Solitary fibrous tumors (SFT) are uncommon mesenchymal neoplasms which can arise at a wide variety of anatomic locations. SFT were described from the pleura firstly,1 but they could arise in extrathoracic sites at abdominal cavity, head and neck or retroperitoneum.2–4 Most of SFT are asymptomatic at presentation, but abdominal neoplasms can associate abdominal pain, urinary symptoms, or palpable mass.4 Malignant behavior is rare but possible.5,6
We present the case of 83-year-old woman with a malignant pelvic SFT who underwent surgery and later adjuvant radiotherapy, with no recurrence at 36 months follow-up.
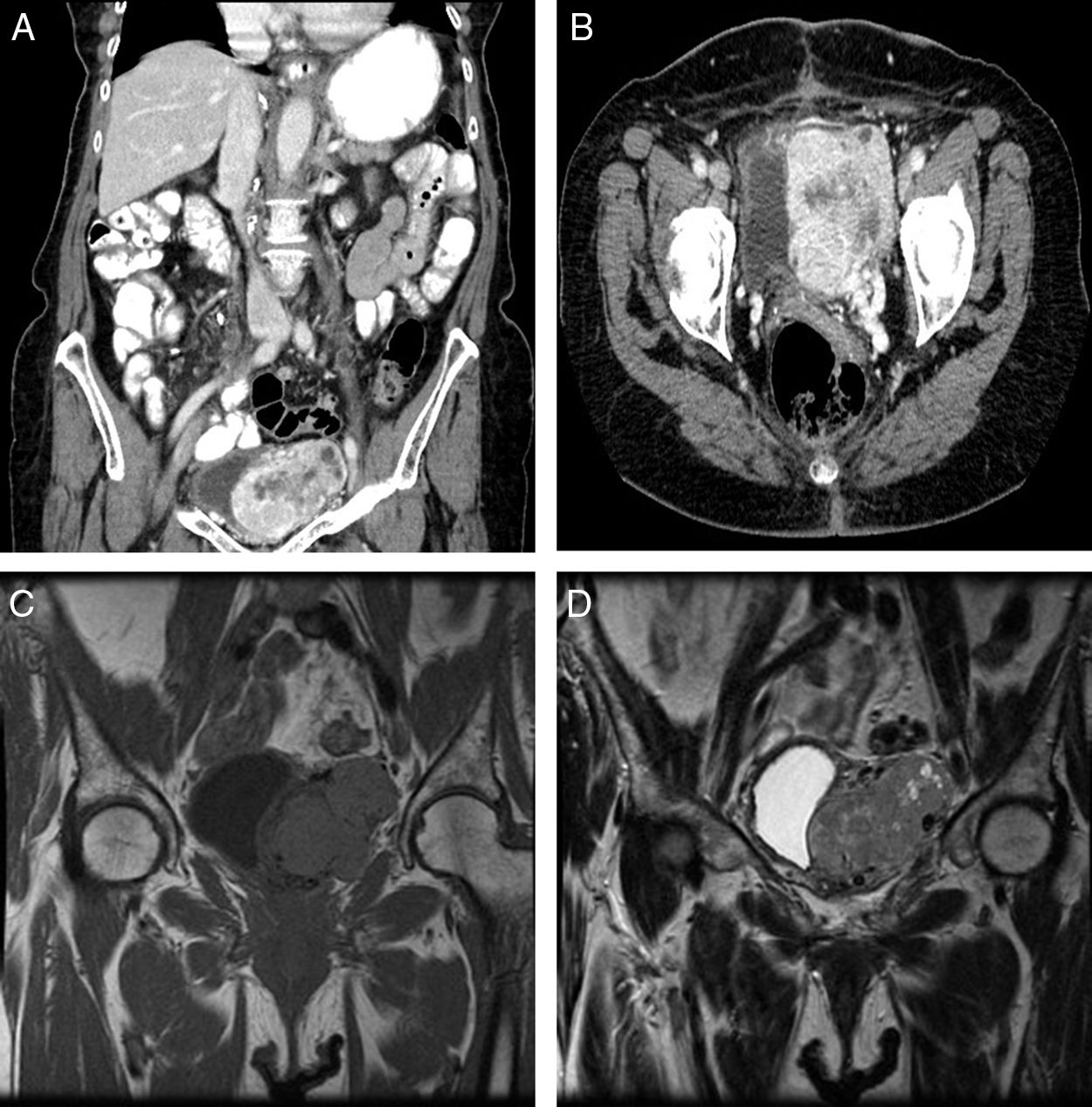
An 83-year-old woman referred to our service because of an incidental finding of a pelvic mass on abdominal ultrasonography ordered by her family physician. She only reported abdominal discomfort for several months in duration, and hypoglycemia was not present at presentation. On physical examination there was the clinical impression of a painless and palpable mass in the left iliac fossa. Laboratory tests were normal. A computed tomographic scan revealed a 7.5cm×5.4cm×4.2cm pelvic mass involving intimate contact with the left wall of the bladder, being this organ laterally displaced. The mass was well-defined, solid and lobulated, with internal necrotic areas and small peripheral cystic compounds. Multiple and dilated vascular structures located inside were identified, these being connected to peripheral vessels and irrigated by iliac internal vessels (Fig. 1A and B). A contrast magnetic resonance imaging (MRI) scan (Fig. 1C and D) showed an heterogeneous solid mass with mild hyperintensity in T2-weighted image, and intense contrast uptake.
(A and B) A computed tomographic scan shows a 7.5cm×5.4cm×4.2cm pelvic mass with intimate contact to the bladder. The mass was well-defined, solid, and with internal necrotic areas and small peripheral cystic compounds. Multiple and dilated vascular structures located inside were identified. (C and D) A contrast magnetic resonance imaging scan reveals a heterogeneous and hypervascularized solid mass. It shows isointensity in T1-weighted image (C), and partial hyperintensity in T2 (D), with intense contrast uptake.
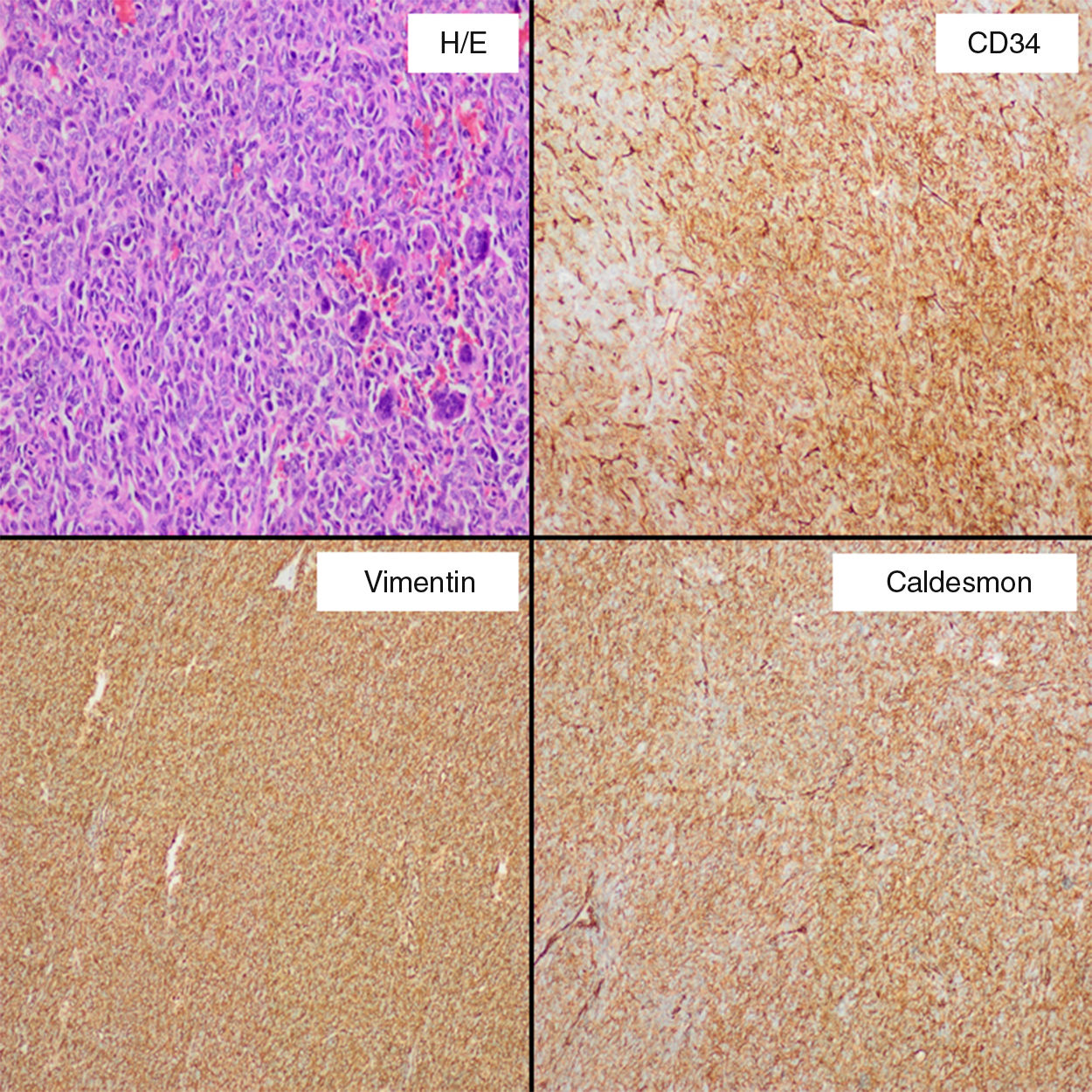
His medical history was uneventful and she underwent an infraumbilical laparotomy at 30 years with a left partial cystectomy for a non-cancerous ovarian cyst. A percutaneous biopsy of the mass was performed to direct the diagnosis. Result of biopsy was compatible with a mesenchymal and hypervascular neoplasm containing spindle cells and isolated atypia. Cells markers CD34 and CD99 were positive. In order to reduce the tumor size, the feeding arteries of the mass were embolized. A laparotomy was performed and there was a difficult dissection because of adhesions to wall of the bladder, left ureter and left intern iliac vein. It was safely resected with blood loss of 200cm3. Microscopically (Fig. 2), the specimen exhibited infiltrative margins and a high cell-density with an arrangement of short bundles and a mixture of necrotic area and dilated vascular structures. The mitosis rate was 4/10 high-power fields and Ki-67 proliferation index around 20%. Immunohistochemical staining showed that cells were positive for CD34, CD99, vimentin, caldesmon, and Bcl-2, and negative for S-100, desmin, CD117 and keratin, which was diagnostic for a malignant SFT. Postoperative course was uneventful. Subsequently, the patient underwent a treatment with adjuvant radiotherapy (60Gy). No recurrence was present at 36 months after surgery.
SFTs are uncommon mesenchymal neoplasms which account for less than 2% of all soft-tissue tumors. SFT was first described in 1931 as pleural mesothelioma,1 but it is now recognized that they can arise at any site, including a wide histologic spectrum with fibroblastic or myofibroblastic characteristics.4 These tumors have overlapping features of other soft-tissue tumors, and most hemangiopericytomas are also classified as SFT.3,4,6
Clinically, SFTs are often asymptomatic and large at presentation with manifestation as a slow-growing mass, being common symptoms like pain and palpable mass. Hypoglycemia may be present in up to 5% of patients, due to an autonomous tumoral secretion of insulin growth factor 2, being described as Doege–Potter syndrome.7 Abdominal distention, bowel obstruction or urinary retention could be present with tumors in the abdomen or pelvis.4 Computed tomography is the initial diagnostic tool of choice, and SFT is present like a hypervascular mass that displaces adjacent organs such as urinary bladder, ureter or vessels. Central hypoenhancing areas may represent necrosis or cystic changes. SFTs are characterized of juxtaposed hyper- and hypocellular spinde cell proliferation, and numerous thin-walled blood vessels. RM equally reveals the hypervascular features and intratumoral changes, T1-weighted image usually shows isointense appearance to adjacent muscle and T2 shows partial o predominant hyperintensity (the hypointense areas imply stromal compounds), including a high contrast uptake. Findings at inmunohistochemical analysis include CD34, Bcl-2, and vimentin positivity, with S100, actin, and keratin negativity.4–6
Malignant SFTs are typically large, with hypercellularity, focal moderate or marked atypia, a mitosis rate ≥4/10 high-power field, calcifications, numerous areas of necrosis and hemorrhage, and infiltrative margins.4–6 The differential diagnosis of malignant SFT is wide, and includes lesions as angiosarcoma, leiomyosarcoma, desmoid tumor, mesothelioma, and uterin neoplasms. Complete surgical resection with negative margins is the treatment of choice and preoperative embolization can reduce intraoperative hemorrhage. Because of location and size, with intimate contact to the bladder and history of an infraumbilical laparotomy we decide to perform a re-laparotomy, although minimally invasive approach has been described to treat abdominal or pelvic SFT, and it could be used safely.8 Neoadjuvant and adjuvant radiotherapy with or without concomitant chemotherapy have been used, with variable success rates.2,4,9
Please cite this article as: Mora-Guzmán I, Valdés de Anca Á, Sanchez-Urdazpal L. Tumor fibroso solitario pélvico maligno: una entidad inusual. Cir Esp. 2019;97:349–351.