The association of preoperative chemoradiotherapy and transanal endoscopic surgery in T2 and superficial T3 rectal cancers presents promising results in selected patients. The main objective is to evaluate the long-term loco-regional and systemic recurrence and, as secondary objectives, to provide results of postoperative morbidity and the correlation between complete clinical and pathological response.
MethodsThis is a retrospective observational study including a consecutive series of patients with T2-T3 superficial rectal cancer, N0, M0 who refused radical surgery (2008–2016). The treatment consisted of preoperative chemotherapy (5-fluorouracil or capecitabine) combined with radiotherapy (50, 4Gy) and transanal endoscopic surgery after 8weeks. Preoperative, surgical, pathological and long-term oncologic results were analyzed.
ResultsTwenty-four patients were included in the study. Two of them required rescue radical surgery for unfavorable pathological results. A local recurrence (4.5%) was observed and 2patients presented systemic recurrence (9%), with a median follow-up of 45 months. A complete clinical tumor response was achieved in 12 patients (50%), and complete pathological tumor response in 9 patients (37.5%). Postoperative complications were observed in 5 patients (20.8%), and they were mild except one. There was no postoperative mortality.
ConclusionsIn this stage of rectal cancer, our results seem to support this strategy, mainly when a complete pathological response is achieved. The complete clinical tumor response does not coincide with the pathological tumor response. Randomized prospective studies should be performed to standardize this treatment.
La asociación de quimiorradioterapia preoperatoria y cirugía endoscópica transanal en el cáncer rectal T2-T3 superficial presenta resultados prometedores en pacientes seleccionados. El objetivo principal es evaluar la recurrencia locorregional y sistémica a largo plazo y los objetivos secundarios son aportar resultados de morbilidad postoperatoria y la correlación entre la respuesta patológica completa y clínica completa.
MétodosEstudio observacional retrospectivo de una serie consecutiva de pacientes diagnosticados de cáncer de recto T2-T3 superficial, N0, M0 que se trataron con quimiorradioterapia neoadyuvante y escisión transanal del tumor (2008-2016). Se recogieron los datos de forma prospectiva. El tratamiento consistió en quimioterapia preoperatoria (5-fluorouracilo o capecitabina) combinada con radioterapia (50,4Gy) y cirugía endoscópica transanal tras 8semanas. Se analizaron las variables preoperatorias, quirúrgicas, patológicas y los resultados oncológicos a largo plazo.
ResultadosDe los 24 pacientes incluidos, 2requirieron rescate a cirugía radical por resultados patológicos desfavorables. Con un seguimiento mediano de 45 meses, se observó recurrencia local en un paciente (4,5%) y 2pacientes presentaron recurrencias sistémicas (9%). La respuesta clínica tumoral completa se logró en 12 pacientes (50%) y la respuesta patológica tumoral completa en 9 pacientes (37,5%). Las complicaciones postoperatorias se apreciaron en 5 pacientes (20,8%), todas leves excepto una. No hubo mortalidad postoperatoria.
ConclusionesEn este estadio del cáncer rectal, nuestros resultados parecen apoyar esta estrategia, principalmente cuando se logra una respuesta patológica tumoral completa. La respuesta clínica tumoral completa no coincide con la respuesta patológica tumoral. Se deben llevar a cabo estudios prospectivos aleatorizados para estandarizar este tratamiento.
Local surgery for clinical stage T2-T3 superficial (T2-3s) N0M0 rectal cancer is limited by lymph node involvement, which reaches 12%–28%.1 Recurrence rates after local excision are greater than 20%, which is inadmissible. Recent meta-analyses on local surgery in these tumors combined with adjuvant treatment have not shown excessively improved results (11%–19%).2 Thus, the treatment of choice for T2-3sN0M0 rectal cancer in the mid and lower third of the rectum continues to be total mesorectal excision (TME), with local recurrence rates between 2% and 11% and systemic rates between 2% and 13%. However, the morbidity and mortality rates associated with this technique are high, at 30–40 and 2%, respectively.3
For almost a decade, the combination of preoperative chemoradiotherapy (CRTx) and local excision have been proposed as a less aggressive alternative to TME, with similar results.4,5 However, several questions remain unanswered; for instance, whether there is a greater percentage of complete pathologic response (cPR) after CRTx in early stages of rectal adenocarcinoma, and whether favorable oncologic results are obtained.6,7 We should also establish whether the complete clinical response (cCR) allows for a “wait-and-see” approach, or whether it is necessary to confirm cPR by means of local resection.8
The main objective of our study is to evaluate long-term locoregional and systemic recurrence in patients with T2-3s rectal cancer treated with CRTx and local excision. As secondary objectives, we provide results for postoperative morbidity and the observed correlation between cPR and cCR.
MethodsThis is a retrospective observational study including a consecutive series of patients diagnosed with T2-3sN0M0 rectal cancer between 2008 and 2016. Data were collected prospectively. The description of the study followed the guidelines found in Strengthening the reporting of observational studies in epidemiology (STROBE) for cohort studies.9 Patients who were candidates for transanal endoscopic surgery were treated in accordance with the protocol of our Unit, which has been previously described.10 The T3 tumors were subdivided into 2 groups: superficial (T3s) with invasion of the mesorectum ≤5mm, or deep.11 T3s disease is believed to have the same prognosis as T2, so both are associated in the same group. As reported in previous studies, patients treated with local excision were classified into 5 preoperative treatment groups10: group I (curative intent) included adenomas at clinical stage (c), cT0, cN0 (benign tumors); group II (also with curative intent) included adenocarcinomas, low-grade stages cT0-1, cN0; group III (consensus group) included adenocarcinomas with low to moderate degree of differentiation, cT2-T3s, cN0; group IV (palliative intent); and group V (atypical indication).
The present analysis includes group III patients who refused radical surgery and accepted local excision after giving their informed consent. Excluded from the study were group III patients included in our multicenter prospective clinical trial,4 those who rejected the indicated treatment strategy or any other preoperative classification.
The established preoperative CRTx was 5-fluorouracil or capecitabine. The 5-fluorouracil was administered intravenously in continuous infusion at a dose of 300mg/m2 per day, 5 days a week for 5 weeks. Capecitabine was administered orally every 12h at a dose of 825mg/m2 on the radiotherapy days. Preoperative radiotherapy was administered at daily fractions of 1.8Gy, 5 days a week for 5 weeks in accordance with the standard protocol.12 The total dose was 45Gy, plus an additional 5.4Gy in the tumor area (total 50.4Gy). The morbidity and mortality associated with neoadjuvant therapy was followed until day 30 after surgery, according to the criteria of the Common terminology criteria for adverse events (CTCAE).13 The 7th week, the clinical response was evaluated, defined according to the difference in size between the magnetic resonance study and the pre- and post-CRTx rectoscopy: reduction ≤50%, reduction >50%, and lack of lesion.14
Surgery was conducted 8 weeks after the end of CRTx. The techniques used for local excision were either transanal endoscopic microsurgery (TEM, Richard Wolf, Knittlingen, Germany)15 or the transanal endoscopic operation (TEO, Karl Storz GmbH, Tüttlingen, Germany). In all cases, the excision was the entire thickness of the rectal wall, as previously described.10 All defects were closed with continuous sutures, as long as they were tension-free, in which case partial sutures were used. Postoperative morbidity was registered after 30 days using the Dindo-Clavien classification system.16
The pathology studies were done by the same pathologist in all patients. The following data were recorded: lesion size, (maximum and minimum diameters in mm), grade differentiations of the adenocarcinoma, grade of response to the CRTx according to the grade of regression (GR) by Bouzourene,17 tumor stage after radiotherapy (ypT), presence of venous, lymphatic or perineural infiltration and margins of the surgical specimen (lateral and deep margins in mm). The resection types (R) were classified in the following manner: R0 (excision with margins >1mm); R≤1 (margins ≤1mm); Rx (fragmentation of the surgical specimen without defined margins) and R1 (affected margins).18
The absence of pathologic response or superior stage (≥ypT3s or GR4) in the resection piece indicated rescue surgery by radical surgery 4 to 6 weeks after surgery. Patient follow-up included rectosigmoidoscopy-biopsy and CEA determinations every 4 months during the first two years. The same tests were repeated every 6 months from the third to the fifth years. Complete colonoscopy, abdominal CT and pelvic magnetic resonance were done annually until the fifth year. The study was approved by the local Ethics Committee and registered in the UIN Research Registry: researchregistry256.
For the description of the variables, SPSS version 20 (Statistical Package for the Social Sciences, Inc, Chicago, Illinois, USA) was used. The quantitative variables were expressed as means with standard deviation and 95% confidence interval when they presented normal distributions. If not, the median and range were calculated. The categorical variables were expressed as absolute numbers and percentages. There were no missing data due to the prospective introduction of the data. Accumulated survival without local recurrence, disease-free survival and overall survival were estimated with the actuarial method and Kaplan–Meier curves.
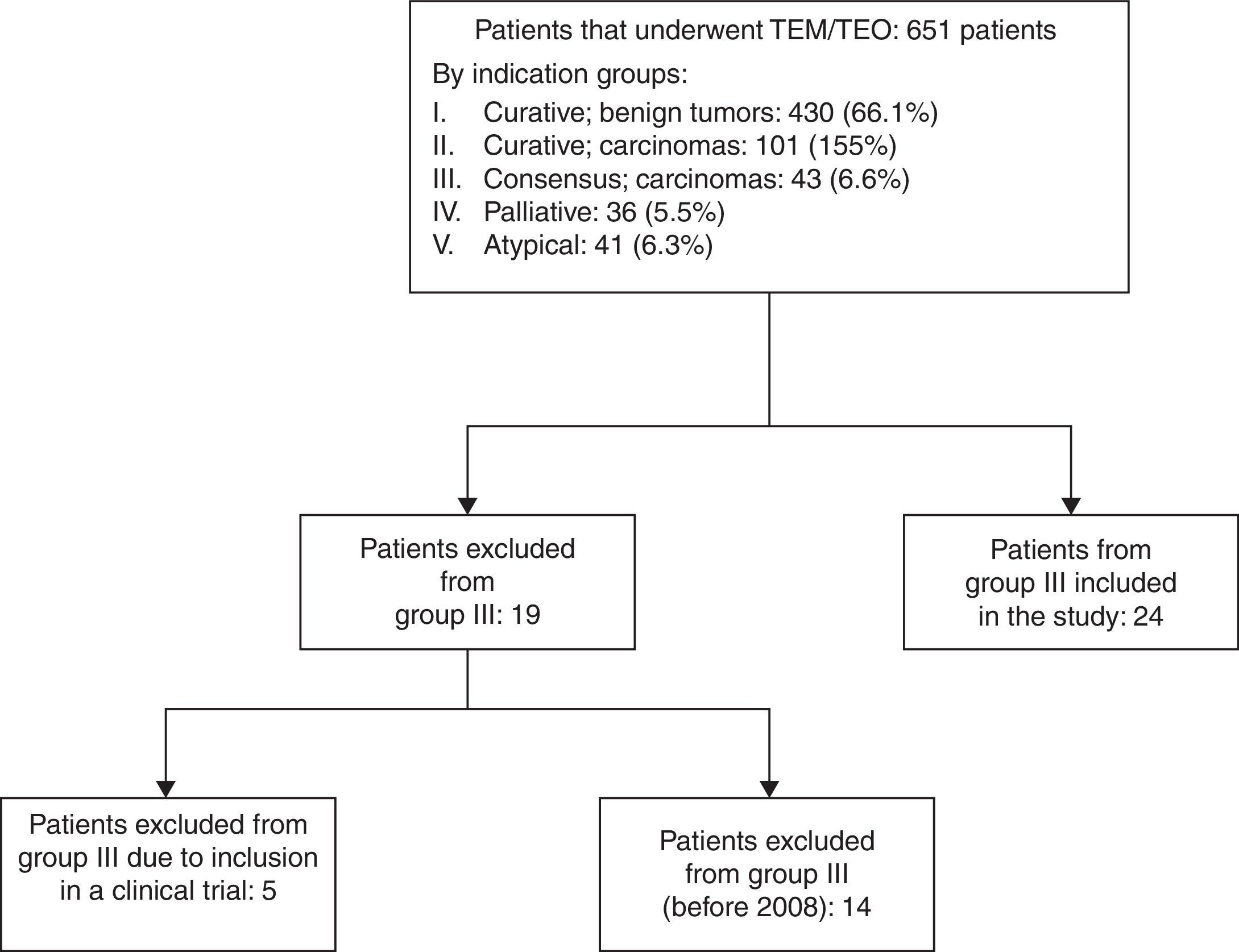
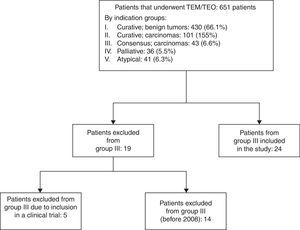
ResultsBetween June 2004 and June 2016, 651 patients were treated by our unit with TEM/TEO. Fig. 1 is a flow diagram of the patients by therapeutic indication groups. In 2008, due to the poor results of group III after having been treated with TEM/TEO and adjuvant therapy,4 it was decided that patients belonging to this group who did not accept the standard indication for radical surgery would be treated with CRTx and local surgery. Out of the 43 patients of group III, 14 were operated on between 2004 and 2008, 5 were included in the multicenter clinical trial, and the 24 remaining patients were analyzed in this study.
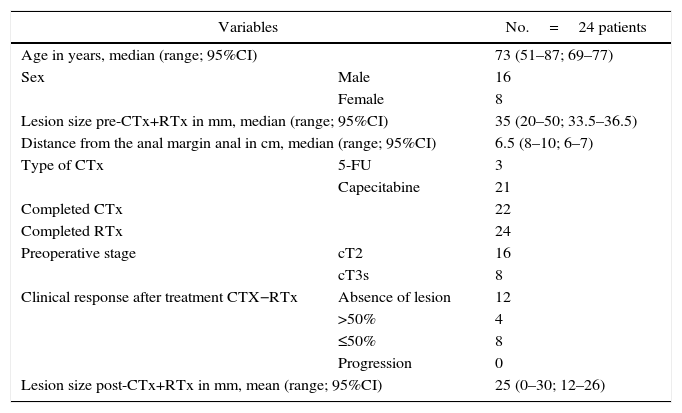
The demographic characteristics of these 24 patients who have received CRTx and TEM/TEO are shown in Table 1. The majority of the patients (21 out of 24) received capecitabine as chemotherapy. All the patients completed neoadjuvant therapy, except 2 who did not complete chemotherapy due to severe adverse effects. The diagnosis in 16 patients was cT2. After CRTx, cCR was observed in 12 of the 24 patients (50%), and in none of the patients was disease progression observed.
Demographic Characteristics and Patient Lesions.
| Variables | No.=24 patients | |
|---|---|---|
| Age in years, median (range; 95%CI) | 73 (51–87; 69–77) | |
| Sex | Male | 16 |
| Female | 8 | |
| Lesion size pre-CTx+RTx in mm, median (range; 95%CI) | 35 (20–50; 33.5–36.5) | |
| Distance from the anal margin anal in cm, median (range; 95%CI) | 6.5 (8–10; 6–7) | |
| Type of CTx | 5-FU | 3 |
| Capecitabine | 21 | |
| Completed CTx | 22 | |
| Completed RTx | 24 | |
| Preoperative stage | cT2 | 16 |
| cT3s | 8 | |
| Clinical response after treatment CTX−RTx | Absence of lesion | 12 |
| >50% | 4 | |
| ≤50% | 8 | |
| Progression | 0 | |
| Lesion size post-CTx+RTx in mm, mean (range; 95%CI) | 25 (0–30; 12–26) | |
5-FU: 5-fluorouracil; cT2: clinical stage T2; cT3: clinical stage T3; 95%CI: 95% confidence interval; RTx: radiotherapy; CTx: chemotherapy.
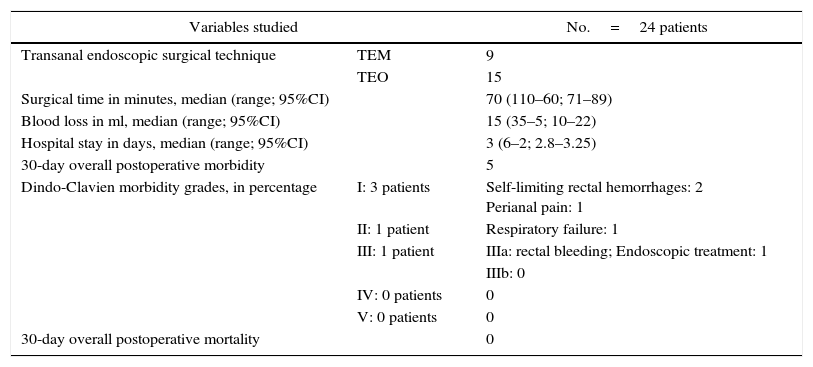
TEM/TEO were done in accordance with one or the other technique. Median surgical time was slightly longer than an hour, with minimal blood loss during the procedure. None of the patients required reconversion to abdominal surgery. Mean hospital stay was 3 days. Only 5 patients out of 24 presented postoperative morbidity at the 30-day follow-up according to the Dindo-Clavien classification,16 and only one patient had a IIIa complication, requiring endoscopic treatment to control the surgical bleeding. None of the patients presented with grades IV or V complications (Table 2).
Intraoperative Variables, Postoperative Morbidity and Follow-Up.
| Variables studied | No.=24 patients | |
|---|---|---|
| Transanal endoscopic surgical technique | TEM | 9 |
| TEO | 15 | |
| Surgical time in minutes, median (range; 95%CI) | 70 (110–60; 71–89) | |
| Blood loss in ml, median (range; 95%CI) | 15 (35–5; 10–22) | |
| Hospital stay in days, median (range; 95%CI) | 3 (6–2; 2.8–3.25) | |
| 30-day overall postoperative morbidity | 5 | |
| Dindo-Clavien morbidity grades, in percentage | I: 3 patients | Self-limiting rectal hemorrhages: 2 Perianal pain: 1 |
| II: 1 patient | Respiratory failure: 1 | |
| III: 1 patient | IIIa: rectal bleeding; Endoscopic treatment: 1 | |
| IIIb: 0 | ||
| IV: 0 patients | 0 | |
| V: 0 patients | 0 | |
| 30-day overall postoperative mortality | 0 | |
95%CI: 95% confidence interval; TEM: transanal endoscopic microsurgery; TEO: transanal endoscopic operation.
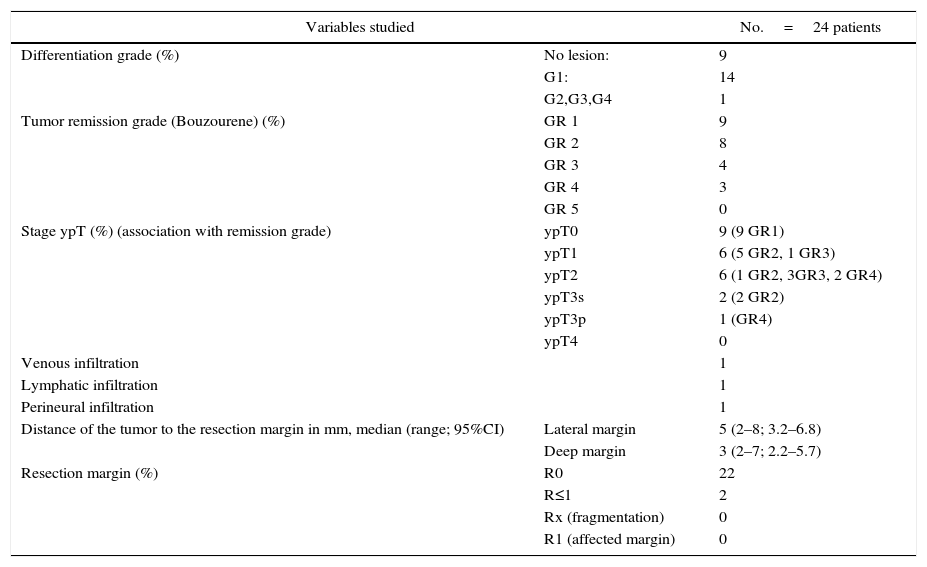
Table 3 describes the anatomical and pathological characteristics of the lesions. There were no affected margins or fragmentation of the surgical specimen in the series studied. Only 2 patients had margins less than 1mm. cPR (ypT0) was seen in 9 of the 24 patients, corresponding with 37.5%. No residual tumor (GR1) or residual tumor cells (GR2) were found in 17 of the 24 patients. No tumor progression was observed in any of the patients (GR5), compared with the preoperative magnetic resonance imaging study. In the 2 patients who did not complete neoadjuvant therapy, one presented GR2 with ypT1, and the other GR4 ypT2.
Anatomical and Pathological Characteristics of the Lesions.
| Variables studied | No.=24 patients | |
|---|---|---|
| Differentiation grade (%) | No lesion: | 9 |
| G1: | 14 | |
| G2,G3,G4 | 1 | |
| Tumor remission grade (Bouzourene) (%) | GR 1 | 9 |
| GR 2 | 8 | |
| GR 3 | 4 | |
| GR 4 | 3 | |
| GR 5 | 0 | |
| Stage ypT (%) (association with remission grade) | ypT0 | 9 (9 GR1) |
| ypT1 | 6 (5 GR2, 1 GR3) | |
| ypT2 | 6 (1 GR2, 3GR3, 2 GR4) | |
| ypT3s | 2 (2 GR2) | |
| ypT3p | 1 (GR4) | |
| ypT4 | 0 | |
| Venous infiltration | 1 | |
| Lymphatic infiltration | 1 | |
| Perineural infiltration | 1 | |
| Distance of the tumor to the resection margin in mm, median (range; 95%CI) | Lateral margin | 5 (2–8; 3.2–6.8) |
| Deep margin | 3 (2–7; 2.2–5.7) | |
| Resection margin (%) | R0 | 22 |
| R≤1 | 2 | |
| Rx (fragmentation) | 0 | |
| R1 (affected margin) | 0 | |
G: grade of differentiation; GR 1: no evidence of residual tumor; GR 2: few residual tumor cells; GR 3: predominance of fibrosis over tumor cells; GR 4: predominance of tumor over fibrosis; GR 5: no tumor regression; R≤1: resection margin ≤1mm; R0: resection margin >1mm; R1: resection margin affected by the tumor; Rx: fragmentation of the piece; ypT: pathologic stage of the tumor after CTx+RTx.
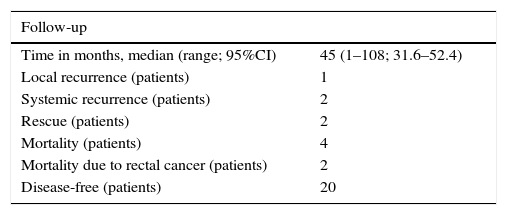
The median follow-up period of the series was 45 months (Table 4). Four patients with unfavorable pathologic results were recommended rescue TME. Two refused (ypT3s), and the other 2 (ypT2-GR4 and ypT3deep GR4) were rescued with radical surgery. Therefore, the total follow-up for CRTx and local surgery is based on 22 patients. One of these patients underwent abdominoperineal resection, with a surgical specimen that was pT0, pN1. In the second, lower anterior resection and ileostomy were done, with a pathology result of pT0, pN0. To date, both patients continue to be disease-free.
Long-Term Oncologic Results After Neoadjuvant Therapy+TEM/TEO.
| Follow-up | |
|---|---|
| Time in months, median (range; 95%CI) | 45 (1–108; 31.6–52.4) |
| Local recurrence (patients) | 1 |
| Systemic recurrence (patients) | 2 |
| Rescue (patients) | 2 |
| Mortality (patients) | 4 |
| Mortality due to rectal cancer (patients) | 2 |
| Disease-free (patients) | 20 |
A total of 22 patients analyzed.
Only one of the 22 patients presented local recurrence (4.5%). This was an 85-year-old patient who completed neoadjuvant treatment without incident; excision of the lesion involved the entire wall, with lateral and deep minimum resection margins of 8mm and 4mm, respectively. The pathology study reported ypT1, with no factors for a poor prognosis. Recurrence was detected on the first follow-up 4 months later. Low anterior resection was carried out with transanal NOTES and protective ileostomy, without incident. Two patients (9%) who completed neoadjuvant therapy, both in stage ypT2 (GR2 and GR3), had developed multiple metastases by the 8 and 12-month follow-up and died after 18 months and 2 years, respectively.
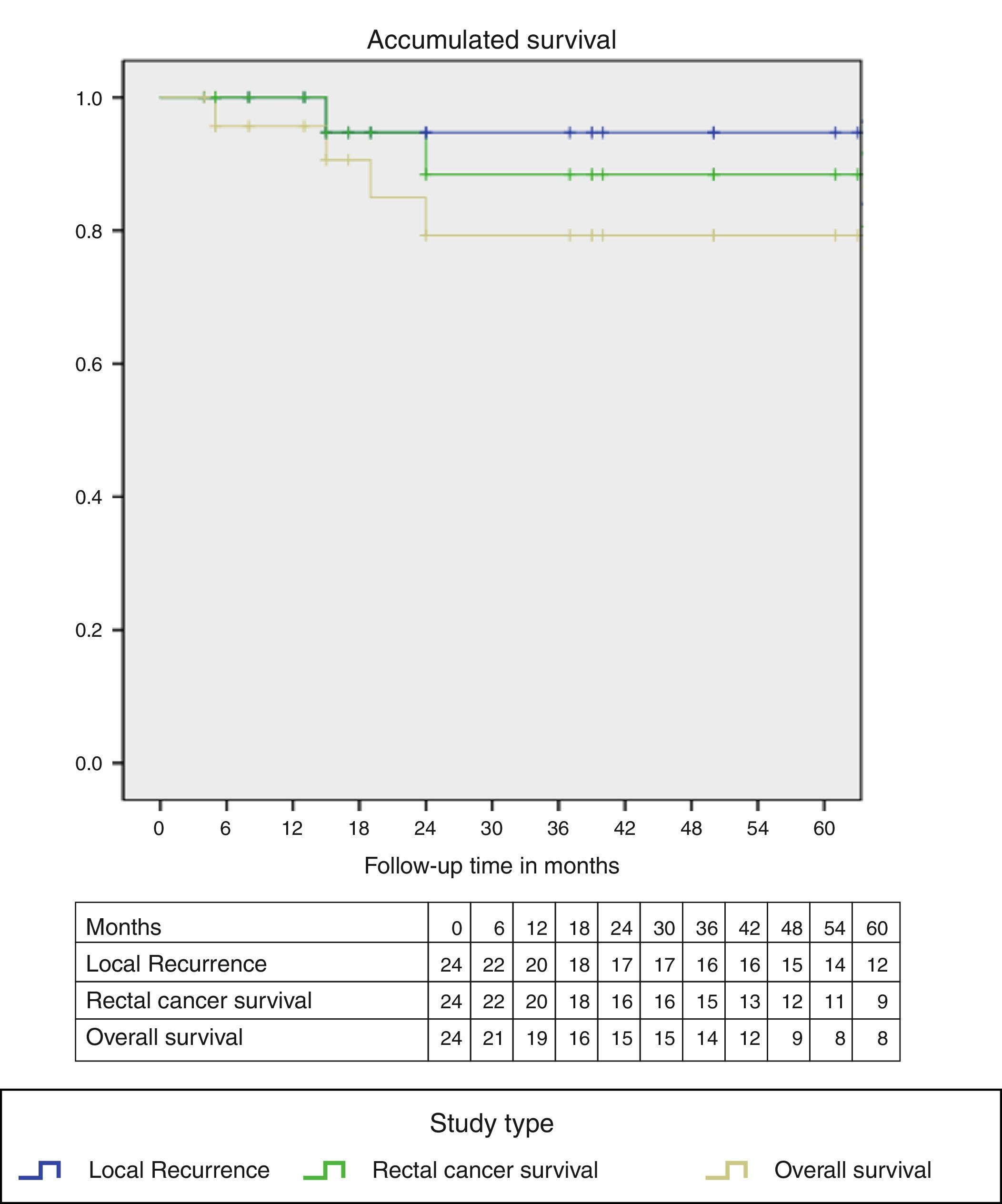
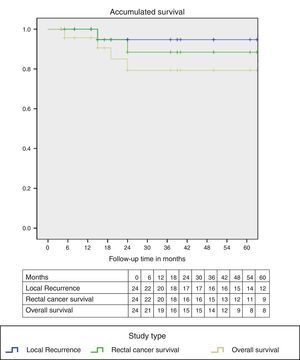
Two patients died due to causes other than rectal cancer. Fig. 2 demonstrates the Kaplan–Meier curves, showing an accumulated survival curve at the end of the follow-up period with absence of local recurrence of 90%; specific rectal-cancer survival was 88% and overall survival 79%.
DiscussionAfter neoadjuvant therapy, the main objective is to achieve cPR. The cPR values published in the literature for locally advanced rectal cancer range between 15% and 27%.19 In T2 and T3, they vary widely from 11.7% to 73%.32 In our study, we found 9 out of 24 patients (37.5%), which is within the published range of values and is superior to the average for locally advanced cancer. The vast majority of these cPR have been published in the literature with long cycles of CRTx, similar to that described in our study. Short cycles of CRTx do not obtain the same results and lead to higher postoperative morbidity.20 What should also be considered are the adverse effects related with the toxicity of CRTx, as we have published previously.21 In our series, 2 patients had to withdraw from treatment. Some authors have changed CRTx regimens in an attempt to improve cPR results. For instance, combining capecitabine at standard doses and oxaliplatin with radiotherapy, García Aguilar et al.22 reached a cPR rate of 48%. However, 44% of patients presented adverse effects of grade ≥3, which required reducing the dose of capecitabine. With the reduced dose, a cPR rate of 36% was obtained, and the incidence of adverse effects in grade ≥3 dropped to 30%. A recent publication about the results of this study, with a mean follow-up of 56 months, observed that in 79 patients there were 6% distant metastases and 4% local recurrence.23
The cCR and cPR do not always coincide. In our series, 12 patients (50%) presented cCR compared to 9 out of 24 patients (37.5%) who presented cPR. Considering our experience, 3 patients out of 24 thought to have with cCR would have relapsed if we had used the “watch and wait” approach, so we consider this strategy dangerous and feel that currently excision of the lesion should be done to confirm cPR. There are studies that have attempted to determine predictive factors (clinical, radiological) of cPR, without being able to give definitive conclusions.22,24,25 In our indications, including T3s, we have obtained results similar to those of T2. Thus, of the 9 patients in whom cPR was obtained, 3 had been T3s and the other 6 cT2. In the literature, there are also good results reported in T3, although not specified as either superficial or deep.26,27
It is the responsibility of the surgeon to perform excision of the complete rectal wall of the lesion, with no fragmentation and margins ≥1mm. When these conditions are not met, the frequencies of local recurrence are much higher. In our series using the TEM/TEO technique, all patients except 2 presented these surgical characteristics. Transanal endoscopic surgery is preferably recommended over conventional local excision, as it achieves better results in terms of resection margins and quality of the sample.28 There are no technical differences with regards to the use of TEM or TEO.29 We have had no postoperative mortality, and hospital stay was short, with a median of 3 days, which is also described in other series.30
Some authors have reported higher morbidity with neoadjuvant therapy and local surgery in these tumors compared to simple excision using TEM/TEO. Pérez et al.31 observed a high morbidity of 44% and 30% re-hospitalization. Coco et al.32 obtained complication percentages of 16% after TEM and 36.4% after neoadjuvant therapy and TEM, although these differences were not statistically significant. In our study, postoperative morbidity was not as important: it was observed in 5 of the 24 patients (20.8%), 4 with Dindo-Clavien grades I-II and just one patient developed a grade IIIa complication. In our experience, the most frequent complication was rectal bleeding, in 3 patients. However, other authors have described suture dehiscence and perianal pain as more important complicaciones.31,33
The combination of neoadjuvant treatment and local surgery (TEM/TEO) in T2-T3s N0M0 rectal cancer seems a possible alternative to TME. In our study, with a mean follow-up period of almost 4 years, only one patient in 24 (4.5%) has presented local recurrence and 2 patients (9%) systemic recurrence. Recent studies in the literature have reported local recurrence below 10%.30 The multicenter study by Yu et al.27 reported 2.5% in 40 patients, and a randomized prospective trial by Lezoche et al.14 found 8% in 50 patients. These same studies reported systemic recurrence at 7.5% and 4%, respectively.
The limitations of the study are derived from its observational design with a limited number of patients, although they are consecutive patients with strict indication criteria. Likewise, the patient inclusion was based on endorectal ultrasound and magnetic resonance, which are not completely reliable for tumor staging (T2-T3s) and also cannot completely rule out the presence of lymphadenopathies (N).
Our results seem to indicate that, in this group of patients, local and systemic recurrences with this type of strategy are similar to these of TME. The cPR after neoadjuvant therapy is higher in these early stages. cCR cannot be equated with cPR, and we therefore do not support the “wait and see” approach in cases of cCR. Postoperative morbidity after neoadjuvant therapy and TEM/TEO, although slightly higher than TEM/TEO, seems to be quite lower than TME.
These results, although positive and hopeful, do not allow for generalizations, as there is not sufficient scientific evidence from prospective and randomized studies. Since 2010, our group has been leading a multicenter clinical trial in order to shed some light on these questions (NCT01308190). Other studies within the European setting, such as the UK-TREK at the University of Birmingham and the French GRECCAR 2, share the same objective, even though their study designs are different. They should all confirm whether this less aggressive therapeutic strategy for a better quality of life is able to achieve the same long-term oncologic results as conventional treatment with TME.
Authorship/CollaborationsXavier Serra-Aracil: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Carlos Pericay: study design, data collection, analysis and interpretation of the results, critical review and approval of the final version.
Laura Mora-Lopez: data collection, analysis and interpretation of the results, critical review and approval of the final version.
Juan Carlos García Pacheco: data collection, analysis and interpretation of the results, critical review and approval of the final version.
José Isaac Latorraca: data collection, analysis and interpretation of the results and approval of the final version.
Julio Ocaña-Rojas: data collection, analysis and interpretation of the results, and approval of the final version.
Alex Casalots: data collection, analysis and interpretation of the results and approval of the final version.
Eva Ballesteros: data collection, analysis and interpretation of the results and approval of the final version.
Salvador Navarro-Soto: data collection, analysis and interpretation of the results, critical review and approval of the final version.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank all the members of our Coloproctology Unit and the Multidisciplinary Committee for Colon and Rectal Tumors at our hospital. Thanks also go to Cristina Gómez Vigo for correcting the composition of the text.
Please cite this article as: Serra-Aracil X, Pericay C, Mora-Lopez L, Garcia Pacheco JC, Latorraca JI, Ocaña-Rojas J, et al. Neoadyuvancia y cirugía endoscópica transanal en neoplasias de recto T2-T3 superficial, N0, M0. Recidiva local, respuesta clínica y patológica completa. Cir Esp. 2017;95:199–207.