in patients in the group using the ERAS protocol.16,17,21,26,27 Kobayashi et al.20 described a lower incidence of statistically significant infections.
DiscussionThe implementation of ERAS programs has grown extensively in the area of colorectal surgery, and favorable results have been obtained,2 such as reduced morbidity, shorter hospital stay, improved patient well-being and reduced costs. However, the application of these protocols in pancreatic surgery is more complex given the greater difficulty of the surgical techniques and elevated postoperative morbidity. Despite improvements in the pancreaticoduodenectomy technique, postoperative morbidity remains high, with an incidence of 40%–50%3,4; consequently, the average hospital stay is longer, estimated at between 14 and 28 days.
Within the existing protocols, the nutritional component is an essential element in perioperative patient management. It is well known that the nutritional status of a patient undergoing surgery will be a prognostic factor, especially if the surgery is oncological. Therefore, it is essential to implement preoperative nutritional screening to detect malnourished patients or those at risk and thus optimize their nutritional status at the time of surgery.7
Nutritional screening and oral overload with carbohydrates up to 2h prior to surgery to avoid preoperative fasting are universal aspects included in ERAS protocols in any surgery. However, the most controversial aspect is the onset and progression of oral intake after the intervention. The high incidence of gastroparesis and pancreatic fistulae means that this progression cannot be similar to those found in ERAS protocols for other surgeries. Gastroparesis is the main cause of morbidity and prolongation of hospital stay, and it is also one of the predominant factors in the progressive rehabilitation of postoperative intake in pancreatic ERAS.5,28
In the analyzed studies, we observed great heterogeneity in the methods used to introduce oral intake, which complicates the analysis of the results. We observed how some studies used fast progression protocols that initiate oral tolerance the same day of surgery, generally with water, then progressing to liquids the day after and finally to tolerance of solids on the second day after surgery.19 Other studies use more conservative progression protocols, in which the patient is maintained nil per os until the third day after surgery and slowly progress to complete oral intake on the eighth day.16 Finally, there are intermediate protocols between these models, in which tolerance is started from the day of surgery but progresses more slowly, reaching tolerance to a complete diet between the fourth and fifth days.17
According to the studies reviewed, not only are no increased morbidity and mortality observed in any of the protocols, but some studies report a reduction in the main complications secondary to pancreatic surgery.15,23,25
In this context, a decrease in the incidence of delayed gastric emptying has been observed in several studies16,17,21,26,27 that report incidences in control groups varying from 24 to 48% and incidences in the ERAS groups from 12 to 26% (with an overall reduction incidence of approximately 50% with ERAS). Furthermore, Kobayashi et al.20 reported a lower incidence of pancreatic fistulae (9 vs 27.8%, P=.001) and infections (14 vs 28.9%, P=.019), and this is the only study that describes the appearance of these complications. The finding by Braga et al.18 is worthy of mention, in which a relationship was observed between the occurrence of complications and the delay in oral tolerance compared to the proposed protocol.
On the other hand, Kobayashi et al20 reported a decrease in the incidence of pancreatic fistulae (9 vs 27.8%, P=.001) and infections (14 vs 28.9%, P=.019), which is the only study to describe the appearance of these complications. Braga et al.18 made a remarkable finding of a correlation between the appearance of complications and delayed oral tolerance according to the protocol proposed.
Similarly, we were not able to correlate compliance to the rate of progression of oral intake. Firstly, this was because few studies report these data, and, secondly, because of the limited data reported by the studies. For example, Zouros et al.27 reported excellent compliance (around 90%) when completing oral intake on the fourth day, while Williamsson et al.26 reported a protocol adherence of only 4% when completing the same progression.
In our opinion, this is a very relevant aspect, since the appearance of postoperative complications is the main limitation in the correct application of ERAS, and this would help establish the appropriate rate of progression for oral intake. However, there is insufficient evidence of an association between the occurrence of post-surgical complications and the rate of dietary progression, making it difficult to choose an adequate diet protocol.
Mean hospital stay is another parameter that has been widely studied in ERAS protocols. Most of the studies analyzed report a decrease in mean hospitalization of 2 to 5 days compared to the control group, with no observed greater reduction in the studies using rapid diet progression protocols. Therefore, conclusions cannot be drawn as to whether earlier or more conservative protocols are associated with a greater decrease in mean hospital stay.
Another aspect to highlight is the indication of artificial nutrition during the oral intake progression pattern. Most studies proposed administering intravenous fluid therapy15–19,22,24,27 until achieving normal dietary intake; however, few indicated initiating artificial nutrition. Out of the studies in which artificial nutrition was proposed, 2 used enteral nutrition and only one used parenteral nutrition. In the case of enteral nutrition, Shao et al.23 administered enteral nutrition with a nasogastric tube for the first 24h, accompanied by water for fluid tolerance. In patients at high risk for developing pancreatic fistula, Sutcliffe et al.24 used enteral nutrition administered through a nasojejunal tube during the first 5 days (combined with water intake), while low-risk patients received enteral nutrition only during the first 24h after surgery. The Spanish Morales group25 proposed parenteral nutrition during the first week post-op until a complete oral diet is reached. The results obtained in these studies do not differ from each other, nor do they differ from groups that do not use artificial nutrition.
None of the studies describe whether it is possible to provide the nutritional requirements necessary to maintain at least a neutral balance and not favor the metabolic and protein catabolism of the patient, which is so relevant in pancreatic surgery. It is coherent to provide only serum therapy if the progression of the diet is rapid and the nutritional requirements of the patient are met within the first 4 days post-op. However, if the protocol is more conservative, we believe that the use of associated artificial nutrition is indicated, as in the case of the Morales et al. group.25 However, the addition of oral nutritional supplements to the diet should also be considered once intake is tolerated, as supplements can be an important aid in meeting nutritional requirements and avoiding the use of artificial nutrition or as a means to withdraw artificial nutrition earlier, thereby avoiding complications associated with its use. However, this aspect was also not valued by any of the studies.
Except for the study by Kobayashi et al.20 describing the evolution of albumin and prealbumin both one and 2 weeks after surgery, with no significant differences being observed in the control group, none of the studies evaluates changes in nutritional parameters, such as weight or visceral proteins. Since the average hospital stay is generally longer than 10 days, these parameters would give us important information about the variation in patient nutritional status.
It is important to note that the Spanish multimodal rehabilitation group (Grupo Español de Rehabilitación Multimodal, GERM)29 proposes different patterns for the progression of oral tolerance depending on the type of surgery performed. In patients undergoing pancreaticoduodenectomy with pancreatogastric anastomosis, they recommend initiating tolerance to water intake on the third day post-op with subsequent progression, whereas, in patients who have undergone pancreaticoduodenectomy with pancreaticojejunal anastomosis or a distal pancreatectomy, they recommend starting water intake 6 h after surgery and achieving normal dietary intake on the third day post-op.
As in any other type of surgery, it is necessary to use preoperative nutritional screening to detect patients who are malnourished or at risk, as well as a protocol for oral carbohydrate overload up to 2h before surgery. In contrast, pancreatic surgery has peculiarities intrinsic to the technique and its complications, which mean that the early onset of postoperative oral intake and its progression should probably be different.
As there are no specific guidelines with a good level of evidence in this area, the studies evaluated in this review are very different from each other in terms of dietary progression, and they generally do not provide sufficient information about aspects as relevant as incidence of complications, such as fistulae, or the correlation of delayed gastric emptying with the rate of progression of the diet (although the application of the ERAS protocol does reduce this). Likewise, there are also insufficient data about patient nutritional status and its evolution, as well as calorie or protein intake, including whether oral nutritional supplements were used.
The results are so disparate that there is no generalized pattern in which patients with more rapid progression present more complications and lower compliance, or if mean hospitalization decreases more than with more conservative protocols. There are also no clear data for the co-administration of artificial nutrition during the first days of oral intake progression, although it seems logical to think that, in the fastest protocols in which daily dietary requirements are reached earlier, isolated fluid therapy may be sufficient.
In conclusion, with the results obtained after the analysis of the studies described, in our setting we propose using early oral tolerance and rapid progression of dietary intake, although more slowly than protocols described for colorectal surgery. Thus, we recommend initiating water tolerance 6h after the end of surgery, but without forcing it upon the patient if he/she is nauseous or drowsy. Subsequently, day one after the surgery would progress to a liquid diet starting at lunch, and a semi-liquid diet would start on the second day, with the reintroduction of oral nutritional supplements if the patient received them prior to surgery. The third postoperative day would be based on an easily digested semi-solid diet, and the fourth day would progress to a bland diet that would continue until discharge. In cases where the proposed protocol was complied with and there was good tolerance to oral progression, we considered it advisable to suspend the therapy on the second day after surgery, not requiring the administration of parenteral or enteral artificial nutrition. Only if there is a complication leading to delayed oral tolerance (so that it is not foreseeable to administer the nutritional requirements of the patient on successive days) do we consider indicated the beginning of such nutrition.
Subsequently, on the first day after surgery, oral intake would progress to liquid diet starting at midday, and on the second day a semi-liquid diet would be started, along with oral nutritional supplements if the patient received them prior to surgery. The third postoperative day would be based on easily digested semi-solid foods, and the fourth day would progress to a bland diet that would be continued until discharge. If this proposed protocol is complied with and good tolerance to oral intake progression is observed, we considered the suspension of fluid therapy indicated the second day after surgery, not requiring the administration of parenteral or enteral artificial nutrition. Only if there is a complication that leads to a delay in oral tolerance, so that it is not foreseeable to administer the nutritional requirements of the patient on successive days, we consider indicated the beginning of such nutrition.
Conflict of InterestsThe authors have received no funding for publishing this article and have no conflict of interests to declare.
Please cite this article as: Márquez Mesa E, Baz Figueroa C, Suárez Llanos JP, Sanz Pereda P, Barrera Gómez MÁ. Manejo nutricional en la rehabilitación multimodal precoz en cirugía pancreática. Cir Esp. 2017;95:361–368.