Solitary fibrous tumours (SFT) of the liver are uncommon neoplastic lesions with benign histological characteristics that develop in the mesenchymal tissue1,2. This type of tumour develops most frequently in locations such as the pleura, peritoneum, thymus or meninges1. Their intrahepatic growth is extremely rare, with only a few dozen cases published in the literature1,2.
This type of tumour has a 5-year survival rate of around 85%, with a 5-year and 10-year risk of metastasis of 26% and 46%, respectively3. The main risk factors for a poor prognosis are tumour size and high mitotic indices.
In 5% of cases, these tumours appear in association with a paraneoplastic syndrome known as Doege-Potter syndrome3, which presents with severe hypoglycaemia and was first described in 19304. This syndrome is more frequent in patients between the ages of 60 and 804 and is associated with a worse prognosis, even more so if the location of the tumour is extrapleural3,4.
This paraneoplastic syndrome presents with severe hypoglycaemia and decreased levels of C-peptide, insulin and IGF-I in the blood due to the secretion of IGF-II by the tumour, which binds to IGF-I receptors4. This union not only causes hypoglycaemia but is also associated with an increase in the number of mitoses in the tumour and its malignant transformation.
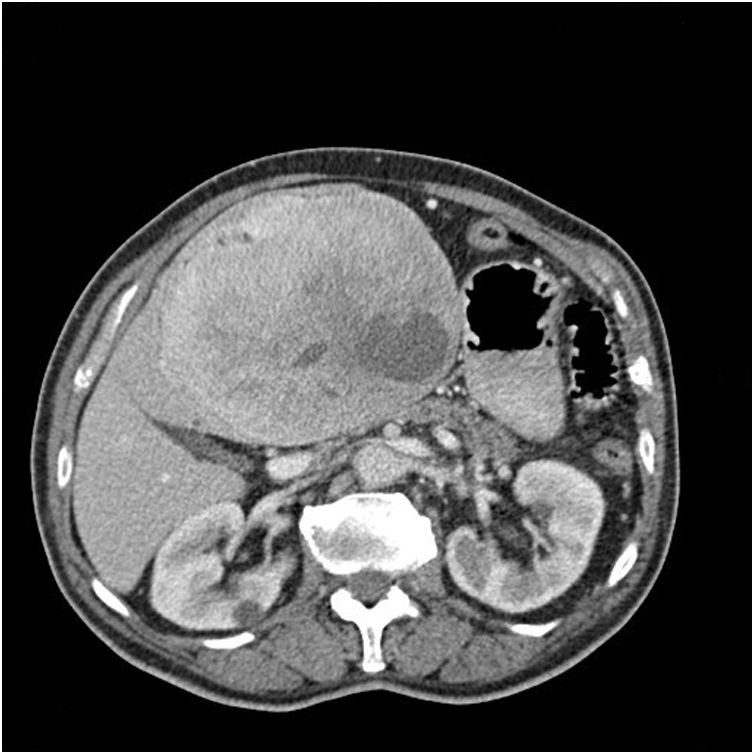
We present the case of an 83-year-old patient with a history of dyslipidaemia and altered baseline fasting glucose who came to the emergency room after a routine blood glucose test of 33 mg/dL and a large indurated epigastric mass. A thoracoabdominal CT scan revealed a mass suggestive of gastric GIST in the gastrohepatic ligament measuring 16 × 12 × 14 cm that was hypervascular and had interior necrotic areas, which exerted a mass effect on the neighbouring organs and caused the portal-splenic axis to collapse, along with signs of portal hypertension (Fig. 1). Gastroscopy was performed, and extrinsic compression was observed at the lesser curvature; biopsies were taken, but not significant. Lab workup to assess hypoglycaemia showed insulin figures of 0.2 U/mL; 0.11 ng/dL of baseline C-peptide and 46.7 ng/mL of IGF-1.
With the suspicion of non-disseminated gastric GIST, we scheduled a surgical intervention, during which we observed a 15 × 15 cm tumour dependent on liver segments 2 and 3, but no invasion of neighbouring structures. We performed a left bisegmentectomy of the liver without incident. During the immediate postoperative period, the patient’s evolution was good; the hypoglycaemic episodes were corrected, and there was even a certain trend towards hyperglycaemia. The postoperative lab workup 7 days after surgery showed that C-peptide and IGF-1 levels had normalized (1.7 ng/mL and 103.1 ng/mL, respectively). The patient was discharged 10 days after surgery without incident.
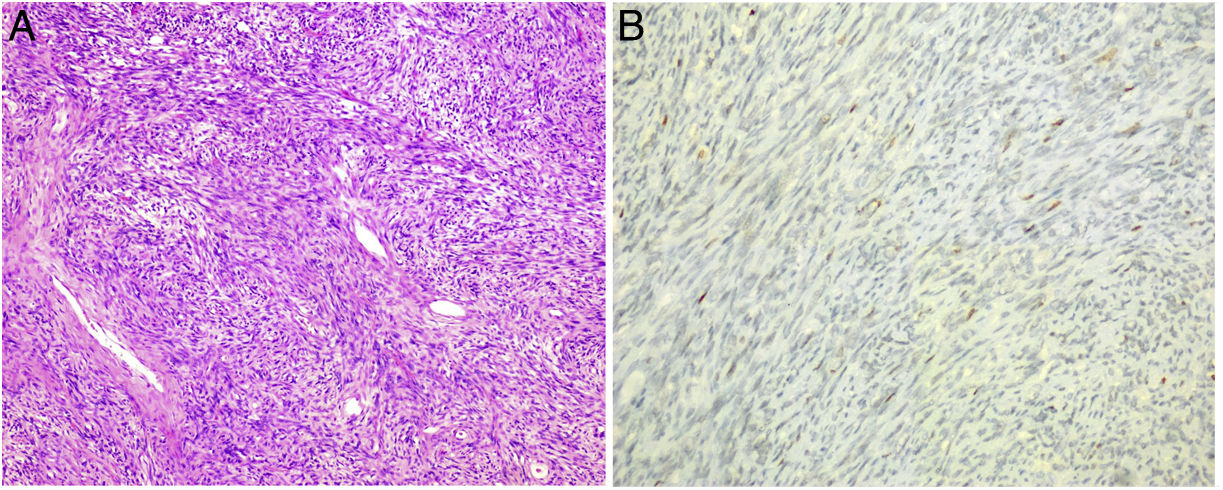
After the analysis of the surgical specimen, the diagnosis of SFT was made since the histological sections showed a mesenchymal neoformation that was arranged in bundles and fascicles with alternating hypercellular and hypocellular areas and the presence of hyalinized collagen bands as well as vascular structures, some of which were branched. The number of mitotic figures was variable (1–3 mitosis/10 cga). The cytoplasms showed poorly defined borders (Fig. 2A). Ischaemic-type necrosis and small calcifications were identified in the central part of the lesion.
The neoformation showed positivity with CD34 (Fig. 2B) and Caldesmon, focal with STAT 6, and negativity with CD117, DOG 1, CK AE1-AE3, S-100, EMA, actin and desmin. The cell proliferation index measured with Ki67 was 5%–10%.
Solitary fibrous tumours are benign neoplasms that have the potential for malignant transformation2. To date, 89 cases have been published of SFT5, including ours. They are usually asymptomatic and may be associated with a paraneoplastic syndrome that occurs with hypoglycaemia (Doege-Potter syndrome), so their growth is progressive and slow, and they are diagnosed incidentally2.
The radiological characteristics on CT and MRI are similar to those of hepatocarcinoma2, although in our case, given its proximity to the stomach, the tumour was diagnosed as a gastric GIST.
Biopsy is not necessary due to the risk of rupture and tumour seeding, as the differential diagnosis includes hepatocarcinoma2. In our case, since the suspected diagnosis was gastric GIST, we decided to take endoscopic biopsies, which were inconclusive. Given that the treatment of choice for both gastric GIST and SFT is surgery, and the patient was symptomatic due to poorly controlled hypoglycaemic episodes, we decided not to carry out further preoperative studies to confirm the highly suspected diagnosis of GIST.
The definitive diagnosis is histopathological, identifying in the sample cell groups separated by extensive bands of connective tissue and myxoid changes, with foci of necrosis and elevated rates of mitosis (≥4/10). These tumours are usually CD34+ (although this is not constant) and STAT6+, which is a much more specific marker1,2,4.
Conflict of interestsNone.
Please cite this article as: Correa Bonito A, Muñoz-Hernández P, de la Hoz Rodríguez Á, Delgado Valdueza J, Martín Pérez E. Síndrome de Doege-Potter secundario a tumor fibroso solitario hepático. Cir Esp. 2022;100:108–110.