In 2007, a multicenter protocol was developed in Catalonia, Spain, combining neoadjuvant chemoradiotherapy and liver transplantation (LT) for those patients with unresectable hilar cholangiocarcinoma (hCCA).
AimTo analyse the effectiveness of the neoadjuvant chemoradiotherapy and LT for those patients enrolled in the protocol based on intention-to-treat.
MethodsObservational multicenter study which includes patients ≤ 68 years-old diagnosed with unresectable, solitary tumors ≤ 3 cm in radial diameter, without evidence of lymph node metastases. The protocol was based on a strategy of neoadjuvant therapy with high-dose radiation (45 Gy in total) plus intravenous fluorouracil (5-FU) given as a daily bolus for the first 3 days of radiation follow by oral capecitabine until transplantation. The patient was included in waiting list for LT if no evidence of disseminated disease was found.
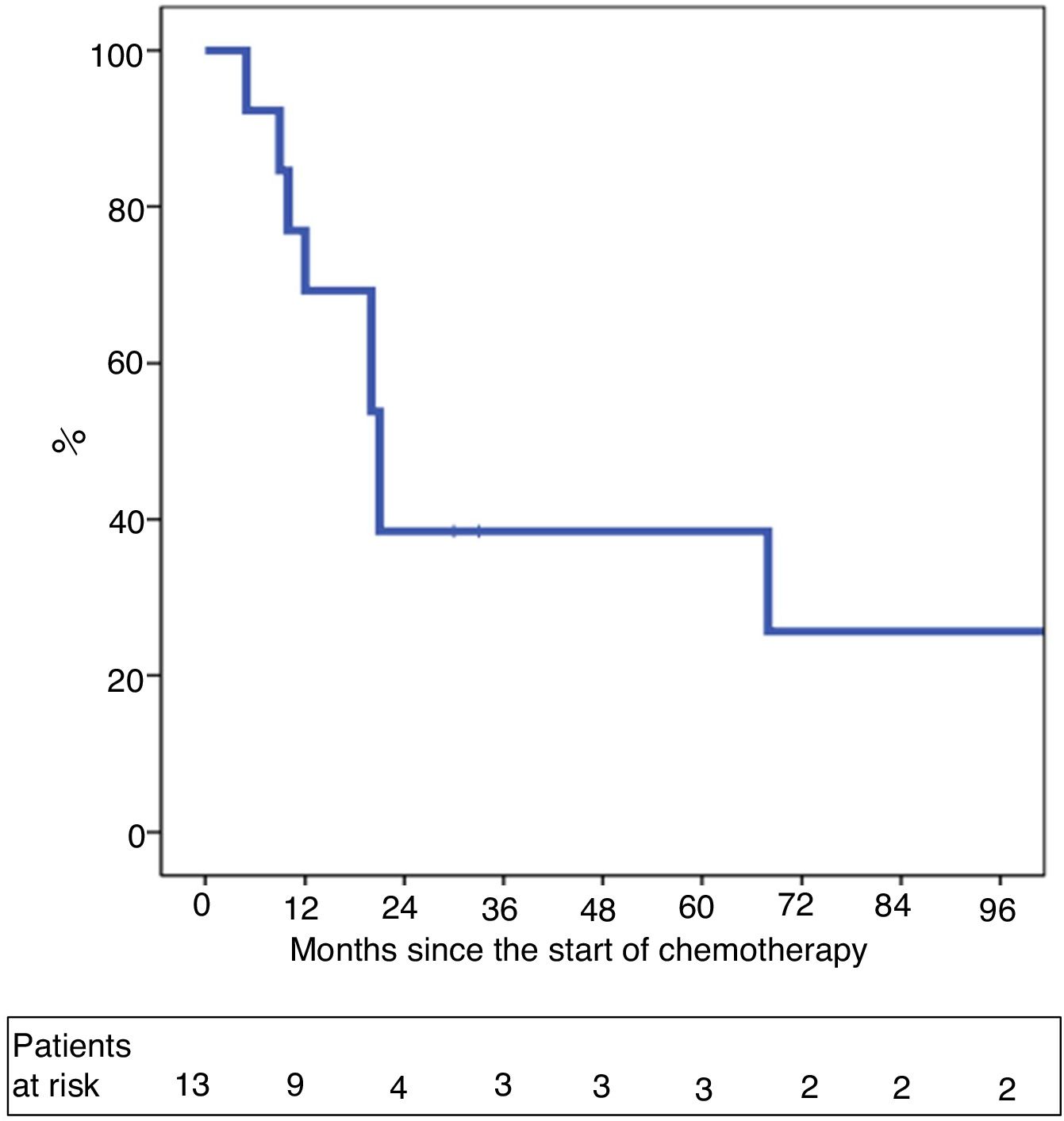
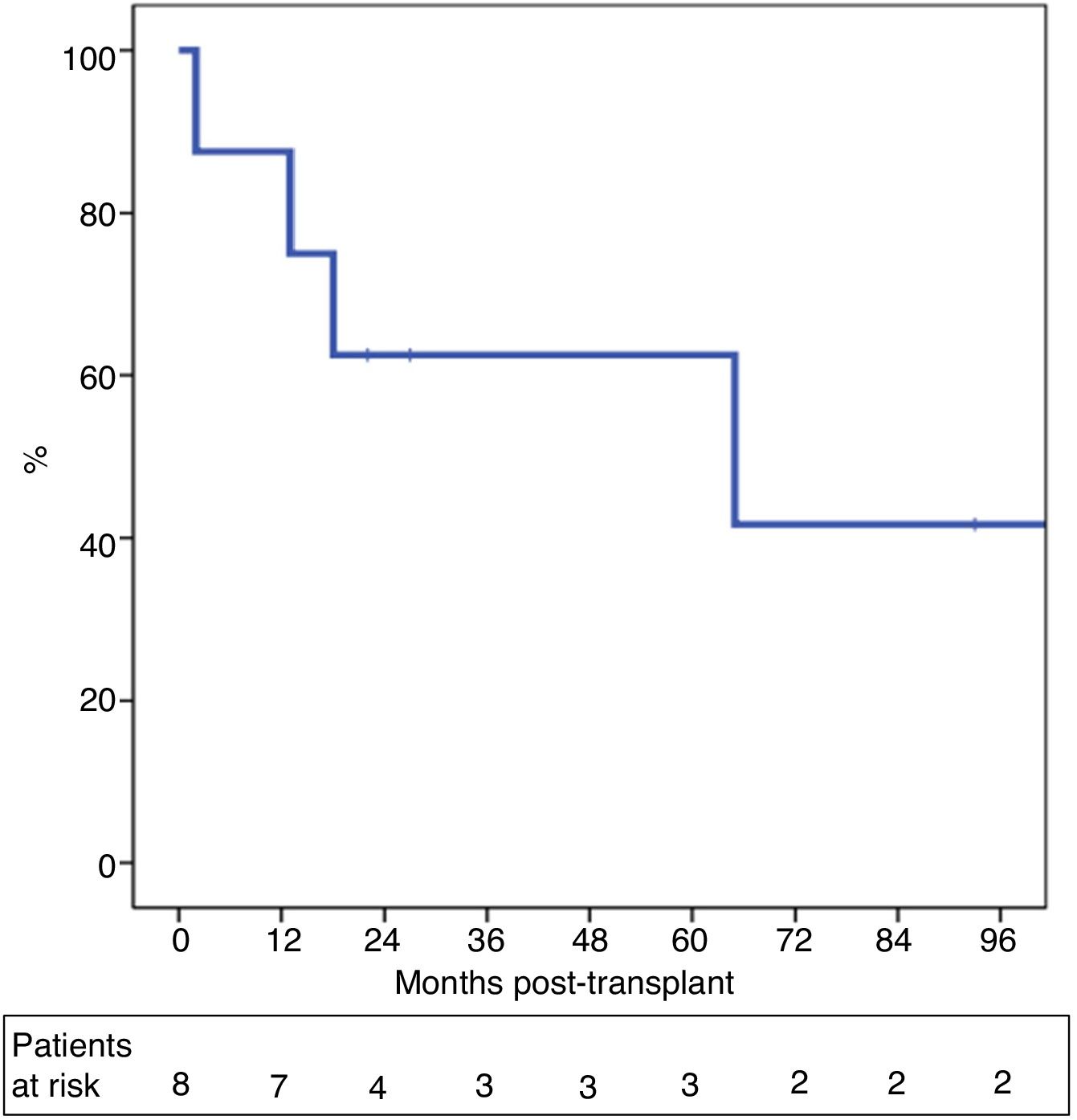
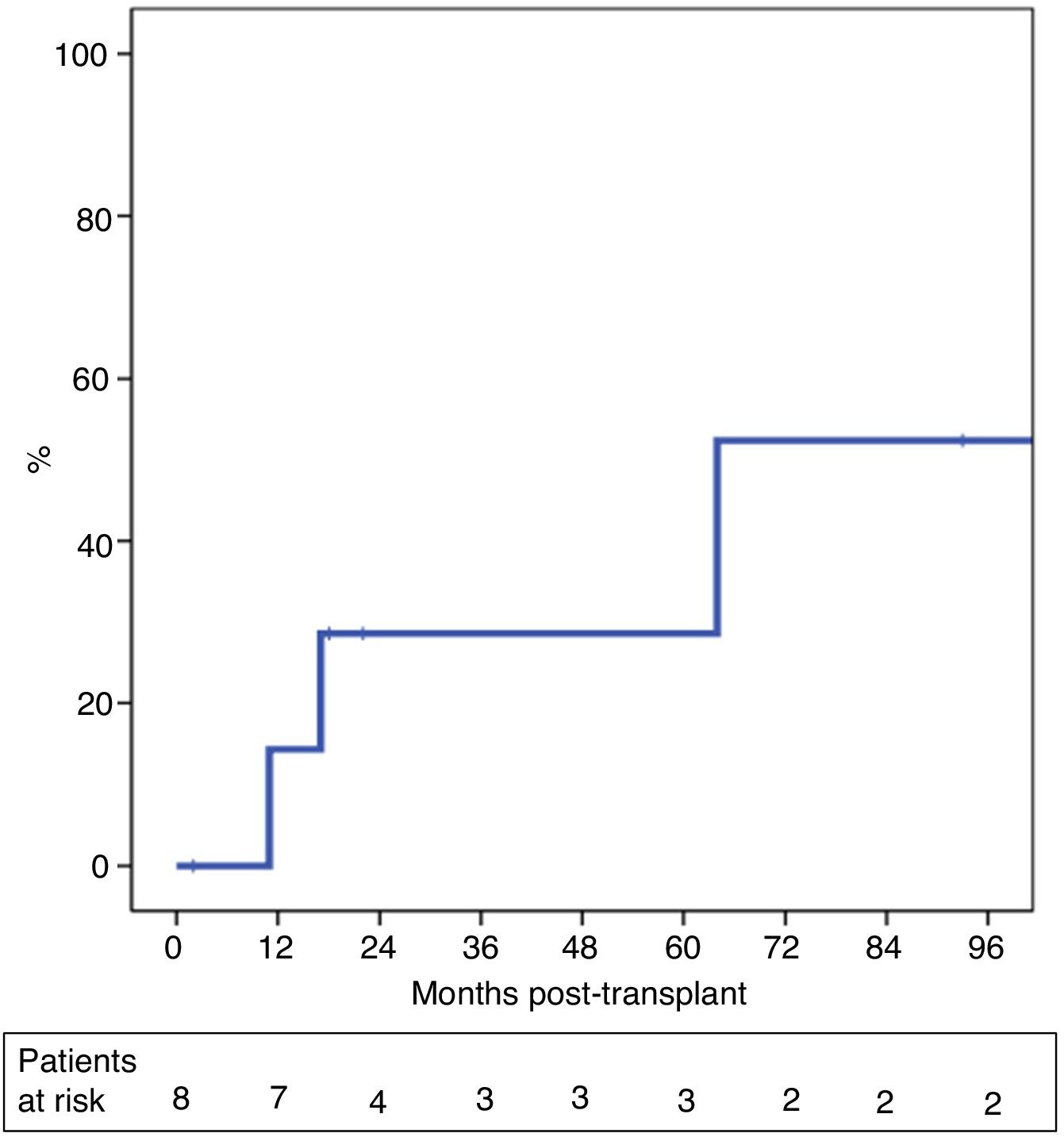
ResultsBetween 2007 and 2018, 13 patients were enrolled in the transplant protocol. Of those, 61% (8/13) of the patients were transplanted. The average time spent on the waiting list was 122 days (range 5–192). Intent-to-treat survival was 69% and 39% at one and 5 years. Post-transplantation overall survival was 87% and 62% and 29% recurrence rate at 5 years.
ConclusionThe suitability of the neoadjuvant chemoradiotherapy and LT protocol was 61% in our series with long-term overall survival and should be considered as an alternative to resection for patients with localized node-negative hCCA.
En 2007 se consensuó un protocolo asistencial entre los tres centros de trasplante hepático (TH) de Cataluña, que contemplaba el trasplante hepático (TH) asociado a quimiorradioterapia neoadyuvante como tratamiento del colangiocarcinoma perihiliar (PCCA) irresecable.
ObjetivoAnalizar la aplicabilidad del TH en los pacientes con PCCA incluidos en el protocolo y la supervivencia por intención de tratamiento.
Material y métodosEstudio observacional multicéntrico que incluye a pacientes de edad ≤ 68 años, diagnosticados de PCCA ≤ 3 cm (diámetro radial), irresecable, sin afectación ganglionar o metástasis a distancia. Los pacientes recibieron tratamiento neoadyuvante basado en radioterapia externa en una dosis total de 45 Gy, asociado con bolos de 5-fluoracilo durante los tres primeros días de irradiación y posteriormente capecitabina oral. Aquellos en los que no se objetivó signos de progresión se incluyeron en la lista de espera para TH.
ResultadosEntre 2007 y 2018, 13 pacientes fueron incluidos en dicho protocolo. Ocho de los 13 pacientes (61%) fueron trasplantados tras un tiempo en lista de espera de 122 días (rango 5–192). La supervivencia por intención de tratamiento a 1 y 5 años fue del 69 y 39%. La supervivencia global post-TH a 1 y 5 años fue del 87 y 62%, con una probabilidad de recidiva del 29% a los cinco años post-TH.
ConclusiónLa aplicabilidad del trasplante hepático combinado con quimiorradioterapia neoadyuvante ha sido del 61% en nuestra serie y debe ser considerado como un tratamiento potencialmente curativo para pacientes seleccionados con PCCA irresecable y sin enfermedad metastásica.
Ebata et al.1 defined perihilar cholangiocarcinoma (pCCA) as any mass encompassing the bile duct at the hilar level, located between the right edge of the umbilical portion of the portal vein and the left edge of the right posterior portal segment. It is a rare tumor and a challenge from a surgical standpoint because of its proximity to the structures of the hepatic hilum, which are often involved, as well as the regional lymph nodes. Surgery is the only potentially curative therapeutic option in the absence of distant metastasis, and the main objective is R0 resection. However, negative margins are only achieved in 40%–80%, and 5-year survival rates range from 20% to 67%,2–9 dropping to nearly 0% in patients with R1 resection.10–12 Currently, the only alternative in cases of unresectable tumors is systemic palliative treatment, with a median survival of 12 months.13
In 2000,14–16 the Mayo Clinic presented the preliminary results of a multimodal protocol for pCCA based on a strategy of neoadjuvant chemotherapy, radiotherapy and brachytherapy, followed by exploratory laparotomy and liver transplantation (LT). Patients included in the protocol with an unresectable perihilar lesion ≤3 cm (radial) and no evidence of lymph node metastasis, and those with resectable pCCA in the context of primary sclerosing cholangitis (PSC), achieved a 5-year survival rate of 82% after liver transplantation.14–18 This strategy was extended to other North American hospitals,19,20 with similar results.
According to what has been published, a care protocol was drawn up in 2007 in Catalonia based on LT with neoadjuvant chemotherapy (CT) and radiotherapy (RT) to treat patients with unresectable pCCA ≤3 cm, with no lymph node or distant metastases. Said protocol was led by the Hospital Clínic (Dr. García Valdecasas) and was created in consensus with the other two LT centers in Catalonia, Hospital Universitario de Bellvitge and Hospital Universitario Vall d’Hebron. The objective of our study was to analyze the applicability of LT and the survival by intention to treat of those patients with pCCA included in said protocol.
MethodsThis is an observational, multicenter study that retrospectively analyzes patients diagnosed with unresectable pCCA included in the management protocol for liver transplantation and neoadjuvant CT-RT, which began in 2007.
All patients signed a consent form to participate in the study, which had been approved by the Ethics Committee of each hospital.
Diagnostic criteria for pCCAAny stenosis or radiological mass located at the hepatic hilum with histological confirmation of adenocarcinoma (by cytology or endoluminal biopsy) and/or a CA 19−9 value greater than 100 U/mL with a total bilirubin value less than 3 mg/dL.
Criteria for unresectabilityMagnetic resonance cholangiopancreatography (MRCP) is the best imaging test to assess the extent of biliary involvement. Unresectable pCCA was defined as a Bismuth IV lesion21 with bilateral extension of the tumor to second-order biliary radicals and those with unilateral ductal involvement to second-order biliary radicals and portal involvement +/− contralateral hepatic lobar atrophy or with involvement of the main portal vein or bilaterally22 or with insufficient liver remnant volume even after portal embolization.
StagingTo rule out distant metastasis and/or lymph node involvement, all patients underwent a computed tomography (CT) scan. Positron emission tomography (PET/CT) was indicated in patients with suspected distant disease. Endoscopic ultrasound-guided biopsy was performed in patients with regional lymphadenopathy suspected of neoplastic involvement.
Liver transplantation protocol combined with neoadjuvant CT + RTInclusion criteria- •
Patients aged ≤68 years.
- •
Unresectable PCCA ≤ 3 cm radial diameter (neither the vascular swelling, nor the length of the biliary involvement, nor the poor definition of the tumor boundaries were contraindications).
- •
Absence of remote illness.
- •
Patients who had received prior chemotherapy or radiotherapy for pCCA.
- •
Previous surgical intervention for pCCA, percutaneous biopsy or intended resection.
- •
Pathological history or associated disease that contraindicates liver transplantation.
- •
Uncontrolled infection or poor general condition.
Neoadjuvant treatment was based on the Mayo Clinic scheme.13 This scheme included external RT at a total dose of 45 Gy (in 30 fractions of 15 Gy, twice a day, for three weeks). Concomitantly, 5-fluorouracil (5-FU) was administered at a dose of 500 mg/m2 as a daily bolus for the first three days of radiation. Brachytherapy was not contemplated in this protocol. Subsequently, the patients received capecitabine at a dose of 2000 mg/m2 per day, two out of every three weeks until transplantation.
Patients with bilirubin values greater than 3 mg/dL underwent a biliary drainage procedure using percutaneous transhepatic cholangiography (PTHC) to achieve bilirubin values lower than 3 mg/dL before initiating neoadjuvant CT-RT.
Criteria for inclusion on the waiting list for liver transplantation- 1
Meet the inclusion criteria specified above.
- 2
Have received neoadjuvant CT-RT.
- 3
Confirmed absence of metastatic lymph node involvement or distant disease by exploratory laparotomy, taking lymph node samples from suspicious lymphadenopathies near the hepatic hilum, celiac trunk, retropancreatic or any suspicious lesion of the abdominal cavity.
In order to be transplanted in less than three months, and in consensus with the rest of the groups and the regional coordination office (OCATT), the patients were added to a common prioritization list with a Model for End-Stage Liver Disease (MELD) score of 19, the same as the score received by patients with hepatocellular carcinoma at high risk of progression on the waiting list.
The follow-up protocol for these patients during their stay on the waiting list included thoracoabdominal CT scan and CA 19−9 every three months.
Liver transplantationAt the time of LT, the abdominal cavity was explored, and an intraoperative biopsy was taken of the lesions suspected of liver metastases or extrahepatic disease, if any. If disease progression was confirmed histologically, continuing with the LT procedure was ruled out, so it was advisable to have a second recipient.
In the event of a negative result, the transplantation was continued in accordance with the hospital’s standard technique, with two considerations:
- 1
Resect the entire main bile duct as an oncological treatment of the biliary tumor, which involves biliary reconstruction using a Roux-en-Y loop and hilar lymphadenectomy.
- 2
Given the frequent involvement of the proper hepatic artery during RT, it is recommended not to use said artery of the recipient for arterial reconstruction in transplantation.
Patients received conventional immunosuppression as stipulated at each hospital, based on calcineurin inhibitors, mycophenolate mofetil and/or steroids.
Post-transplantation follow-upPost-LT follow-up was standard, with periodic lab work and thoracoabdominal-pelvic CT and CA 19−9 tumor marker levels every three months during the first two years, and every six months thereafter. Post-LT systemic treatment was only indicated in patients with evidence of disease recurrence.
Statistical studyDemographic data, neoadjuvant therapy, intraoperative and pathology study data, and patient follow-up were recorded retrospectively. A descriptive analysis presented the categorical variables as total number and percentage, and the quantitative variables as medians and range. Survival was analyzed by intention to treat, overall post-transplant survival, and disease-free survival of the series using a Kaplan-Meier analysis. Intention-to-treat survival time was calculated from the date of initiation of neoadjuvant systemic treatment to the date of death or last follow-up. Overall survival was calculated from the date of LT until death or last follow-up, and disease-free time until the appearance of recurrence or last follow-up. Statistical analysis was performed using SPSS® Statistics Version 22.
ResultsBetween January 2007 and August 2018, thirteen patients from the three centers that perform transplantation in Catalonia (Hospital Vall d'Hebron, Hospital Bellvitge, Hospital Clínic) were diagnosed with unresectable pCCA and evaluated for inclusion in the CT-RT and LT protocol. We collected follow-up data for these patients until December 2019.
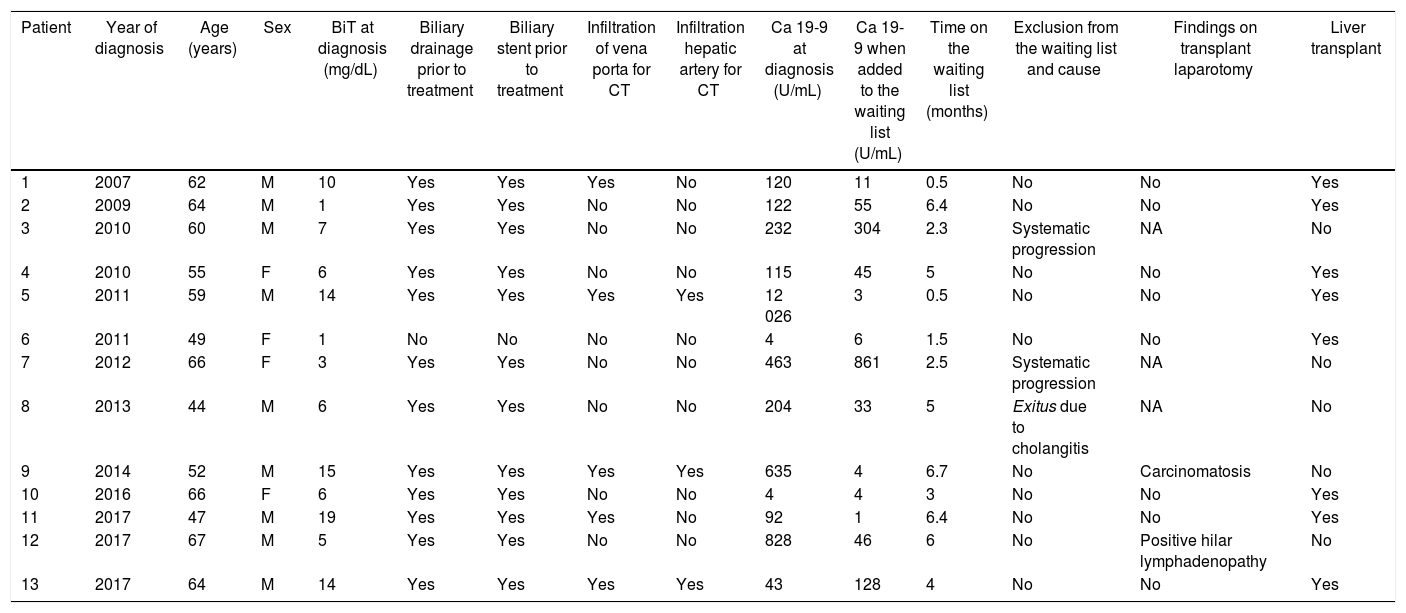
The characteristics of the study group are reflected in Table 1.
Characteristics of the patient and pre-transplant.
| Patient | Year of diagnosis | Age (years) | Sex | BiT at diagnosis (mg/dL) | Biliary drainage prior to treatment | Biliary stent prior to treatment | Infiltration of vena porta for CT | Infiltration hepatic artery for CT | Ca 19-9 at diagnosis (U/mL) | Ca 19-9 when added to the waiting list (U/mL) | Time on the waiting list (months) | Exclusion from the waiting list and cause | Findings on transplant laparotomy | Liver transplant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2007 | 62 | M | 10 | Yes | Yes | Yes | No | 120 | 11 | 0.5 | No | No | Yes |
| 2 | 2009 | 64 | M | 1 | Yes | Yes | No | No | 122 | 55 | 6.4 | No | No | Yes |
| 3 | 2010 | 60 | M | 7 | Yes | Yes | No | No | 232 | 304 | 2.3 | Systematic progression | NA | No |
| 4 | 2010 | 55 | F | 6 | Yes | Yes | No | No | 115 | 45 | 5 | No | No | Yes |
| 5 | 2011 | 59 | M | 14 | Yes | Yes | Yes | Yes | 12 026 | 3 | 0.5 | No | No | Yes |
| 6 | 2011 | 49 | F | 1 | No | No | No | No | 4 | 6 | 1.5 | No | No | Yes |
| 7 | 2012 | 66 | F | 3 | Yes | Yes | No | No | 463 | 861 | 2.5 | Systematic progression | NA | No |
| 8 | 2013 | 44 | M | 6 | Yes | Yes | No | No | 204 | 33 | 5 | Exitus due to cholangitis | NA | No |
| 9 | 2014 | 52 | M | 15 | Yes | Yes | Yes | Yes | 635 | 4 | 6.7 | No | Carcinomatosis | No |
| 10 | 2016 | 66 | F | 6 | Yes | Yes | No | No | 4 | 4 | 3 | No | No | Yes |
| 11 | 2017 | 47 | M | 19 | Yes | Yes | Yes | No | 92 | 1 | 6.4 | No | No | Yes |
| 12 | 2017 | 67 | M | 5 | Yes | Yes | No | No | 828 | 46 | 6 | No | Positive hilar lymphadenopathy | No |
| 13 | 2017 | 64 | M | 14 | Yes | Yes | Yes | Yes | 43 | 128 | 4 | No | No | Yes |
M: male; F: female; BiT: total bilirubin; NA: not applicable.
All the cases in our series were de novo pCCA, with no history of PSC. It was necessary to drain the bile duct and place bilateral stents using PTHC in 12 patients. Four patients (numbers 6, 10, 11 and 13) required endoluminal cytology for the definitive diagnosis because they presented radiological images of a hilar mass but tumor marker levels were not elevated.
CT-RT treatment was completed in 12 patients. Oral capecitabine was discontinued in patient 11 due to associated morbidity. After exploratory laparotomy, all patients were placed on the waiting list for LT. Pre-LT CA19−9 levels were higher than 500 U/mL in only one case, as shown in Table 1. The median time between diagnosis and inclusion on the list was five months (r: 3–11 months). Patient number 12 presented several episodes of recurrent cholangitis despite biliary drainage, which delay the start of neoadjuvant treatment and, therefore, inclusion on the waiting list (11 months after diagnosis).
Removal from the waiting listDuring the period on the waiting list, 2 patients had to be excluded due to tumor progression in the form of cutaneous metastases 2 months later (patient number 3) and the appearance of liver metastases 3 months later (patient number 7). A third patient died four months later from septic shock due to cholangitis, with no signs of progression (Table 1).
At the time of transplantation, the procedure was suspended in two patients after the finding of carcinomatosis during laparotomy in one case, and lymphadenopathic involvement in the second. The time on the waiting list for these two patients was 6.7 and 6 months (Table 1).
In the end, eight of the thirteen patients were transplanted, which represents an applicability of 61%. The median waiting list time for these patients had been 122 days (r: 5–192 days).
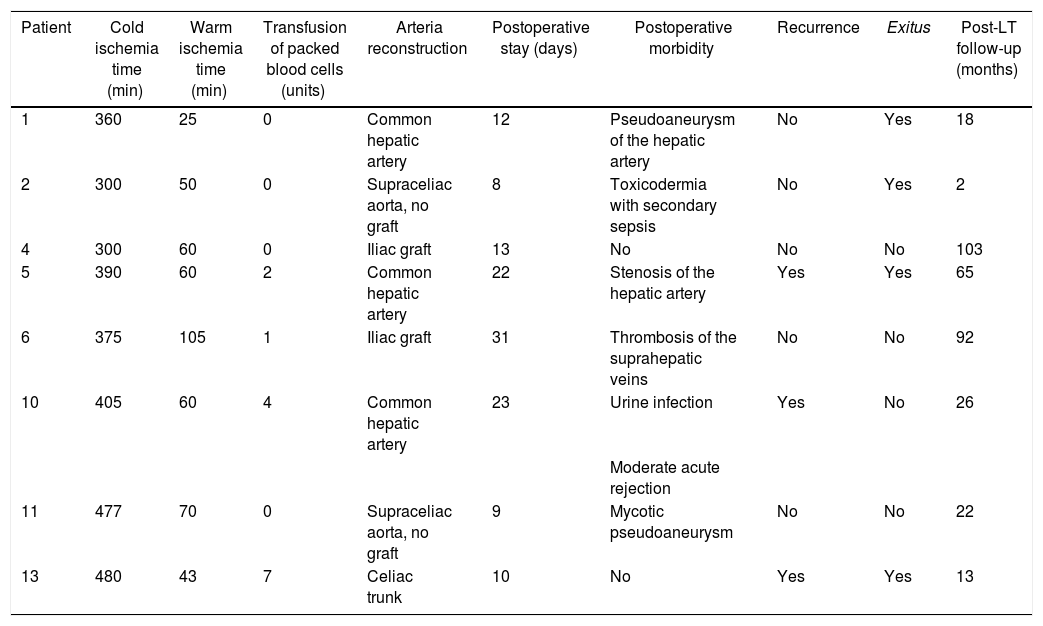
Liver transplantationTable 2 shows the main characteristics of the surgery and follow-up. All patients received a graft from a brain-dead donor with a median age of 53 (r: 33–78 years). Cold ischemia time was 382 min (r: 300−480 min). In three of the eight transplanted patients, the common hepatic artery was used for arterial reconstruction (Table 2).
Characteristics of surgery and post-transplant.
| Patient | Cold ischemia time (min) | Warm ischemia time (min) | Transfusion of packed blood cells (units) | Arteria reconstruction | Postoperative stay (days) | Postoperative morbidity | Recurrence | Exitus | Post-LT follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 360 | 25 | 0 | Common hepatic artery | 12 | Pseudoaneurysm of the hepatic artery | No | Yes | 18 |
| 2 | 300 | 50 | 0 | Supraceliac aorta, no graft | 8 | Toxicodermia with secondary sepsis | No | Yes | 2 |
| 4 | 300 | 60 | 0 | Iliac graft | 13 | No | No | No | 103 |
| 5 | 390 | 60 | 2 | Common hepatic artery | 22 | Stenosis of the hepatic artery | Yes | Yes | 65 |
| 6 | 375 | 105 | 1 | Iliac graft | 31 | Thrombosis of the suprahepatic veins | No | No | 92 |
| 10 | 405 | 60 | 4 | Common hepatic artery | 23 | Urine infection | Yes | No | 26 |
| Moderate acute rejection | |||||||||
| 11 | 477 | 70 | 0 | Supraceliac aorta, no graft | 9 | Mycotic pseudoaneurysm | No | No | 22 |
| 13 | 480 | 43 | 7 | Celiac trunk | 10 | No | Yes | Yes | 13 |
Two patients (2/3) in whom the common hepatic artery was used for the anastomosis had a pseudoaneurysm at the level of the anastomosis that was surgically repaired 34 days post-LT and a stenosis of the anastomosis that required the placement of a stent six months post-LT. One patient with a supraceliac anastomosis presented a mycotic pseudoaneurysm 41 days post-LT, requiring placement of an aortic endoprosthesis and subsequent vascular surgical reconstruction. None of these cases resulted in graft loss (Table 2).
Patient number 6 developed a thrombosis of the anastomosis of the three suprahepatic veins secondary to torsion during the immediate postoperative period. Long-term surgical repair was required, with no complications (Table 2).
Four of the eight transplanted patients died during follow-up: 2 patients due to disease recurrence 13 months and 65 months post-LT. A third patient presented with liver failure due to portal hypertension secondary to late portal anastomotic stenosis, which was the cause of death 18 months post-LT, with no evidence of recurrence. A fourth patient developed toxicodermia with bullous dermatosis and subsequent Klebsiella sepsis, with no evidence of recurrence, at two months post-LT (Table 2).
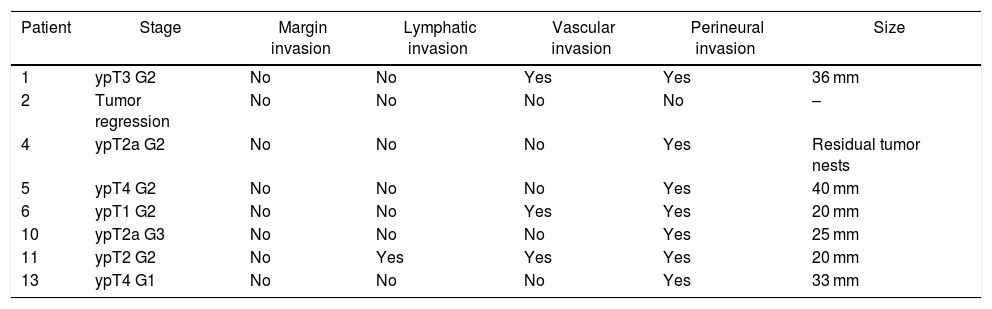
Histological examination of the explanted liverFor the pathological anatomy study, the pTNM classification of the American Joint Committee on Cancer, 8th edition, was used.23
The histological findings are presented in Table 3. It is necessary to highlight the presence of tumor in all the surgical specimens except in one case of tumor regression and the absence of involved margins. There were no signs of liver disease in the explant specimen in any case.
Histological characteristics of the tumor in the explant specimen.
| Patient | Stage | Margin invasion | Lymphatic invasion | Vascular invasion | Perineural invasion | Size |
|---|---|---|---|---|---|---|
| 1 | ypT3 G2 | No | No | Yes | Yes | 36 mm |
| 2 | Tumor regression | No | No | No | No | – |
| 4 | ypT2a G2 | No | No | No | Yes | Residual tumor nests |
| 5 | ypT4 G2 | No | No | No | Yes | 40 mm |
| 6 | ypT1 G2 | No | No | Yes | Yes | 20 mm |
| 10 | ypT2a G3 | No | No | No | Yes | 25 mm |
| 11 | ypT2 G2 | No | Yes | Yes | Yes | 20 mm |
| 13 | ypT4 G1 | No | No | No | Yes | 33 mm |
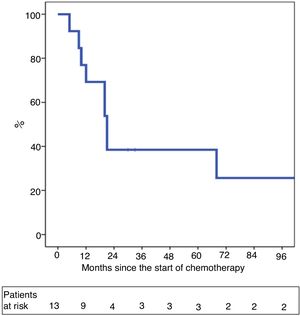
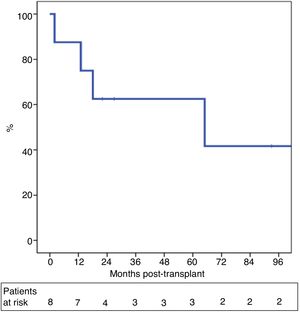
One-year and 5-year intention-to-treat survival rates were 69% and 39%, respectively (Fig. 1). After a median post-LT follow-up of 24 months (r: 2–103 months), overall one-year and 5-year survival rates were 87% and 62%, respectively (Fig. 2).
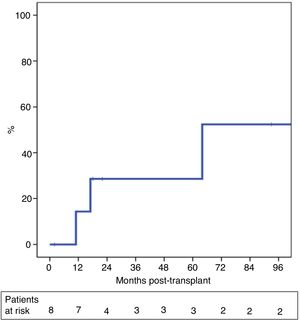
The probability of recurrence within five years was 29% (Fig. 3). One patient presented systemic recurrence 64 months post-LT, which was the cause of death shortly thereafter. A second patient died 13 months post-LT due to upper gastrointestinal bleeding secondary to local recurrence with infiltration of the celiac trunk diagnosed 11 months post-LT. And, finally, a third patient presented relapse in the form of an anterior gastric focal implant 17 months after LT, which was resected. The patient is currently alive and receiving systemic treatment.
DiscussionPCCA continues to be one of the main challenges in the field of hepatobiliary-pancreatic surgery. Due to the invasive nature of this type of tumors, it is difficult to determine the margin preoperatively by imaging tests with the intention of achieving R0, in addition to the high morbidity and mortality associated with surgical complexity. The Ebata et al. group, which has the most experience in the world in this pathology, described an overall 5-year survival of 67% and disease-free survival of 58% in R0 resectable pCCA with no lymphatic or vascular involvement.9 However, for a selected group of patients with unresectable pCCA, LT associated with neoadjuvant CT-RT has become a promising therapeutic alternative (as we have demonstrated in this study), achieving global and disease-free survival rates similar to resectable tumors. This approach is still just another entity within the concept of Transplant Oncology, which is taking shape from the combined principles of oncological surgery, liver transplantation and neoadjuvant chemotherapy.24,25
In the year 2000, De Vreede et al. from the Mayo Clinic published a series of 25 patients with pCCA included within a protocol of CT-RT combined with brachytherapy. Twelve patients (63%) received transplants, with a survival rate of 91% after a median follow-up of 44 months.14 In 2005, Rea et al18 compared the results of transplanted patients with cases that were resected, and the authors demonstrated an improvement in overall one-, 3- and five-year survival rates in the LT group (92%, 82% and 82%, respectively) compared to those who underwent curative-intent resection (82%, 48%, and 21%, respectively).
In an update of the Mayo Clinic series published by Croome et al.,26 54 resected patients were compared with 99 patients included in the neoadjuvant protocol and LT. A gain in overall survival was observed in the transplant group after one, 3, and 5 years (90%, 71%, and 59% vs 81%, 53%, and 36% in the resected group). We should note that 30% of the transplanted patients did not present residual cancer in the explant specimen, which they justify as a result of the neoadjuvant therapy. Subsequently, Lehrke et al.27 reported complete/near complete response after neoadjuvant treatment in 56% of cases. Also, patients with PSC (56% of the series) had a higher percentage of complete response than patients without PSC (71% vs. 31%), which could cast doubt on the existence of a tumor prior to the start of treatment, confusing typical benign stenotic lesions in PSC with malignant lesions. This is the main criticism of the Mayo Clinic protocol, as histological confirmation was not necessary for inclusion in the protocol; this, however, is justified due to the high risk of dissemination during transperitoneal biopsy.
The results of our series are similar to results published in the literature, to which we must add two differential facts regarding the American series: that all cases were de novo pCCA with no history of PSC, and that brachytherapy was excluded from the neoadjuvant therapy, with a waiting list time shorter than that described by the United Network of Organ Sharing (UNOS) (<6 months).
With regards to the de novo pCCA diagnostic criteria, we have followed the same Mayo Clinic criteria, based on radiological stenosis at the hilar level and histological confirmation of adenocarcinoma (by cytology or endoluminal biopsy) and/or a CA 19−9 value greater than 100 U/mL. It is important to discuss these cases in a multidisciplinary committee with radiologists who are experts in hepatobiliary-pancreatic pathology, to make a precise description of the extension of the tumor, confirm its unresectability, and define the radial size of the lesion (which must be ≤3 cm – one of the main prognostic factors published in the literature).18,28 The difficulty lies in cases in which histological confirmation is not possible, when tumor marker levels are below 100 U/mL. Along this line, at the last consensus meeting of the International Liver Transplantation Society (ILTS) in February 2019, the presence of a hilar ‘mass’ in the context of radiological stenosis was accepted as an inclusion criterion.
Although the published series do not specify the mean time on the waiting list for LT, a drop-out risk due to disease progression is estimated at 12% for every three months on the list, reaching 46% after 12 months from inclusion. Thus, in 2009, the UNOS recommended an adjustment of the MELD score to give extra points for prioritizing these patients and minimizing the risk of progression.28 The three Catalan hospitals agreed to prioritize these patients with a MELD score of 19. Three patients were excluded from the list: two patients due to tumor progression (15%), and one death due to sepsis in the context of severe cholangitis. Two patients were excluded at the time of transplantation due to the presence of carcinomatosis and regional lymphatic involvement, without having observed findings in the exploratory laparotomy prior to inclusion, although the time on the list was higher than the average. Finally, 61% of the patients received a transplant after a median time on the waiting list of four months, which was similar to the results of the most recent series published by Ethun et al.,20 without exceeding 6.7 months on the list in any case. Darwish et al.28 described CA19-9 concentrations ≥ 500 U/mL as one of the main risk factors for drop-out. In fact, of the 13 patients in our series, CA19-9 values had increased slightly since diagnosis in the two cases that progressed while being on the list, and one of them had CA19-9 values higher than 500 U/mL at the time of inclusion. However, there is insufficient evidence in the literature to define the real value of this marker and its impact.
Histologically, the presence of a tumor in the explant specimen was confirmed in all cases except one (12%), probably due to tumor regression. Although radiologically it was compatible with pCCA, we do not have pre-transplant histological confirmation. This percentage is considerably lower than that published in the literature and could be attributed both to a strict selection of cases and to the absence of a history of PSC, unlike other series. The resection was R0 in 100% of the cases, compared to the 90% reported by Ethun et al.,20 and much higher than reports for resectable pCCA of 70%–80%.9,20,26
Although the probability of reaching R0 with LT versus surgery is indisputable, another point to consider is perioperative morbidity and mortality. Surgery for resectable pCCA usually includes complex procedures, associating extended hepatectomy with biliary and/or vascular reconstructions. Postoperative morbidity after surgery exceeds 60%, some 45% of which are major complications.4,20,29,30 Postoperative mortality is 2%–15% in Western series,4,20–30 but Nagino et al.5 reported a mortality of 11% before 1990 and 1.4% after 2005. Compared to LT, the main disadvantage is early post-LT arterial thrombosis associated with intimal damage caused by radiotherapy, which is why De Vreede et al.14 has recommended the interposition of an iliac graft between the donor artery and the infrarenal aorta. Our series had 50% major complications, all of which were vascular: three arterial problems resolved with surgery or stents, and one problem with drainage of the suprahepatic veins that was repaired surgically. Although the arterial graft was not used in a standard manner to create the anastomosis, in no case was the patient’s own artery used. Thus, the high incidence of arterial problems in our series should make us reconsider the arterial graft. Although mortality during the immediate postoperative period was nil, the 90-day post-LT mortality was 12.5%. These results, which are in line with what has been published in the literature, show that LT continues to present morbidity and mortality rates similar to resectable pCCA surgery.
After a median follow-up of 24 months, the overall survival of our series was 62%, with a probable 5-year recurrence of 29%. The most recent series by Ethun et al.20 demonstrated an overall survival of 54% in the transplanted group without PSC and better than resected patients with the same characteristics (32%) (P = .049). The recurrence rate was 31% in the transplanted patients compared to 29% in the operated pCCA (P = .1) after a median follow-up of 23 months for the transplanted cohort vs. 15 for the resected group.
The intention-to-treat survival analysis probably gives us a more realistic idea of the effectiveness of neoadjuvant treatment combined with LT, since LT is delayed until the completion of the study and after intense systemic treatment. Along these lines, Ethun et al.20 also performed an intention-to-treat subanalysis of all patients diagnosed with unresectable pCCA who were included in the neoadjuvant protocol and LT and were compared with all those patients with resectable pCCA <3 cm/N0. Even so, the transplant patients had better survival even if we excluded patients with PSC (5-year survival 41% vs 27%; P = .049). Our results demonstrated a 5-year intention-to-treat survival of 39%, which was similar to the rate reported by Ethun et al.20
The other point to consider is the role of immunosuppression. Without being the objective of our study, the tendency was to use calcineurin inhibitor minimization guidelines as is done in patients who undergo transplantation for hepatocarcinoma at high risk of recurrence. However, more studies would be necessary to be able to analyze its impact on the evolution of the disease.
Our series is one of the few European studies published about the management of unresectable pCCA within a combined treatment protocol of neoadjuvant CT-RT and LT. The modest European experience is limited to very old series31–34 with poor 3-year survival rates of 30%–38% and a high rate of disease recurrence. This is probably justified by a lack of patient selection and the absence of neoadjuvant treatment that would allow not only a control of tumor growth but also to contemporize its behavior. Recently, an Irish group35 published their experience of LT with the Mayo Clinic protocol. They included 37 patients, 26 of which were eventually transplanted. Only 8% of the cases were Bismuth type IV, 88% presented PSC, and 62% of the cases achieved a complete pathological response. Five-year overall survival was 55%, which is similar to ours, but disease recurrence was 23%. This may be justified by the high complete pathological response, but once again it calls into question whether there really was a tumor at diagnosis in all patients.
An ongoing prospective, randomized French study, TRANSPHIL (ClinicalTrials.Gov NCT02232932) compares LT and neoadjuvant chemoradiotherapy versus conventional surgery. The inclusion criteria are like those defined by the Mayo Clinic group (the 3-cm rule), including potentially resectable patients and excluding patients with PSC. The primary endpoint is 5-year intention-to-treat survival, and it is likely to provide more definitive answers.
As for the limitations of our study, the strict patient selection must be highlighted, as demonstrated by the small number of patients recruited. This has also allowed the impact on the waiting list for other indications to be minimal. According to data provided by the OCATT, an average of 169 transplants were performed in Catalonia per year from 2007 to 2019, so that LT for pCCA represents 0.36% of the total number of transplanted patients since 2007.36 We should also mention that arterial complications continue to be the Achilles heel of this procedure, which should be minimized by avoiding the hepatic artery to create the arterial anastomosis.
Therefore, we conclude that the applicability of LT, combined with neoadjuvant CT-RT, was 61% in our series. This strategy should be included in the therapeutic algorithm for a group of selected patients with unresectable pCCA and no metastatic disease. These data should be confirmed with future prospective multicenter studies.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Dr. Hessheimer for her help in collecting data. Thanks also go to Abiguei Torrents, OCATT technician, for the information provided.
Please cite this article as: Dopazo C, Lladó L, Fondevila C, Macarulla T, Navalpotro B, Ramos E, et al. Aplicabilidad y resultados del trasplante hepático combinado con quimiorradioterapia neoadyuvante en el tratamiento del colangiocarcinoma perihiliar irresecable. Cir Esp. 2021;99:190–199.