Aortoenteric fistula is a rare entity, which consists of abnormal communication between the aorta and the gastrointestinal tract. The most common site of communication is the duodenum, but it can communicate with other organs, such as the oesophagus1,2.
In addition to aortic prosthetic surgery, there are a number of risk factors for the development of fistula, including digestive neoplasms3, complications of gastric surgery3–5 and the placement of prostheses or foreign bodies in the gastrointestinal tract5–8.
We present a clinical case of a patient with an aorto-oesophageal fistula after surgical treatment for gastric neoplasia.
A 75-year-old man consulted for significant weight loss and was diagnosed with subcardial adenocarcinoma of the gastric adenocarcinoma by endoscopy. After completing the study with echoendoscopy without evidence of infiltration of the muscularis propria layer and extension CT scan without discordant findings, and with the diagnostic suspicion of early gastric cancer (uT1bN0), total gastrectomy, omentectomy and laparoscopic D2 lymphadenectomy were performed. For the reconstruction of the transit, a mechanical end-to-side oesophagojejunal anastomosis was performed with a circular endograpator, ascending the transmesocolic alimentary loop; incidentally, during the diagnostic process, an appendicular mucocele was detected, so an appendicectomy was performed at the same time. The pathological anatomy showed appendiceal adenocarcinoma gastricum pT4N0 and appendiceal mucinous adenocarcinoma pT2 with an affected margin.
On the eighth postoperative day, a small dehiscence of the oesophago-jejunal anastomosis was observed with little clinical and analytical repercussions. With the aim of speeding up recovery and early treatment in view of the already known pathological anatomy (right hemicolectomy and adjuvant chemotherapy with or without radiotherapy), it was decided to place an oesophageal prosthesis covered by endoscopy. After this, the patient progressed favourably and was discharged 12 days later.
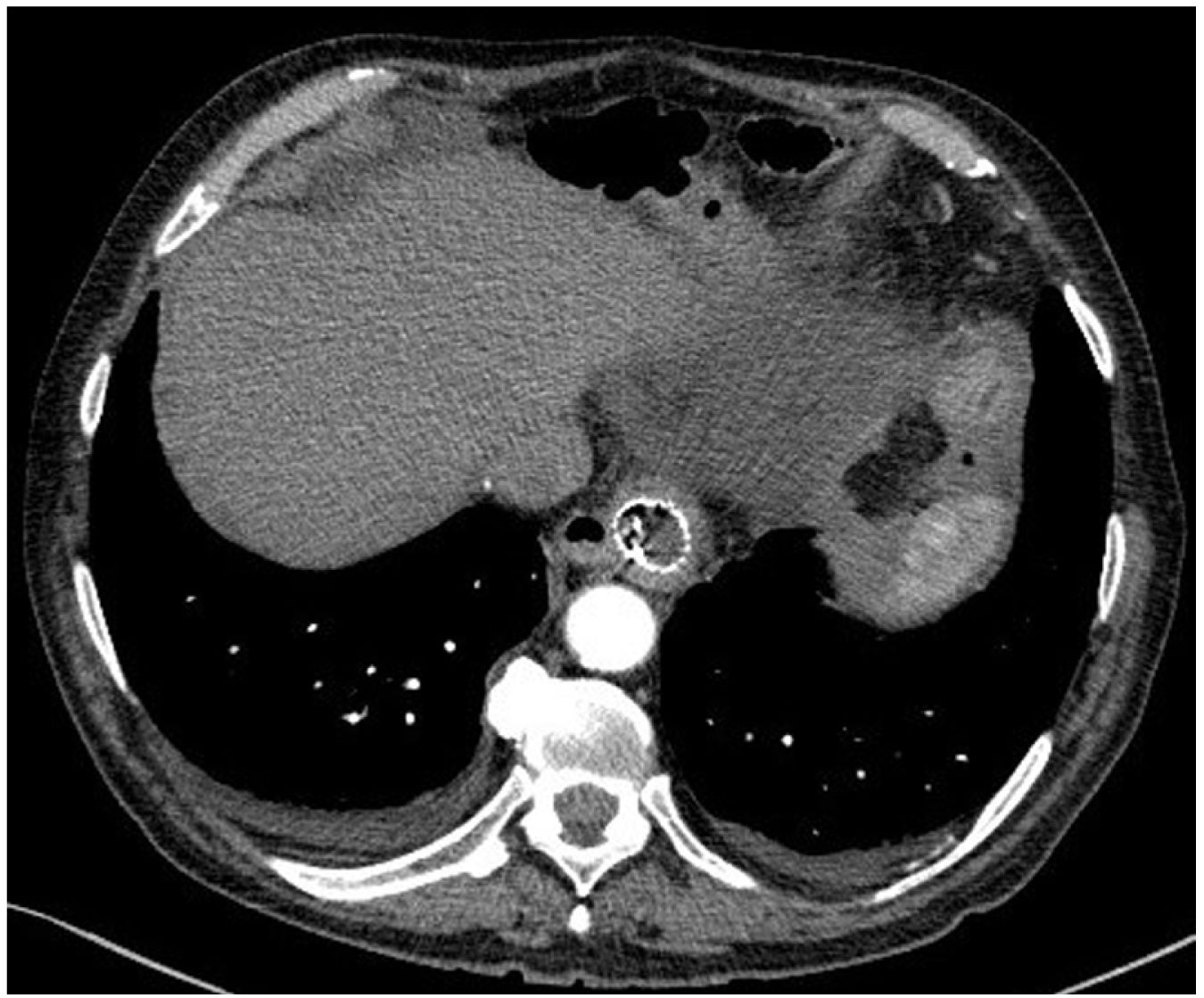
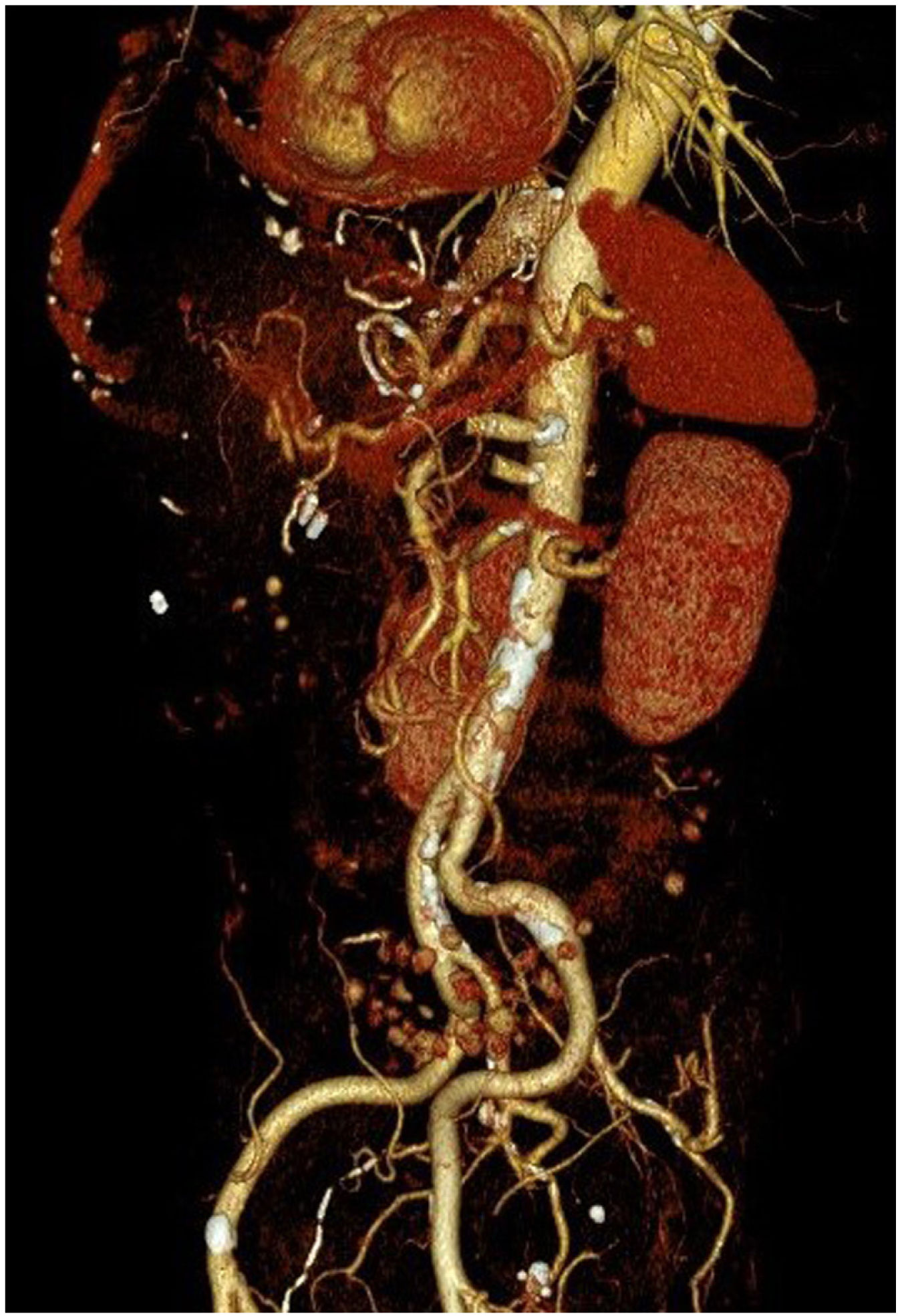
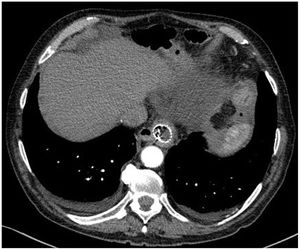
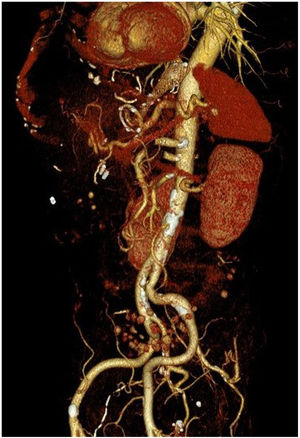
However, 24 h later, he was readmitted for haematemesis with normal CT angiography (Fig. 1), and endoscopy was performed to control the haemorrhage by means of clips without adequately visualising the endoprosthesis. After a new endoscopy at 8 h, the endoprosthesis was removed and haemostasis was checked, apparently adequate. Five days later, the patient presented with a new haemorrhage with instability, and endoscopic control was not achieved, so urgent open surgery was decided on suspicion of aorto-oesophageal fistula. The review of the CT angiography, prior to removal of the endoprosthesis, supports the diagnosis by showing the proximity of the proximal portion of the endoprosthesis to the aorta, as shown in the 3D reconstruction (Fig. 2). During surgery, the fistula was confirmed and repaired with direct aortic suture. For visualisation, the oesophago-jejunal anastomosis had to be undone and oesophagectomy, cervical oesophagostomy and feeding jejunostomy were performed.
The patient evolved favourably, with good tolerance to enteral nutrition, and a control angio-CT scan was performed one week later with no alterations in the aortic wall, for which he was discharged. However, after discharge, he died suddenly at home due to unknown causes, as no autopsy was performed.
Aortoenteric fistula is an uncommon entity, which very rarely occurs at the oesophagus level1,2. For this reason, although there are many published studies on aortoenteric fistula in general, references in the literature are much less frequent in the specific case of aorto-oesophageal fistula, with only a few clinical cases published in recent years. These studies describe several risk factors specifically associated with this location, such as radiotherapy in advanced tumours9, radical gastric surgery10 or the type of anastomosis performed. Two of them propose contact with the aorta of the stapling line of the oesophagojejunal anastomosis, performed with a linear stapler, as the causative agent3,4. The most widely described aetiological factor for the appearance of fistulas in this location is the placement of oesophageal prostheses5–8.
The most common form of presentation of aortoenteric fistulas of any location is haemorrhage, which frequently begins with so-called “herald” or sentinel haemorrhages, prior to the appearance of massive haemorrhages3,10. The characteristic clinical features of the oesophageal location are the Chiari triad, consisting of the onset of centrothoracic pain followed by sentinel haemorrhage and, subsequently, massive haemorrhage3.
Angio-CT is the diagnostic test of choice, although in unstable patients the diagnosis will most often be made in the operating theatre1–3. Other useful tests include endoscopy and digital subtraction angiography1,3. Due to its complexity and rapid onset, it is not uncommon for death to occur before the diagnosis is made4,5,10.
Treatment, as in other locations, is based on 3 fundamental pillars: resuscitation and haemodynamic stabilisation, prolonged antimicrobial therapy and fistula repair1,2. Although open surgical repair was considered the repair of choice, endovascular management is playing an increasingly important role. Years ago, endovascular treatment was often used as a bridge to open surgery, especially in unstable patients, as it was believed that endovascular management alone increased rebleeding1,2. Nowadays, with the development of endovascular techniques and the decrease in postoperative complications and hospital stay, it is often used as definitive treatment with good results2,6–8. However, some authors maintain the need to treat the oesophageal defect (and, if necessary, remove the prosthesis in it) to avoid recurrence of bleeding and the risk of infection8, manoeuvres that did not prevent the fatal outcome in our case.
Therefore, despite being an uncommon entity, given its severity and high mortality, which can exceed 50%, an aorto-oesophageal fistula should be suspected in the presence of upper gastrointestinal bleeding in patients with risk factors for its occurrence1–3.
FundingThis research has not received specific support from public sector agencies, the commercial sector or non-profit organisations.