In this observational study we reviewed the efficacy and side effects of different antiemetic combinations used in our hospital for postoperative nausea and vomiting (PONV) prophylaxis in high-risk women undergoing highly emetogenic surgery.
MethodsAfter reviewing retrospectively the medical records of patients undergoing highly emetogenic elective surgeries under general anaesthesia, we selected 368 women whose Apfel risk score was≥3 and receiving a combination of 2 antiemetics for PONV prophylaxis. We analysed the incidence of PONV at 2, 6, 12 and 24h after surgery, antiemetic rescue requirements, pattern of occurrence of PONV, side effects and level of sedation were also assessed. The main goal was complete response defined as no PONV within 24h after surgery.
ResultsOndansetron 4mg i.v. plus dexamethasone 8mg i.v. (O&Dex), haloperidol 1mg i.v. (O&Hal1), haloperidol 2mg i.v. (O&Hal2) or droperidol 1.25mg i.v. (O&Dro) were the combinations most frequently used. The complete response was better in groups O&Dex: 68.5% (CI: 58–78), O&Hal2: 64.1% (CI: 53–74) and O&Dro 63% (CI: 52–73) than in group O&Hal1: 41.3% (CI: 31–52) (P<.01). Peak incidence of PONV occurred within the 2–6h period. The incidence of side effects was higher in group O&Hal2.
ConclusionIn high risk patients for PONV who underwent highly emetogenic surgeries, the efficacy of low-dose haloperidol (1mg) in combination is limited. Higher doses (2mg) are more effective but its use is associated with a high incidence of side effects.
El objetivo de este estudio fue evaluar la eficacia y los efectos secundarios de distintas combinaciones de antieméticos para la profilaxis de náuseas y vómitos postoperatorios (NVPO) en pacientes propicios a presentarlos tras cirugía muy emetógena.
MétodosTras revisar retrospectivamente las historias clínicas de pacientes sometidos a cirugía electiva muy emetógena bajo anestesia general durante el periodo 2009 a 2011, seleccionamos 368 mujeres con puntuación de Apfel ≥3 y que recibieron una combinación de 2 antieméticos como profilaxis. Analizamos la incidencia de NVPO a las 2, 6, 12 y 24h del postoperatorio, rescates antieméticos, patrón de aparición de NVPO, efectos secundarios y nivel de sedación. Valoramos la respuesta completa como ausencia de NVPO en las primeras 24h.
ResultadosOndansetrón 4mg i.v. en combinación con dexametasona 8mg i.v. (O&Dex), haloperidol 1mg i.v. (O&Hal1), haloperidol 2mg i.v. (O&Hal2) o droperidol 1,25mg i.v. (O&Dro) fueron las combinaciones más empleadas. La respuesta completa fue mayor en los grupos O&Dex: 68,5% (IC: 58-78); O&Hal2: 64,1% (IC: 53-74) y O&Dro 63% (IC: 52-73) que en el grupo O&Hal1: 41,3% (IC: 31-52) (P<0,01). La máxima incidencia de NVPO ocurrió entre las 2 y 6h del postoperatorio. La incidencia de efectos secundarios fue mayor en el grupo O&Hal2.
ConclusionesEn pacientes con elevado riesgo de NVPO sometidos a cirugía muy emetógena, la eficacia de dosis bajas de haloperidol (1mg) en combinación con ondansetrón es escasa. Dosis mayores (2mg) son altamente eficaces, pero se asocian a una alta incidencia de efectos secundarios.
Despite the development of new antiemetics with a stronger and safer profile, postoperative nausea and vomiting (PONV) continues to be a problem for surgical patients, primarily for those with an increased risk of developing this complication; among whom the incidence rate can reach 80%, even after prophylactic administration of antiemetics.1–3 Good control of PONV increases patient satisfaction, reduces postoperative complications and allows for the development of outpatient and fast-track surgery.4–7 Clinical guidelines recommend an antiemetic prophylaxis that is proportional to the patient's risk, using combinations of antiemetics with different mechanisms of action in high-risk patients.8
The effectiveness and safety of ondansetron, dexametasone and droperidol, alone and in combination, have been demonstrated in several studies.9–12 Droperidol has been used in anaesthesia for many years, but since the FDA released an alert in 2001 regarding the risk of arrhythmia associated with its use, haloperidol has been increasingly used as an alternative, as it also belongs to the butyrophenone group and shares a mechanism of action (blocks D2 receptors).13,14 It has been used as an antiemetic since its approval as an antipsychotic in 1967 and its use is recommended by current clinical guidelines.8,15
Considering these criteria, we studied the effectiveness and safety of the combinations of antiemetics that were most commonly used in our normal clinical practice for prophylaxis of PONV in patients with an increased risk of presenting these symptoms. The primary hypothesis was that all combinations of antiemetics are equally effective, without any noticeable side effects.
Patients and MethodsThe study, approved by the Navarra Ethics Committee, was designed as a retrospective cohort study.16 All patients were selected using our digitised clinical record. Starting in September 2009 and working in chronological order, we revised all the patients that had undergone surgical interventions with an increased risk of postoperative vomiting (colorectal, gynaecological, breast, thyroid, and cholecystectomies), performed on women older than 18 and whose score on the Apfel scale was≥3 (woman, non-smoker, previous history of PONV/kinetosis, postoperative use of opioids). We chose patients who had received a combination of 2 antiemetics during surgery as prophylaxis for PONV. We excluded patients who had undergone outpatient surgery or emergency surgery, as well as those who had received loco regional anaesthesia or total intravenous anaesthesia (TIVA).
Once the patient was approved for inclusion in the study, we contacted her to obtain informed consent. As we will explain later, we needed 92 patients per prophylactic group, and so we included in each group the first 92 patients that we reviewed who met the eligibility criteria and gave consent. The most recent patient underwent surgery in December 2011.
We obtained all the variables needed to complete the database through the digitised clinical record, in which variables related to PONV were recorded daily, and so it was possible to access a reliable source of data. During surgery, the patient's vital signs are transferred electronically from the monitor to their clinical record, and information on the periods of time spent in surgery and under anaesthesia, the medication given and the fluid therapy administered are recorded directly by the anaesthetist.
In the Post-Anaesthesia Recovery Unit (PARU), the vital signs are transferred electronically, and every 30min until discharge the nurse anaesthetist records the length of hospitalisation, medication, fluid therapy and other variables, such as pain intensity or the presence of nausea or emetic episodes (EE). On the general ward, the nurse records this information every 6h. The notes from each ward round run by the doctor or nurse are written in the clinical record.
The primary end-point was a complete response to the antiemetic medication: an absence of nausea and EE in the first 24h after surgery (from the skin closure). The following data were recorded in PARU (0–2h) and at 6, 12 and 24h: incidence of nausea (yes/no); incidence of emetic episodes (EE) (retching or vomiting) (yes/no); pain intensity when at rest: mild (VAS<3), moderate (VAS 3–7) or severe (VAS>7); heart rate, blood pressure and oxygen saturation (pulse oximetry), as well as rescue antiemetic requirements, secondary effects and the time of first intake. Data on fluid therapy and the presence of arrhythmias were recorded in surgery and in the PARU. In PARU, the level of sedation was recorded: completely awake, eyes open (4); drowsy, eyes closed (3); sleeping, responds to voice (2); sleeping, responds to touch (1); no response (0).
Statistical AnalysisAccording to the incidence of PONV in studies carried out in our hospital using ondansetron 4mg+dexametasone 8mg (close to 20%) and in order to have an 80% probability of achieving an absolute reduction of 15% of PONV in the first 24h after surgery, with an alpha level of 5% (two-tailed), each group required 92 patients. Student's t-test and the Mann–Whitney U test were used to compare the differences between groups. The ¿2 test (with Bonferroni correction) was used for categorical variables. The value P<.05 was considered statistically significant. The analysis was performed using SPSS 17.0 software.
ResultsIn order to obtain a cohort of 368 patients, we reviewed 1288 patients who had undergone highly emetogenic surgery. We excluded 266 males and minors under the age of 18, as well as 259 patients for having an Apfel score of <3, 46 for not having received the combination of 2 antiemetics, 52 for having received regional anaesthesia/TIVA and 115 for having been emergency/outpatient cases. In order to include in chronological order (by date of surgery) the first 92 patients that gave consent so as to complete each prophylactic group, it was necessary to review 550 patients who met the eligibility criteria.
The most frequently used combinations (96.2%), and therefore those included in the analysis, were ondansetron 4mg+dexametasone 8mg (O&Dex), ondansetron 4mg+haloperidol 1mg (O&Hal1), ondansetron 4mg+haloperidol 2mg (O&Hal2), and ondansetron 4mg+droperidol 1.25mg (O&Dro). Other combinations were also used (3.8%), but these were not analysed.
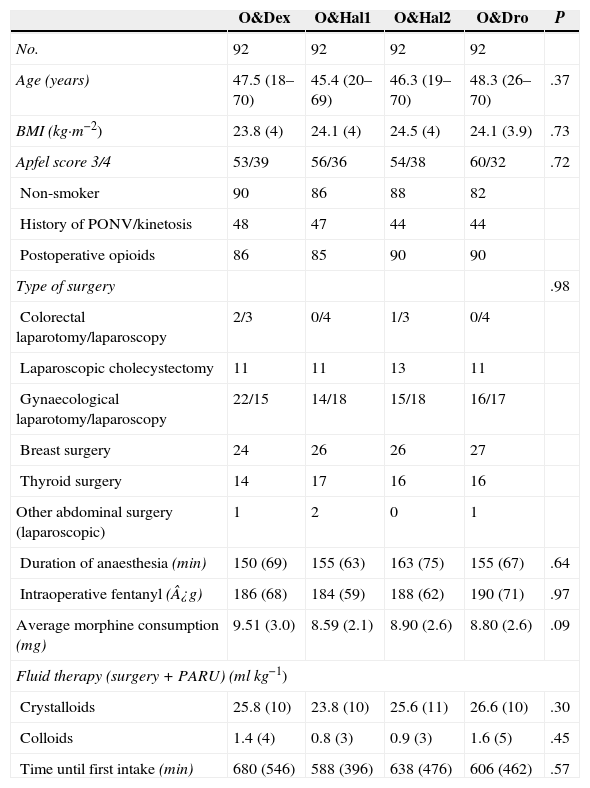
We did not find any differences between the groups with regard to patient characteristics nor to factors related to the surgery or anaesthesia that could have affected the occurrence of PONV (Table 1).
Baseline Characteristics of Patients and Factors Related to the Surgery and Anaesthesia.
| O&Dex | O&Hal1 | O&Hal2 | O&Dro | P | |
|---|---|---|---|---|---|
| No. | 92 | 92 | 92 | 92 | |
| Age (years) | 47.5 (18–70) | 45.4 (20–69) | 46.3 (19–70) | 48.3 (26–70) | .37 |
| BMI (kg·m−2) | 23.8 (4) | 24.1 (4) | 24.5 (4) | 24.1 (3.9) | .73 |
| Apfel score 3/4 | 53/39 | 56/36 | 54/38 | 60/32 | .72 |
| Non-smoker | 90 | 86 | 88 | 82 | |
| History of PONV/kinetosis | 48 | 47 | 44 | 44 | |
| Postoperative opioids | 86 | 85 | 90 | 90 | |
| Type of surgery | .98 | ||||
| Colorectal laparotomy/laparoscopy | 2/3 | 0/4 | 1/3 | 0/4 | |
| Laparoscopic cholecystectomy | 11 | 11 | 13 | 11 | |
| Gynaecological laparotomy/laparoscopy | 22/15 | 14/18 | 15/18 | 16/17 | |
| Breast surgery | 24 | 26 | 26 | 27 | |
| Thyroid surgery | 14 | 17 | 16 | 16 | |
| Other abdominal surgery (laparoscopic) | 1 | 2 | 0 | 1 | |
| Duration of anaesthesia (min) | 150 (69) | 155 (63) | 163 (75) | 155 (67) | .64 |
| Intraoperative fentanyl (¿g) | 186 (68) | 184 (59) | 188 (62) | 190 (71) | .97 |
| Average morphine consumption (mg) | 9.51 (3.0) | 8.59 (2.1) | 8.90 (2.6) | 8.80 (2.6) | .09 |
| Fluid therapy (surgery+PARU) (mlkg−1) | |||||
| Crystalloids | 25.8 (10) | 23.8 (10) | 25.6 (11) | 26.6 (10) | .30 |
| Colloids | 1.4 (4) | 0.8 (3) | 0.9 (3) | 1.6 (5) | .45 |
| Time until first intake (min) | 680 (546) | 588 (396) | 638 (476) | 606 (462) | .57 |
The values are expressed either as a mean (standard deviation [SD] or range) or in numbers of patients.
BMI: body mass index; min: minutes; PONV: postoperative nausea and vomiting; O&Dex: ondansetron 4mg IV in combination with dexametasone 8mg IV; O&Dro: in combination with droperidol 1.25mg IV; O&Hal1: in combination with haloperidol 1mg IV; O&Hal2: in combination with haloperidol 2mg IV; PARU: post-anaesthesia recovery unit.
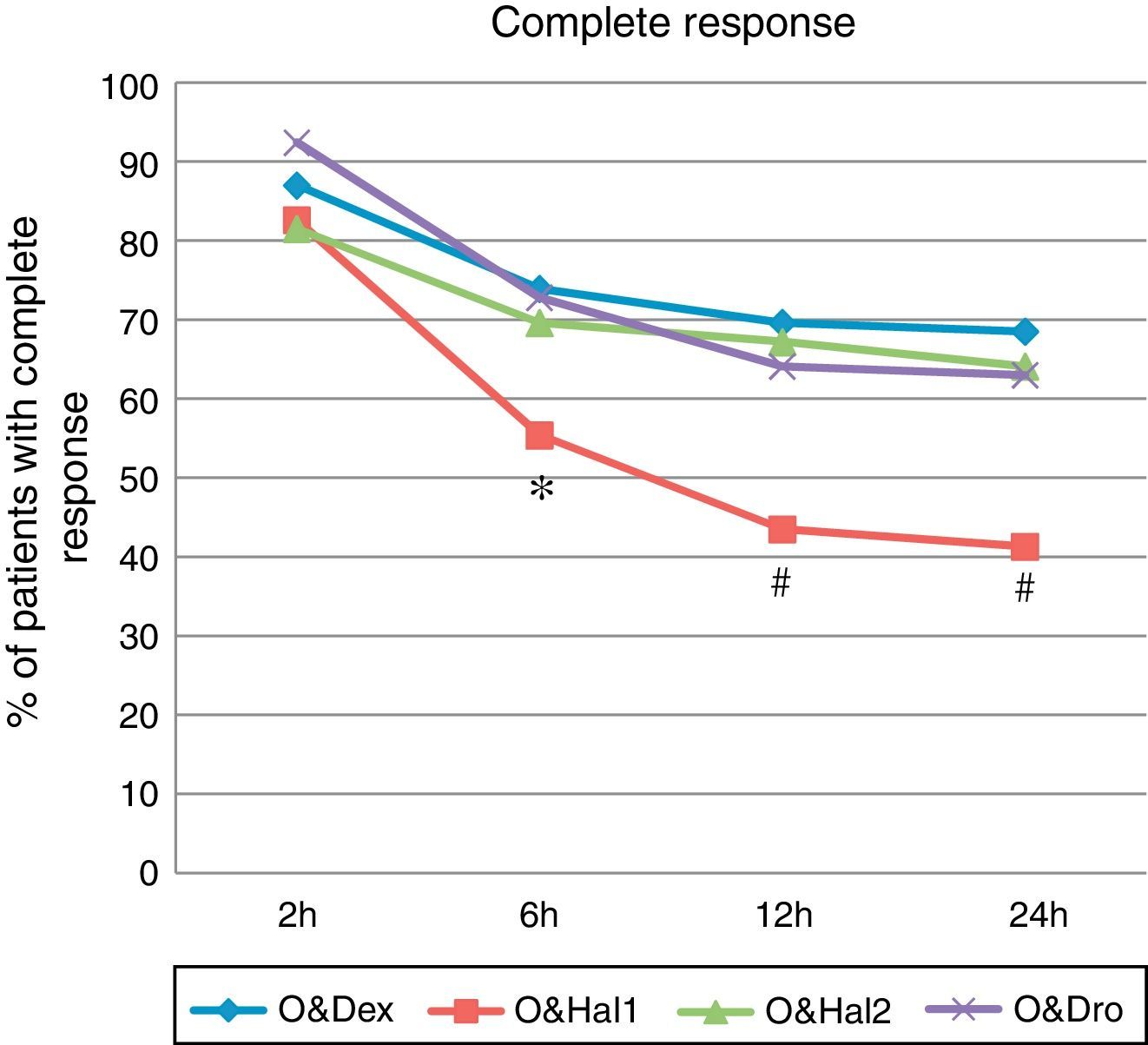
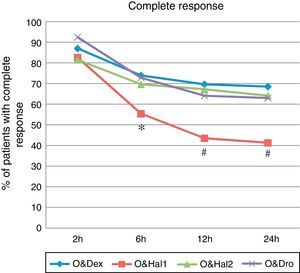
A complete response was achieved in 41% of patients in the O&Hal1 group (CI 95%: 31–52) vs 68% (CI: 58–78), 64% (CI: 53–74) and 63% (CI: 52–73) in patients in groups O&Dex, O&Hal2 and O&Dro respectively (P=.001). These significant differences were observed from the 2 to 6h interval (Fig. 1).
Percentage of patients with complete response (cumulative incidence) at each point of the assessment. O&Dex: ondansetron 4mg IV in combination with dexametasone 8mg IV; O&Dro: in combination with droperidol 1.25mg IV; O&Hal1: in combination with haloperidol 1mg IV; O&Hal2: in combination with haloperidol 2mg IV. * Significant differences (P<.05) between groups. Using the Bonferroni correction: # Significant differences (P<.01) compared with O&Dex, O&Hal2 and O&Dro.
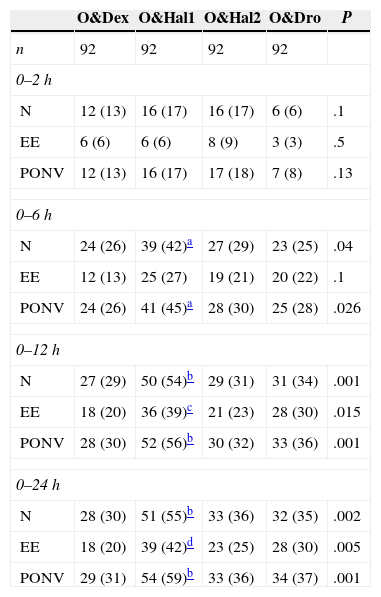
In the O&Hal1 group, 55% (CI: 45–66) of patients suffered from nausea at some stage vs 30% (CI: 21–41), 36% (CI: 26–46) and 35% (CI: 25–45) in groups O&Dex, O&Hal2 and O&Dro respectively (P=.002). These significant differences were observed from the 2 to 6h interval (Table 2).
Cumulative Incidence of Nausea, Emetic Episodes and Postoperative Nausea and Vomiting.
| O&Dex | O&Hal1 | O&Hal2 | O&Dro | P | |
|---|---|---|---|---|---|
| n | 92 | 92 | 92 | 92 | |
| 0–2h | |||||
| N | 12 (13) | 16 (17) | 16 (17) | 6 (6) | .1 |
| EE | 6 (6) | 6 (6) | 8 (9) | 3 (3) | .5 |
| PONV | 12 (13) | 16 (17) | 17 (18) | 7 (8) | .13 |
| 0–6h | |||||
| N | 24 (26) | 39 (42)a | 27 (29) | 23 (25) | .04 |
| EE | 12 (13) | 25 (27) | 19 (21) | 20 (22) | .1 |
| PONV | 24 (26) | 41 (45)a | 28 (30) | 25 (28) | .026 |
| 0–12h | |||||
| N | 27 (29) | 50 (54)b | 29 (31) | 31 (34) | .001 |
| EE | 18 (20) | 36 (39)c | 21 (23) | 28 (30) | .015 |
| PONV | 28 (30) | 52 (56)b | 30 (32) | 33 (36) | .001 |
| 0–24h | |||||
| N | 28 (30) | 51 (55)b | 33 (36) | 32 (35) | .002 |
| EE | 18 (20) | 39 (42)d | 23 (25) | 28 (30) | .005 |
| PONV | 29 (31) | 54 (59)b | 33 (36) | 34 (37) | .001 |
EE: emetic episodes; N: nausea; PONV: postoperative nausea and vomiting; O&Dex: ondansetron 4mg IV in combination with dexametasone 8mg IV; O&Dro: in combination with droperidol 1.25mg IV; O&Hal1: in combination with haloperidol 1mg IV; O&Hal2: in combination with haloperidol 2mg IV.
Number of patients (%) from the end of surgery until each assessment point.
In the O&Hal1 group, 42% (CI: 32–53) of patients suffered from EE vs 20% (12–29), 25% (16–35) and 30% (21–41) in groups O&Dex, O&Hal2 and O&Dro respectively (P=.005). These significant differences were observed in groups O&Dex and O&Hal1 from the 6 to 12h interval (Table 2).
Of the patients who suffered from PONV, 72% required rescue treatment: 17% of the O&Dex group, 33% of O&Hal1, 26% of O&Hal2 and 24% of O&Dro, but these differences were not found to be significant (P=.156).
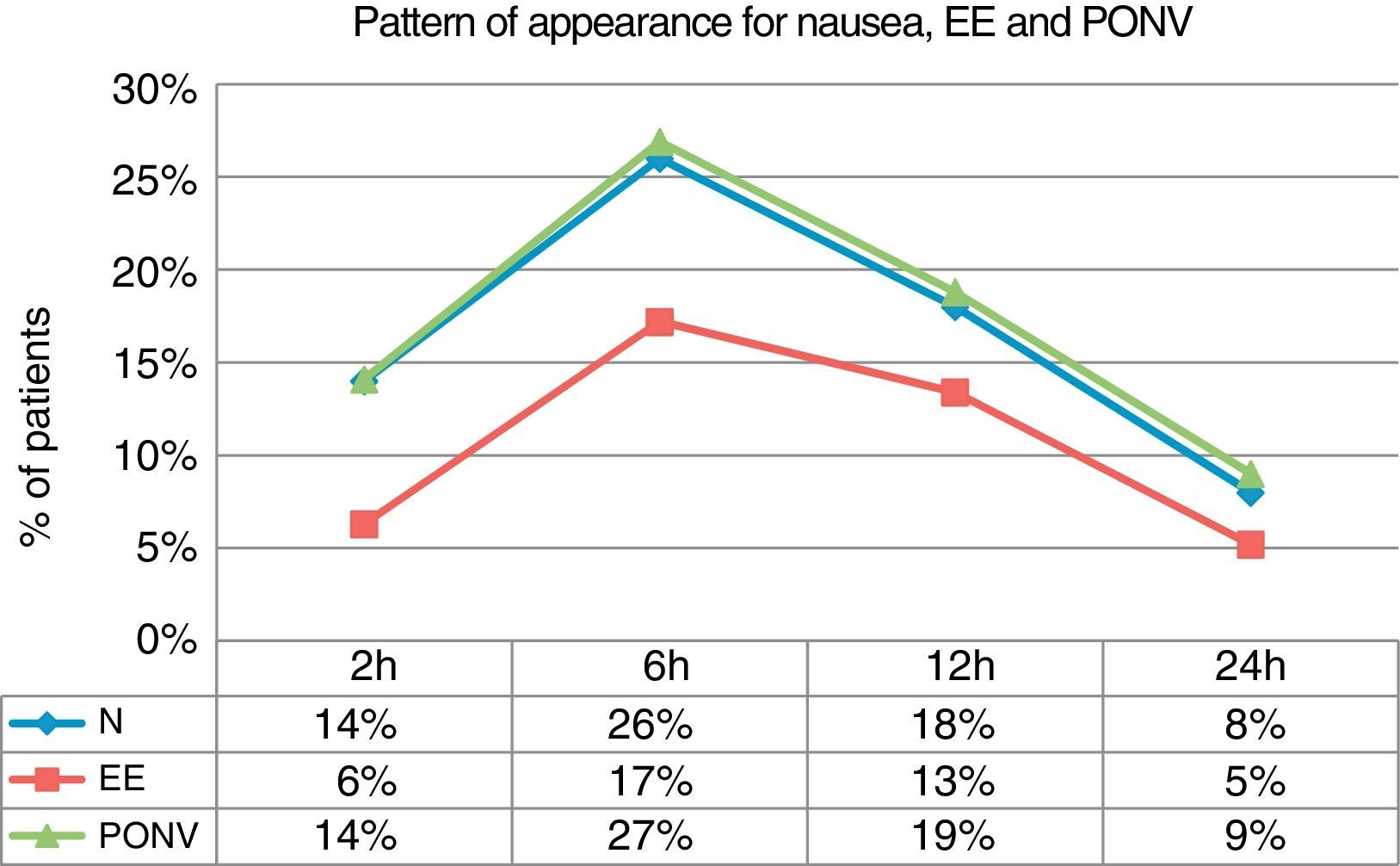
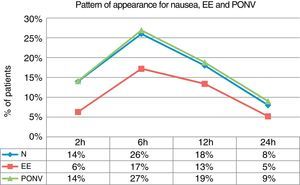
If we examine the global incidence of nausea, EE and PONV within each time interval, we can see that the greatest incidence is detected within the 2–6h interval, in which 27% of patients suffered from PONV, 26% from nausea and 17% from EE (Fig. 2).
To evaluate the influence of different confounding factors on the occurrence of PONV, we analysed the cases of PONV according to type of surgery, pain intensity and opioid requirements. No differences were found in the incidence of PONV according to type of surgery by anatomical location (P=.74). Due to the low number of patients with severe pain and in order to facilitate the statistical analysis, patients with moderate and severe pain were grouped together. No differences were found between groups regarding pain intensity, except at 24h, when a greater number of patients from the O&Hal2 group reported mild pain compared with the O&Dex group. However, opioid requirements did not differ between groups.
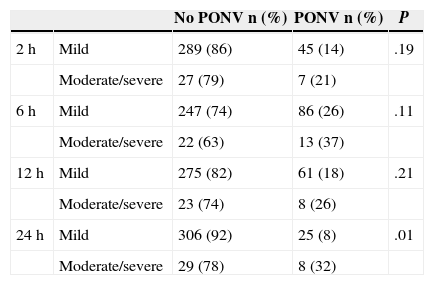
We analysed PONV incidence within each time interval according to the pain intensity during that period. We found differences in PONV incidence at 24h, with 32% of patients with moderate/severe pain suffering from PONV compared with 8% of patients with mild pain (P=.01) (Table 3). There was no significant difference in the quantity of morphine received among patients who suffered from PONV compared with non-sufferers. Average consumption was 8.81mg vs 9.06mg respectively (P=.38).
Incidence of Postoperative Nausea and Vomiting According to Pain Intensity Within Each Time Interval.
| No PONV n (%) | PONV n (%) | P | ||
|---|---|---|---|---|
| 2h | Mild | 289 (86) | 45 (14) | .19 |
| Moderate/severe | 27 (79) | 7 (21) | ||
| 6h | Mild | 247 (74) | 86 (26) | .11 |
| Moderate/severe | 22 (63) | 13 (37) | ||
| 12h | Mild | 275 (82) | 61 (18) | .21 |
| Moderate/severe | 23 (74) | 8 (26) | ||
| 24h | Mild | 306 (92) | 25 (8) | .01 |
| Moderate/severe | 29 (78) | 8 (32) |
PONV: postoperative nausea and vomiting.
We found statistical differences in the level of sedation in PARU between the groups. A significantly greater number of patients in the O&Dex group were completely awake (level 4) after 2h in PARU compared with patients in the O&Dro group (P=.007).
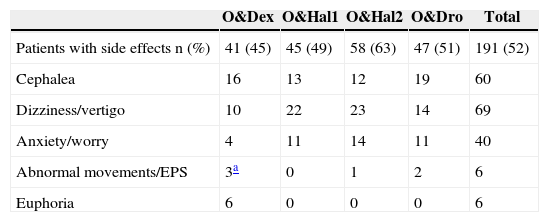
There was a greater incidence of secondary effects in the O&Hal2 group: 63% vs 45%, 49% and 51% in groups O&Dex, O&Hal2 and O&Dro respectively, although these results were not significant (P=.075). We found specific secondary effects for each prophylactic group, such as euphoria in the O&Dex group and abnormal movements/extrapyramidal symptoms (EPS), anxiety or dizziness/vertigo in the groups that were given butyrophenones (Table 4).
Number of Patients With Side Effects in Each Group and Number and Type of the Most Relevant Side Effects in Each Prophylactic Group.
| O&Dex | O&Hal1 | O&Hal2 | O&Dro | Total | |
|---|---|---|---|---|---|
| Patients with side effects n (%) | 41 (45) | 45 (49) | 58 (63) | 47 (51) | 191 (52) |
| Cephalea | 16 | 13 | 12 | 19 | 60 |
| Dizziness/vertigo | 10 | 22 | 23 | 14 | 69 |
| Anxiety/worry | 4 | 11 | 14 | 11 | 40 |
| Abnormal movements/EPS | 3a | 0 | 1 | 2 | 6 |
| Euphoria | 6 | 0 | 0 | 0 | 6 |
O&Dex: ondansetron 4mg IV in combination with dexametasone 8mg IV; O&Dro: in combination with droperidol 1.25mg IV; O&Hal1: in combination with haloperidol 1mg IV; O&Hal2: in combination with haloperidol 2mg IV; EPS: extrapyramidal symptoms.
No differences were found between the groups regarding the haemodynamic or respiratory variables. No arrhythmias were described during surgery or in the first 2h of the postoperative period.
DiscussionThe problem of PONV control in high-risk patients, along with the fact that droperidol, the most frequently used antiemetic in recent decades, was withdrawn from the market and then returned to hospitals a few years later at a higher price and subject to restrictions, led us to start using haloperidol as an antiemetic. In this context, we decided to review the effectiveness of the combinations of antiemetics used for high-risk patients.
The types of surgery chosen were those procedures considered to be highly emetogenic that are performed regularly in our medical centre.17–20 The Apfel scale for predicting the risk of PONV in adults has been validated and is the most commonly used21; it is applied systematically in the postoperative period to calculate the risk and guide prophylaxis. We used the scale in this study to find patients to include in our cohort.
We excluded patients who had undergone outpatient/emergency surgery because the anaesthetic and postoperative procedures used in these circumstances are very different from the standard procedures, which would have complicated the task of finding a uniform cohort and performing a postoperative follow-up. Patients who received TIVA/regional anaesthesia were also excluded as these techniques reduce the baseline risk of PONV.
When the analysis of factors related to the patient, the surgery and the anaesthesia that affect the occurrence of PONV were performed, no differences were found between the groups, and therefore the differences found in the complete response could be attributed to the prophylaxis received.
The antiemetic effectiveness of dexametasone, ondansetron and droperidol is well known,9–12 as is that of combinations that include ondansetron.22–25 Droperidol was the most frequently used antiemetic around the world,26 and, since the FDA alert, the use of haloperidol has increased. Evidence of its antiemetic effect has existed since the 1970s and it possesses a longer half-life than droperidol (12–36h vs 2.5h). In the only systematic review to have examined the antiemetic effectiveness of haloperidol, it was concluded that it is effective between 0.5 and 4mg with a minimal toxicity.15 But many of the studies included were not satisfactorily designed. The results of the clinical studies using haloperidol are controversial. An in-depth review of studies since the 1970s shows that higher doses of haloperidol have demonstrated greater effectiveness.27–30 Various studies using haloperidol 1mg have shown that it is effective15,28,31,32 but other studies have not achieved the same results.33,34 Even so, clinical guidelines recommend the use of haloperidol 1 or 2mg in combination.8,27,35,36 In the studies that showed haloperidol to be effective, patients were either not high-risk or were not observed for a sufficient length of time.27,31,35
O&Hal2, O&Dex and O&Dro proved to be more effective than O&Hal1. The complete response observed was worse than that obtained in other studies, possibly because of the patients’ high risk of PONV and the types of surgery included.
The peak of PONV incidence occurs within the 2–6h interval, and incidence remains elevated for the 6–12h period. The cause of this pattern is multifactorial, and could be explained by the late secondary effect of postoperative opioids, transfer from PARU to the general ward and the mobilisation of patients (with increased sensitivity of the vestibular system caused by the opioids), the start of oral tolerance, worse control over pain or the decreased effect of certain antiemetics. If a postoperative visit is not performed, it can lead to the belief that the prophylaxis used has been effective, unaware of the fact that the patient later suffered from PONV on the ward.37
Although several studies have shown a lower intensity of postoperative pain after administering corticosteroids, within the 12–24h interval a greater number of patients from the O&Hal2 group defined their pain as mild compared with patients from the O&Dex group. This fact may have influenced the greater incidence of PONV in the O&Dex group within this interval, even though morphine consumption did not increase and both types of prophylaxis were the most effective.
During the first 2h, a lower level of consciousness was observed in patients in the O&Dro group; this could perhaps explain the strong anti-nausea effect of droperidol in PARU.
Patients in the O&Hal2 group suffered from a greater number of side effects. It is important to underline the high frequency of side effects caused by neuroleptics and the sedative effect associated with “high” doses of haloperidol. Extremely high doses were not used in this study; however, it appears that this dose has a very marked effect on patients without delirium or with a high sensitivity to neuroleptics.
The retrospective design of this study created limitations due to the impossibility of controlling all the factors that could affect the occurrence of PONV, and the reliability of certain variables could be called into question. However, uniform groups of patients were found for analysis, and by excluding those patients who had received TIVA/regional anaesthesia or outpatient/emergency surgery, we eliminated certain important factors that could have affected PONV incidence. At the same time, using the Apfel scale, patients were excluded if they did not classify as high risk (Apfel≥3), thereby controlling any possible selection bias created by the inclusion criteria. No differences were found between the groups regarding factors related to surgery and anaesthesia, and therefore these did not affect the results. The fact that our centre is highly interested in PONV and has a complete digitised clinical record made it possible for us to access many variables related to PONV, which allowed us to have a complete and reliable database.
After having studied PONV in our centre, we know that it occurs primarily after discharge from PARU, highlighting the importance both of finding an effective and long-lasting prophylaxis and of carrying out postoperative visits. We did not achieve good control over PONV in high-risk patients using haloperidol 1mg in combination; we therefore decided to withdraw its use from our clinical practice. In any case, there is a need for more prospective studies that are designed with the aim of confirming our results and of finding the minimum effective dose of haloperidol and its tolerance.
In patients at high risk of PONV who have undergone highly emetogenic surgery, the O&Hal1 combination is not effective. Despite its efficacy, the high incidence of side effects associated with the use of O&Hal2 must be investigated before deciding to use this combination.
FundingThis study was funded by grant no. TRA-107 awarded by the Ministry of Health and Social Policy.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Veiga-Gil L, López-Olaondo L, Pueyo J, Callejas R, Duque P, Carrascosa F. Dosis bajas de haloperidol en combinación con ondansetrón no son eficaces para la profilaxis de náuseas y vómitos postoperatorios en pacientes propicios a esta complicación. Cir Esp. 2015;93:110–116.
Preliminary results from this study were presented at the Euroanaesthesia Congress in Paris in June 2012.