About 25%–35% of polytraumatized patients have a profound alteration of hemostasis on arrival at the hospital (acute traumatic coagulopathy [CAT]). Viscoelastic tests (ROTEM®) measure the hemostatic capacity and provide an early detection of CAT. The objectives of this study are to describe the initial thromboelastogram of these patients and to determine the prevalence of CAT according to predefined thromboelastographic profiles.
MethodsSingle-center, observational, prospective study in polytraumatic patients. Initial blood and thromboelastographic test (ROTEM®) were made, and pre-hospital, hospital, transfusion, initial surgical/angiographic interventions, cardiac arrest and mortality data were collected. ROTEM®-based, patients were classified as: normal, hypercoagulable, hypocoagulable, hypocoagulable+hyperfibrinolytic and isolated hyperfibrinolysis.
ResultsOne hundred and twenty-three patients were analyzed. 32 cases (26%) with CAT: 15 patients with hypocoagulability, 9 with hyperfibrinolysis alone and 8 with hypocoagulability+hyperfibrinolysis. The CAT group, related to the normal group, presented higher ISS (23 vs 16, P<.01), higher blood products transfusion (2.5 vs 0; P=.001), more cardiac arrest (19 vs 1%, P<.01), and higher mortality (34 vs 5%, P<.01). The subgroup with hypocoagulability/hyperfibrinolysis, related to the groups with hypocoagulability or hyperfibrinolysis alone, presented a higher ISS (41 vs 25 vs 15, P<.01), higher angiographic procedures (62% vs 13% vs 0%, P<.01) and higher mortality (75% vs 33% vs 0%, P=.05).
ConclusionsTwenty-six percent of the polytrauma patients presented early coagulopathy assessed by thromboelastography. It is associated with higher consumption of blood products and lower survival. The presence of hypocoagulability+hyperfibrinolysis is associated with greater severity and a higher requirement of blood products.
El 25-35% de los pacientes politraumatizados presentan profundas alteraciones de la coagulación a su llegada al hospital (coagulopatía aguda traumática [CAT]). Los test viscoelásticos (ROTEM®) valoran rápidamente la capacidad hemostática y detectan precozmente la CAT. Los objetivos de este estudio son describir el tromboelastograma inicial de estos enfermos y determinar la prevalencia de CAT según unos perfiles tromboelastográficos predefinidos.
MétodosEstudio unicéntrico, observacional y prospectivo en pacientes politraumatizados. Se realizó analítica, prueba tromboelastográfica (ROTEM®) y se registraron datos prehospitalarios y hospitalarios, transfusiones, intervenciones quirúrgicas/arteriografía iniciales, paradas cardiorrespiratorias y fallecimientos. Los pacientes fueron clasificados en grupos según su ROTEM® inicial: «normal», «hipercoagulabilidad», «hipocoagulabilidad», «hipocoagulabilidad+hiperfibrinólisis» e «hiperfibrinólisis aislada».
ResultadosSe analizaron 123 pacientes. En 32 casos (26%) se objetivó CAT: 15 pacientes presentaron hipocoagulabilidad, 9 hiperfibrinólisis aislada y 8 hipocoagulabilidad+hiperfibrinólisis. El grupo con CAT, respecto al grupo «normal», presentó mayor ISS (23 vs 16; P<0,01), mayor transfusión de hemoderivados (2.5 vs 0; P=0,001), más episodios de PCR (19 vs 1%, P<0,01) y mayor mortalidad (34 vs 5%, P<0,01). El subgrupo con hipocoagulabilidad+hiperfibrinólisis, respecto a los grupos con hipocoagulabilidad o hiperfibrinólisis aislada, presentó mayor ISS (41 vs 25 vs 15, P<0,01), mayor necesidad de arteriografía (62% vs 13% vs 0%, P<0,01) y mortalidad superior (75% vs 33% vs 0%, P=0,05).
ConclusionesEl 26% de los enfermos politraumatizados presenta coagulopatía precoz evaluada mediante tromboelastografía, asociada a mayor consumo de hemoderivados y menor supervivencia. El perfil combinado de «hipocoagulabilidad+hiperfibrinólisis» se asocia a mayor gravedad y necesidades superiores de hemoderivados y arteriografía.
Trauma hemorrhage is the main cause of preventable mortality in polytrauma patients (PTP).1 Several studies have shown that 25%–35% of PTP present profound coagulation alterations upon arrival at the admission center2; this phenomenon, intrinsically associated with severe trauma and with a decisive prognostic value, has been termed “acute coagulopathy of trauma” or “acute traumatic coagulopathy” (ATC).3 This entity should be considered an independent factor – but with a synergistic effect – of the altered hemostasis derived from hemodilution (excessive fluid therapy) and the so-called “lethal triad” (hypothermia, metabolic acidosis and coagulopathy).3
Classically, the degree of coagulopathy is monitored by conventional coagulation tests based on plasma reactions (plasma-based tests: TP, INR, aPTT) and on the concentration of platelets and fibrinogen. These parameters only reflect the amount of thrombin generated during the initial phase of coagulation, without providing information on the interaction of platelets with coagulation factors, clot formation-stability-lysis, or the overall state of hyperfibrinolysis (characteristic of severe PTP). Therefore, “viscoelastic tests” (TEG® and ROTEM®) have recently acquired an increasing role in the assessment of the hemostatic capacity of polytrauma patients and the early detection of ATC.4,5 Rotational thromboelastometry (ROTEM®) and conventional thromboelastography (TEG®) provide qualitative and quantitative information on the overall balance between clot formation and destruction, being able to discriminate the pathophysiological mechanism of severe hemorrhage and more precisely guide PTP hemostatic resuscitation in an individualized manner.6,7 However, the prototypical thromboelastogram pattern in severe polytrauma patients treated at Spanish reference hospitals has not yet been defined.
The main objectives of our study are to describe the initial thromboelastogram of polytrauma patients treated at our hospital and to determine the prevalence of ATC based on predefined thromboelastometric profiles of normal coagulation, hypocoagulability and/or hyperfibrinolysis. As secondary objectives, we analyzed the clinical, analytical, transfusional, therapeutic and prognostic differences between the groups that present a normal ROTEM® pattern versus a coagulopathic pattern (ATC). Finally, we describe the association between the different thromboelastometric profiles and trauma severity (hemodynamic characteristics, Glasgow and Injury Severity Score [ISS]), transfusion and therapeutic needs (surgery, arteriography) and mortality.
MethodsOurs is an observational prospective study conducted at a single hospital between October 2012 and October 2013. The study was approved by the Clinical Research Ethics Committee of the Hospital Universitario of Bellvitge, Barcelona (reference PR205/12).
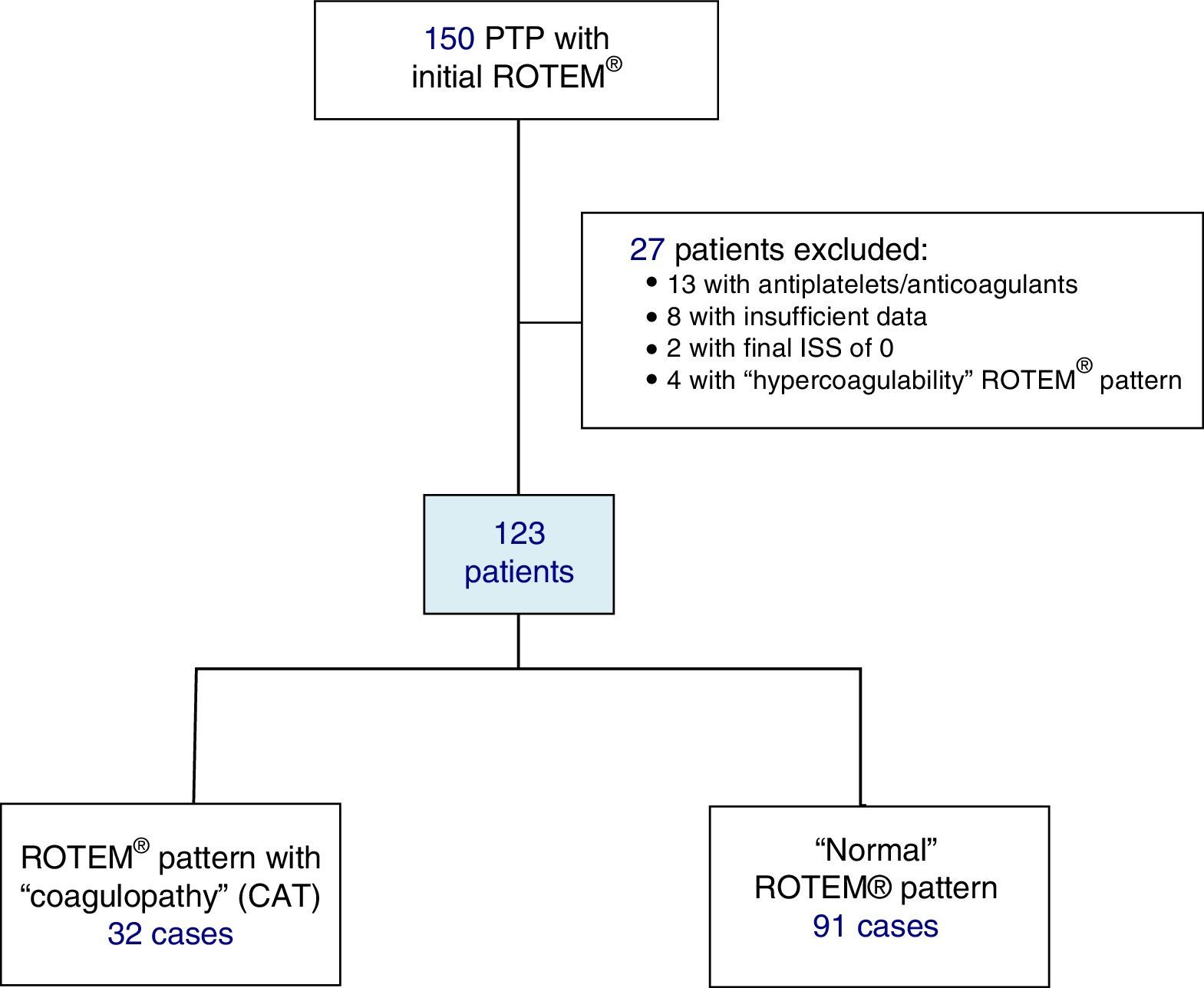
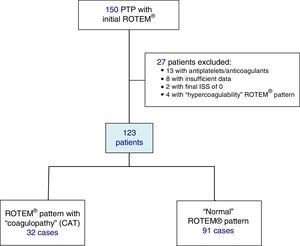
Included in the study were polytrauma patients treated in the Emergency Room after the pre-hospital activation of the “polytrauma patient” code (PTP code) and primary transfer. Excluded from the study were patients receiving antiplatelet and/or anticoagulant therapy, patients with insufficient pre-hospitalization data, and those with an ISS of 0 (Fig. 1).
All patients were initially treated according to standardized guidelines for the management of polytrauma patients (ATLS®).
Trauma severity was assessed with an initial estimation of the Injury Severity Score (ISS) in order to differentiate between patients with mild trauma (ISS<15) or severe trauma (ISS<15).
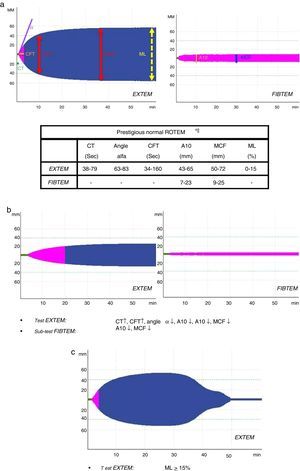
All patients underwent complete immediate lab work-up (acid–base balance, lactate, complete blood count, biochemistry and coagulation) and assessment of coagulation by means of ROTEM® thromboelastometry (EXTEM test: evaluation of the extrinsic pathway); depending on the result (A10-EXTEM≤35) and ISS (ISS≥15), fibrinogen assessment was included (FIBTEM subtest).
Initially, severe PTP (ISS≥15) received 1g of tranexamic acid (iv bolus) together with 1g in continuous perfusion for 8h (CRASH-2 protocol)8; less severe patients (ISS<15) were given only the iv bolus. Patients received 2g of iv fibrinogen if the A10-FIBTEM level was ≤7mm (indicative of an insufficient role of fibrinogen in clot formation; normal range: 7–23mm). If the ROTEM® registered an insufficient platelet effect (A10-FIBTEM parameters >7mm and A10-EXTEM≤35mm; normal range: 43–65mm), a platelet pool was administered.
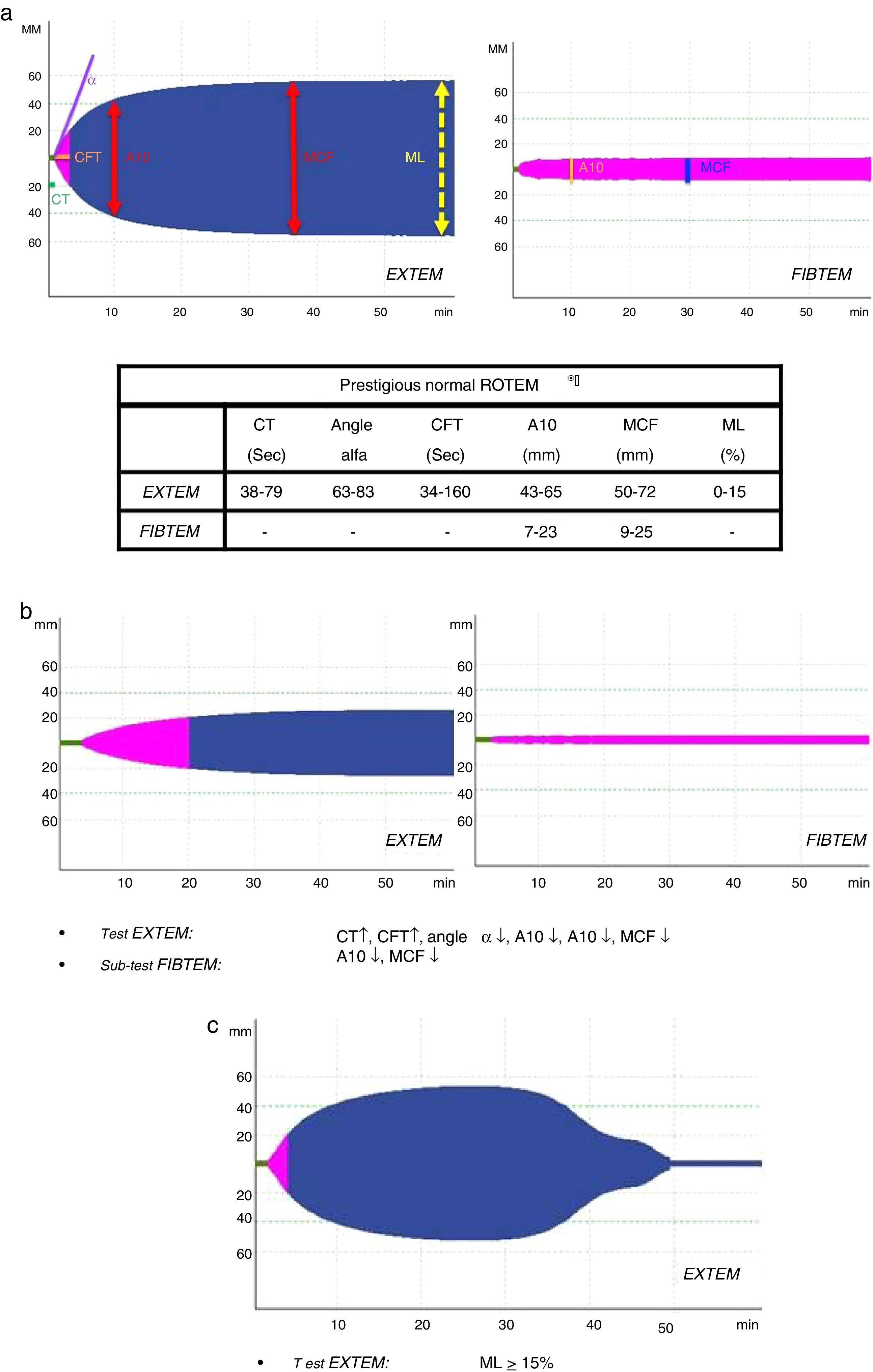
Several variables were collected, including demographic variables (age, sex), ISS score, type of trauma (closed vs open), prehospitalization and hospital clinical variables: systolic blood pressure (SBP), heart rate (HR), respiratory rate (RR), oxygen saturation (SatO2) and Glasgow coma scale (GCS); lab work data: hemoglobin, hematocrit, platelets, activated partial thromboplastin time (aPTT), prothrombin time (PT/INR), fibrinogen, lactate, pH, base excess; parameters of the different ROTEM® tests: coagulation time (CT-EXTEM), clot formation time (CFT-EXTEM), maximum clot firmness (MCF-EXTEM and MCF-FIBTEM), clot amplitude at 10min (A10-EXTEM and A10-FIBTEM), “alpha angle” (α-EXTEM angle) and maximum clot lysis in 1h (ML-EXTEM) (Fig. 2a–c).
According to the assessment of the initial thromboelastogram, patients were classified into five “ROTEM® profiles”: “normal”, “hypocoagulability”, “hypocoagulability and hyperfibrinolysis”, “isolated hyperfibrinolysis” and “hypercoagulability”. Following the criteria previously described by Kaufmann et al.,9 ROTEM® with “hypocoagulability” was defined as the presence of two or more of the following factors: lengthening of CT or CFT, decrease of the “alpha angle” or reduction of MCF/A10; “normal” ROTEM® was considered when all these parameters were within normal ranges; ROTEM® with “hyperfibrinolysis” was considered if the ML parameter was ≥15% (normal value ML-EXTEM<15%); ROTEM® with “hypercoagulability” was defined as the presence of two or more of the following factors: increase of the “alpha angle”, decrease of CT or CFT or increase of MCF/A10.4
The presence of ATC was considered in all patients who presented any ROTEM® pattern other than that defined as “normal” (ROTEM® group with “coagulopathy” vs “normal” ROTEM® group).
We registered the early transfusion (≤24h) of packed red cells, platelets, fresh frozen plasma and fibrinogen; the need for laparotomy, thoracotomy, cranial surgery, trauma surgery of extremities/pelvis and angioradiology intervention in the first 24h; episodes of cardiac arrest (CA) and deaths during admission.
The data were analyzed by means of the SPSS® 15.0 statistical program (SPSS Inc, Chicago, USA). The categorical variables were reported with absolute frequency and percentage, and the continuous variables by means and standard deviation (or median and range). The chi-squared or Fisher's tests were used for the analysis of the categorical variables, and the Student's t test for continuous variables. The homogeneity of the continuous variables was checked by the Kolmogorov test, and the Mann–Whitney U was applied if the distribution was not normal. A p<0.05 was accepted as statistically significant.
ResultsDuring the study, 150 PTP patients were recorded. A total of 23 cases were excluded: 13 with antiplatelet/anticoagulant treatment, 8 with incomplete data, and 2 with an ISS score of 0. The remaining 127 patients were classified according to their initial ROTEM® profile: 91 patients with a “normal” thromboelastometric pattern, 32 with “hypocoagulability and/or hyperfibrinolysis” (ATC) and 4 with “hypercoagulability”. Patients with “hypercoagulability” were excluded from the analysis because they represented a very small sample, so that the final study population was defined by 123 patients.
In 32 cases (26%), some form of ATC was observed according to the ROTEM® data: hypocoagulability associated with hyperfibrinolysis was recorded in 8 patients, isolated hypocoagulability (without hyperfibrinolysis) in 15, and isolated hyperfibrinolysis (without hypocoagulability) in 9.
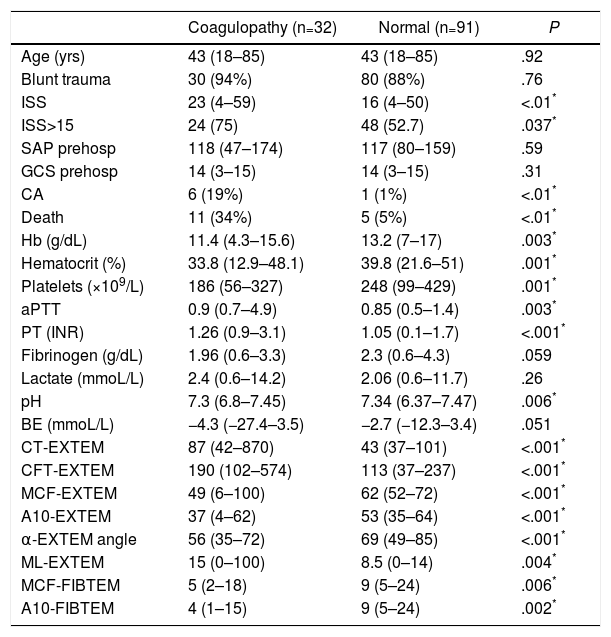
The two groups of patients – with and without thromboelastometric alterations – were comparable in terms of age, sex and mechanism of injury. Significant differences were observed in terms of trauma severity, with higher ISS (23 vs 16) and higher percentage of severe injuries (ISS≥15) in the ATC group (75% vs 52.7%) (Table 1).
Demographic and Clinical Variables, Laboratory Data and ROTEM® Test (EXTEM Test and FIBTEM Sub-test) in the ROTEM® Groups “With Coagulopathy” and “Normal” ROTEM®.
| Coagulopathy (n=32) | Normal (n=91) | P | |
|---|---|---|---|
| Age (yrs) | 43 (18–85) | 43 (18–85) | .92 |
| Blunt trauma | 30 (94%) | 80 (88%) | .76 |
| ISS | 23 (4–59) | 16 (4–50) | <.01* |
| ISS>15 | 24 (75) | 48 (52.7) | .037* |
| SAP prehosp | 118 (47–174) | 117 (80–159) | .59 |
| GCS prehosp | 14 (3–15) | 14 (3–15) | .31 |
| CA | 6 (19%) | 1 (1%) | <.01* |
| Death | 11 (34%) | 5 (5%) | <.01* |
| Hb (g/dL) | 11.4 (4.3–15.6) | 13.2 (7–17) | .003* |
| Hematocrit (%) | 33.8 (12.9–48.1) | 39.8 (21.6–51) | .001* |
| Platelets (×109/L) | 186 (56–327) | 248 (99–429) | .001* |
| aPTT | 0.9 (0.7–4.9) | 0.85 (0.5–1.4) | .003* |
| PT (INR) | 1.26 (0.9–3.1) | 1.05 (0.1–1.7) | <.001* |
| Fibrinogen (g/dL) | 1.96 (0.6–3.3) | 2.3 (0.6–4.3) | .059 |
| Lactate (mmoL/L) | 2.4 (0.6–14.2) | 2.06 (0.6–11.7) | .26 |
| pH | 7.3 (6.8–7.45) | 7.34 (6.37–7.47) | .006* |
| BE (mmoL/L) | −4.3 (−27.4–3.5) | −2.7 (−12.3–3.4) | .051 |
| CT-EXTEM | 87 (42–870) | 43 (37–101) | <.001* |
| CFT-EXTEM | 190 (102–574) | 113 (37–237) | <.001* |
| MCF-EXTEM | 49 (6–100) | 62 (52–72) | <.001* |
| A10-EXTEM | 37 (4–62) | 53 (35–64) | <.001* |
| α-EXTEM angle | 56 (35–72) | 69 (49–85) | <.001* |
| ML-EXTEM | 15 (0–100) | 8.5 (0–14) | .004* |
| MCF-FIBTEM | 5 (2–18) | 9 (5–24) | .006* |
| A10-FIBTEM | 4 (1–15) | 9 (5–24) | .002* |
A10-EXTEM: clot amplitude after 10min (mm, range 43–65); A10-FIBTEM: clot firmness after 10min in the FIBTEM test (mm, range 7–23); α-EXTEM angle: alpha angle (grades, range 63–83); CFT-EXTEM: clot formation time (seconds, range 34–160); CT-EXTEM: clotting time (seconds, range 38–79); BE: base excess; GCS prehosp: prehospital Glasgow coma score; Hb: hemoglobin; ISS: Injury Severity Score; MCF-EXTEM: maximum clot firmness (mm, range 50–72); MCF-FIBTEM: maximum clot firmness in FIBTEM test (mm, range 9–25); ML-EXTEM: maximum clot lysis (%, range 0–15); SAP prehosp: prehospital systolic arterial pressure (mmHg); CA: cardiac arrest; PT: prothrombin time (INR); aPTT: activated partial thromboplastin time.
Data expressed as median and range (or percentage) between parentheses.
The analytical tests showed significant differences between both groups. The group with coagulopathy recorded by ROTEM® presented lower levels of hemoglobin, hematocrit, platelets and pH, and higher values of aPTT and TP/INR, with no significant differences in the concentrations of fibrinogen, lactate or base excess (Table 1).
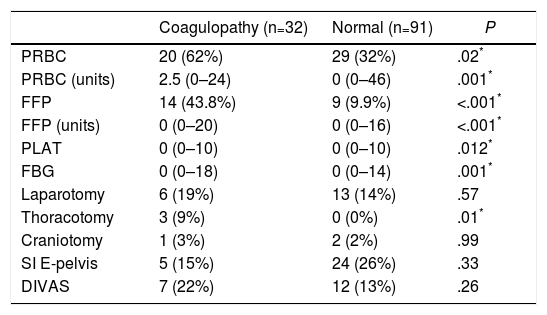
The transfusion of blood products was significantly higher in the “coagulopathy” group, both in the percentage of transfused patients and in the number of blood products administered (Table 2). Regarding the initial intervention, only a greater need for exploratory thoracotomy was observed in the group with coagulation alterations (Table 2).
Data for Transfusion of Blood Products and Initial Interventions (24h) in the ROTEM® Groups “With Coagulopathy” and “Normal” ROTEM®.
| Coagulopathy (n=32) | Normal (n=91) | P | |
|---|---|---|---|
| PRBC | 20 (62%) | 29 (32%) | .02* |
| PRBC (units) | 2.5 (0–24) | 0 (0–46) | .001* |
| FFP | 14 (43.8%) | 9 (9.9%) | <.001* |
| FFP (units) | 0 (0–20) | 0 (0–16) | <.001* |
| PLAT | 0 (0–10) | 0 (0–10) | .012* |
| FBG | 0 (0–18) | 0 (0–14) | .001* |
| Laparotomy | 6 (19%) | 13 (14%) | .57 |
| Thoracotomy | 3 (9%) | 0 (0%) | .01* |
| Craniotomy | 1 (3%) | 2 (2%) | .99 |
| SI E-pelvis | 5 (15%) | 24 (26%) | .33 |
| DIVAS | 7 (22%) | 12 (13%) | .26 |
PRBC: patients transfused (packed red blood cells); PRBC (units): units of packed red blood cells/patient (only in transfused patients); DIVAS: Digital Intravenous Angiography by Substraction; FBG: grams of fibrinogen administered; SI E-pelvis: surgical intervention on extremities and/or pelvis; FFP: patients transfused (fresh frozen plasma); FFP (units): units of plasma/patient (only transfused patients); PLAT: platelet apheresis.
Data expressed as median and range (or percentage) between parentheses.
The development of CA (19% vs 1%) and mortality (34% vs 5%) were significantly higher in the group with altered ROTEM® profile (ATC) (Table 1).
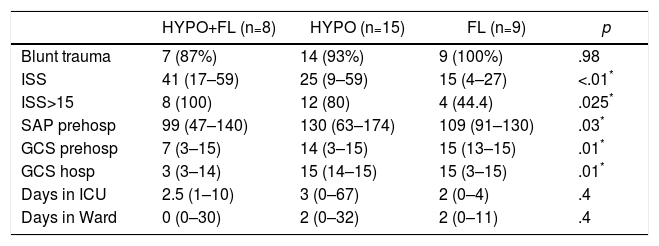
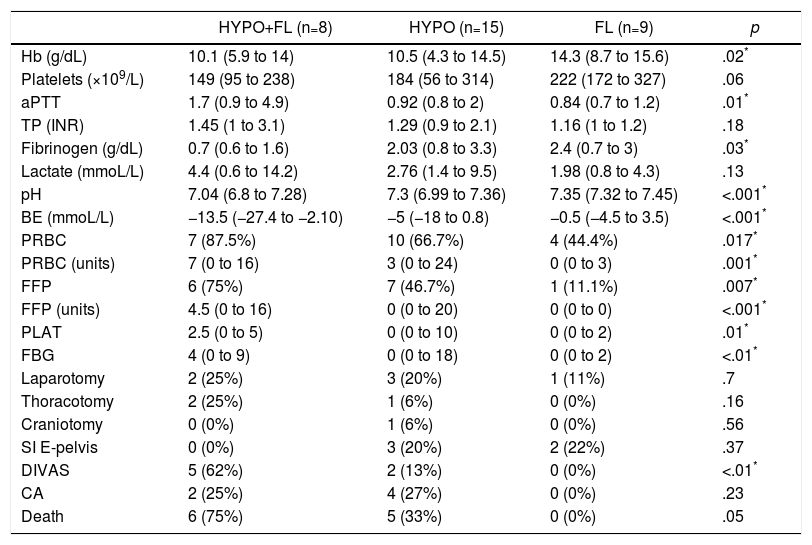
In the “coagulopathy” group (ATC, 32 cases), the subgroup analysis showed that patients with a “combined” pattern of hypocoagulability associated with hyperfibrinolysis (8 cases) presented a more severe ISS than the rest, as well as a lower Glasgow score (pre-hospital and hospital) (Table 3). The percentage of patients with severe trauma was also significantly higher in this subgroup (100% of the cases), compared with 80% in the “isolated hypocoagulability” group and 44% in the “hyperfibrinolysis” group. Blood pressure was also lower in this subgroup, but only at the prehospital level. Likewise, this subgroup with a combined pattern presented lower initial levels of hemoglobin, fibrinogen and pH, and higher values of aPTT and base deficit; this group also registered a greater need for all types of blood products and early arteriography than the other ATC subgroups (Table 4). The median hospital stay in critical care units or conventional hospital wards did not show significant differences between the different subgroups.
Demographics and Clinical Variables Between the Different Subgroups With Coagulopathy (ROTEM® Patterns).
| HYPO+FL (n=8) | HYPO (n=15) | FL (n=9) | p | |
|---|---|---|---|---|
| Blunt trauma | 7 (87%) | 14 (93%) | 9 (100%) | .98 |
| ISS | 41 (17–59) | 25 (9–59) | 15 (4–27) | <.01* |
| ISS>15 | 8 (100) | 12 (80) | 4 (44.4) | .025* |
| SAP prehosp | 99 (47–140) | 130 (63–174) | 109 (91–130) | .03* |
| GCS prehosp | 7 (3–15) | 14 (3–15) | 15 (13–15) | .01* |
| GCS hosp | 3 (3–14) | 15 (14–15) | 15 (3–15) | .01* |
| Days in ICU | 2.5 (1–10) | 3 (0–67) | 2 (0–4) | .4 |
| Days in Ward | 0 (0–30) | 2 (0–32) | 2 (0–11) | .4 |
FL: ROTEM® pattern with hyperfibrinolysis; GCS hosp: hospital Glasgow coma scale; GCS prehosp: prehospital Glasgow coma scale; HYPO: ROTEM® pattern with hypocoagulability; HYPO+FL: ROTEM® pattern with hypocoagulability and hyperfibrinolysis; ISS: Injury Severity Score; SAP prehosp: prehospital systolic arterial pressure (mmHg); ICU: intensive care unit.
Data expressed as median and range (or percentage) between parentheses.
Laboratory Data, Transfusion of Blood Products (24h), Initial Intervention (24h) and Evolution Between the Different Subgroups With Coagulopathy (ROTEM® Patterns).
| HYPO+FL (n=8) | HYPO (n=15) | FL (n=9) | p | |
|---|---|---|---|---|
| Hb (g/dL) | 10.1 (5.9 to 14) | 10.5 (4.3 to 14.5) | 14.3 (8.7 to 15.6) | .02* |
| Platelets (×109/L) | 149 (95 to 238) | 184 (56 to 314) | 222 (172 to 327) | .06 |
| aPTT | 1.7 (0.9 to 4.9) | 0.92 (0.8 to 2) | 0.84 (0.7 to 1.2) | .01* |
| TP (INR) | 1.45 (1 to 3.1) | 1.29 (0.9 to 2.1) | 1.16 (1 to 1.2) | .18 |
| Fibrinogen (g/dL) | 0.7 (0.6 to 1.6) | 2.03 (0.8 to 3.3) | 2.4 (0.7 to 3) | .03* |
| Lactate (mmoL/L) | 4.4 (0.6 to 14.2) | 2.76 (1.4 to 9.5) | 1.98 (0.8 to 4.3) | .13 |
| pH | 7.04 (6.8 to 7.28) | 7.3 (6.99 to 7.36) | 7.35 (7.32 to 7.45) | <.001* |
| BE (mmoL/L) | −13.5 (−27.4 to −2.10) | −5 (−18 to 0.8) | −0.5 (−4.5 to 3.5) | <.001* |
| PRBC | 7 (87.5%) | 10 (66.7%) | 4 (44.4%) | .017* |
| PRBC (units) | 7 (0 to 16) | 3 (0 to 24) | 0 (0 to 3) | .001* |
| FFP | 6 (75%) | 7 (46.7%) | 1 (11.1%) | .007* |
| FFP (units) | 4.5 (0 to 16) | 0 (0 to 20) | 0 (0 to 0) | <.001* |
| PLAT | 2.5 (0 to 5) | 0 (0 to 10) | 0 (0 to 2) | .01* |
| FBG | 4 (0 to 9) | 0 (0 to 18) | 0 (0 to 2) | <.01* |
| Laparotomy | 2 (25%) | 3 (20%) | 1 (11%) | .7 |
| Thoracotomy | 2 (25%) | 1 (6%) | 0 (0%) | .16 |
| Craniotomy | 0 (0%) | 1 (6%) | 0 (0%) | .56 |
| SI E-pelvis | 0 (0%) | 3 (20%) | 2 (22%) | .37 |
| DIVAS | 5 (62%) | 2 (13%) | 0 (0%) | <.01* |
| CA | 2 (25%) | 4 (27%) | 0 (0%) | .23 |
| Death | 6 (75%) | 5 (33%) | 0 (0%) | .05 |
PRBC: patients transfused (packed red blood cells); PRBC (units): units of packed red blood cells/patient (only transfused patients); DIVAS: Digital Intravenous Angiography by Substraction; BE: base excess; FBG: grams of fibrinogen administered; FL: ROTEM® pattern with hyperfibrinolysis; Hb: hemoglobin; HYPO: ROTEM® with hypocoagulability; HYPO+FL: ROTEM® pattern with hypocoagulability and hyperfibrinolysis; SI E-pelvis: surgical intervention of extremities and pelvis; CA: cardiac arrest; FFP: patients transfused (fresh frozen plasma); FFP (units): units plasma/patient (only in transfused patients); PLAT: platelet apheresis; PT: prothrombin time (INR); aPTT: activated partial thromboplastin time.
Data expressed as median and range (or percentage) between parentheses.
When we analyzed the causes and temporal distribution of mortality in the entire sample, none of the 5 deaths registered in the group with normal ROTEM® pattern occurred during the first 24h, while 7 of the 11 deaths in the group of patients with initial coagulopathy occurred during the first 24h (2 due to neurological causes and 5 due to hemorrhagic shock). In the analysis by subgroups with ATC, mortality was higher (although not statistically significant) in the subgroup with a combined pattern (“hypocoagulability and hyperfibrinolysis”) (75%, 6 out of the 8 cases) compared with the “isolated hypocoagulability” subgroup (33%, 5 out of the 15 cases) and the “isolated hyperfibrinolysis” subgroup (no deaths of the 9 cases) (Table 4).
DiscussionThe initial ROTEM® profile and the prevalence of ATC in polytrauma patients treated at our hospitals have not yet been fully defined. This article describes different thromboelastometric patterns and estimates the prevalence of ATC–according to ROTEM® criteria–in a PTP population treated at a third-level center; secondarily, we have defined and compared three thromboelastometric ATC profiles by clinical parameters, transfusion and therapeutic needs and survival.
In our series, 26% of polytrauma patients presented coagulopathy identified by thromboelastometry (ROTEM®) at the time of admission. This datum coincides with the prevalence (25%–35%) of ATC reported by other series since its original description.3,6,10 The overall mortality of the sample (13%, 16 cases) is similar to other reports.2,6
Viscoelastic tests (TEG® thromboelastography and ROTEM® rotational thromboelastometry) assess patient hemostatic capacity more quickly and completely than conventional coagulation tests (TP, INR, aPTT), offering dynamic information–“in real time” and at bedside–about platelet–coagulation factor interactions and about clot formation, stability and lysis. Several studies have confirmed the usefulness of transfusion protocols based on viscoelastic techniques (compared to the usual coagulation tests). The advantages obtained after their introduction in elective surgery (early detection of coagulopathy, reduction of blood products and re-operations due to bleeding, increased survival) have promoted their application in the context of PTP, with promising initial results.4,10–12 These techniques provide more precise diagnosis of coagulopathy and a “directed” correction according to the type and intensity of the specific alterations detected, optimizing the use of resources and blood products. This would allow for a more individualized therapeutic approach (hemostatic resuscitation), with potentially increased survival, as suggested by some recent PTP management guidelines.1,5,7,13,14
In our series, patients who presented an initial ROTEM® pattern with “coagulopathy” were associated with a general profile of more severe trauma and higher ISS (23 vs 16). Many studies in this field correlate the state of coagulation and/or the development of ATC with the trauma severity. For example, Johansson et al.4 recorded associations between hypercoagulability and “moderate” trauma, hypocoagulability and “severe” trauma, and hyperfibrinolysis and “massive” trauma.
Compared to the group without coagulopathy, the group of patients with ATC had higher transfusion requirements, while also being associated with a higher percentage of CA and a significantly higher mortality rate (34% vs 5%). These data coincide with other studies published about ATC.3,10 Regarding the temporal distribution of mortality, most of the deaths in the group with coagulopathy occurred early in the first 24h of hospitalization (7 of the 11 deaths), as opposed to the group without coagulopathy (5 deaths, all of them >24h).
Our results confirm the presence of three different thromboelastometric profiles that reflect an early stage of ATC and determine the prognosis to a large extent: isolated hypocoagulability (15 cases), isolated hyperfibrinolysis (9 cases) and hypocoagulability associated with hyperfibrinolysis (8 cases).6,9
Patients with a combined ROTEM® pattern of “hypocoagulability and hyperfibrinolysis” were associated with higher ISS, lower Glasgow score and increased need for blood products and early arteriography. The mortality of this sub-group was also higher than that of the others, although it did not reach statistical significance. We can infer that the combined thromboelastometric pattern of “hypocoagulability and hyperfibrinolysis” represents an expression of maximum trauma severity, indicating patients who may need massive blood product replacement and a higher use of resources (logistical and therapeutic).
It should be noted that 9 patients (7%) presented a profile of isolated hyperfibrinolysis, with no consequences in terms of the transfusion of blood products or mortality. Our results contrast with those obtained by other groups, where the presence of hyperfibrinolysis is associated with greater transfusion needs and poorer prognosis.13,15
Recently, a variable spectrum of responses of the fibrinolytic system to severe trauma has been described16 using viscoelastic tests. At one end of the spectrum is hyperfibrinolysis, which is less prevalent (7%–18%) but more serious (mortality 34%–70%) and intrinsically associated with hemorrhagic shock. At the other end is resistance or inhibition of fibrinolysis (fibrinolysis shutdown), which is more frequent (46%–64%), with lower associated mortality (17%–23%), and is probably related to tissue injury.17,18 In our series, we excluded the hypercoagulability pattern as it is a minority (3%), although other studies have recorded it as the most frequent pattern, associated with mild trauma.6 Although it has not been considered in our study, the existence of an inhibited fibrinolytic profile (shutdown) raises the possibility of a “selective” administration of antifibrinolytics, while suggesting a possible relationship with the subsequent development of multiple organ failure and/or thromboembolic phenomena.17,18
Limitations of the study include the small sample size, the lack of uniformity of criteria in the literature to define the different thromboelastographic patterns, not having assessed the progression of the coagulopathy over time nor having made a series of ROTEM® determinations, and the absence of registries suggestive of hypofibrinolysis.
In conclusion, 26% of PTP patients present early coagulopathy evaluated by ROTEM® thromboelastography, associated with a higher consumption of blood products and lower survival rates. The combined profile of “hypocoagulability+hyperfibrinolysis” is associated with greater severity and higher requirements for blood products and arteriography.
Conflict of InterestThe authors have no conflict of interests to declare.
Please cite this article as: Bonet A, Madrazo Z, Koo M, Otero I, Mallol M, Macia I, et al. Perfil tromboelastométrico y coagulopatía aguda del paciente politraumatizado: implicaciones clínicas y pronósticas. Cir Esp. 2018;96:41–48.