The use of bilateral mastectomy with immediate reconstruction is increasing. Radiotherapy increases complications; however, its uses have been extended. We evaluate the profile of the complications and long-term failure of reconstruction through a comparative analysis with a cohort without radiotherapy.
MethodsRetrospective analysis of patients with breast cancer who underwent mastectomy with immediate reconstruction during 2000–2016. Three groups were evaluated: (1) patients who received radiotherapy and posterior breast reconstruction; (2) patients with bilateral mastectomy and immediate reconstruction following adjuvant radiotherapy; (3) patients who did not receive radiotherapy at all. Demographic variables, surgical techniques and postoperative morbidity were assessed.
Outcomes and complications were compared between cohorts. Analysis was done with SPSS Statistics.
Results296 bilateral mastectomies with immediate reconstruction. Mean age 48.4±9. No differences in comorbidity in the different groups.
Group 1: 125 patients. Radiotherapy given 21.69 months before, on average. Complication rate: 20%. Failure of reconstruction rate: 20%. Reoperation rate: 33.6%.
Group 2: 71 patients. Radiotherapy after reconstruction: mean 134.2 days. Complication rate: 36.7%. Failure of reconstruction rate: 21.1%. Reoperation rate: 16.9%.
Group 3: 100 patients. Complication rate: 25%. Failure of reconstruction rate: 21%. Reoperation rate: 20%.
Morbidity published in patients after radiotherapy before or after reconstruction is higher than complications in patients who did not receive radiotherapy. Even so, in our series they were similar.
We found a higher sequelae rate in group 1, with almost double the rate of reoperation.
ConclusionsPatients who underwent radiotherapy before reconstruction had a higher risk of developing failure of reconstruction and needing reoperation than those patients who received radiotherapy after breast reconstruction or did not receive radiotherapy at all.
La mastectomía bilateral con reconstrucción inmediata (MB+RMI) está aumentando. La radioterapia incrementa las complicaciones, pero se han ampliado los criterios de administración. Queremos evaluar las tasas de complicaciones/secuelas realizando un análisis comparativo con una cohorte sin radioterapia.
MétodosAnálisis observacional analítico de cohortes retrospectivo de pacientes tratadas mediante MB como tratamiento de cáncer de mama con RMI entre 2000 y 2016. Se evalúan 3grupos: grupo 1: pacientes previamente tratadas con cirugía local y radioterapia, y MB+RMI posterior; grupo 2: pacientes con MB+RMI y radioterapia posterior por un cáncer de novo, y grupo 3: pacientes con MB+RMI sin radioterapia previa ni posterior.
Evaluamos variables demográficas, técnicas quirúrgicas y morbilidad postoperatoria.
ResultadosSe intervinieron un total de 296 MB+RMI.
Grupo 1: 125 pacientes con radioterapia previa, administrada 21,69 meses antes de media. Tasa de complicaciones del 28,8%, secuelas 33,6% y reintervención 33,6%.
Grupo 2:71 pacientes con radioterapia tras reconstrucción 134,2 días de media. Tasa de complicaciones del 29,6%, secuelas 19,9% y reintervención 16,9%.
Grupo 3: 100 pacientes. Tasa de complicaciones del 30%, secuelas 21% y reintervención 20%.
Encontramos más secuelas en el grupo 1, con casi el doble de reintervenciones que en el grupo 2 (33,6% vs 16,9%; p=0,067).
ConclusionesLa tasa de complicaciones a largo plazo y la tasa de reintervenciones es mayor en el grupo MB+RMI con radioterapia previa que en los grupos MB+RMI con radioterapia posterior o MB+RMI sin radioterapia.
Despite therapeutic advances, about 45% of patients with breast cancer will undergo mastectomy,1 and 20%–40% of these procedures will be associated with some type of reconstruction technique to improve patient quality of life and reduce the socio-psychological impact of mastectomy.2
Over the last decade, bilateral mastectomy rates have been increasing. The same has been true for breast reconstruction rates,3 both deferred and immediate, although the latter involves an increased risk of postoperative complications.
Radiotherapy has been shown to increase complication rates, including infection, skin necrosis, capsular contracture, asymmetry and implant loss.4 However, the criteria for performing skin-sparing mastectomies that allow for immediate reconstruction have been extended; this procedure now seems safe even in patients with previous radiation as part of the conservative treatment of breast cancer or those who need postmastectomy adjuvant radiotherapy.5,6
The impact of prior radiotherapy or radiotherapy administered after mastectomy with immediate reconstruction continues to be infrequently reported. The objective of this study is to evaluate the complications, sequelae and reoperation rates and to conduct a comparative analysis with a cohort of patients treated by mastectomy and immediate reconstruction without associated radiotherapy.
Our aim was to determine which specific aspects in this patient population improved the quality of preoperative assessment and anticipate possible early complications and those arising during the long-term follow-up.
MethodsWe conducted a retrospective, observational analysis of a cohort of patients treated with bilateral mastectomy for breast cancer and immediate reconstruction using a direct prosthesis at our hospital from 2000 to 2016. We identified patients treated with radiation after breast-conserving surgery and subsequent reconstruction, as well as patients with mastectomy and immediate reconstruction who later required radiotherapy. We also selected a cohort of patients treated by mastectomy and immediate reconstruction without previous or subsequent radiotherapy.
Inclusion criteria for the group of patients diagnosed with local recurrence or new primary tumour (group 1): the indications were those stated above, and the following:
- –
Desire of the patient to complete the mastectomy after primary breast-conserving surgery.
- –
Impossibility to complete radiotherapy after conservative surgery and prior radiotherapy.
- –
Need for contralateral symmetry.
And the inclusion criteria for patients with de novo breast cancer (group 2 and group 3) were:
- –
Multicentric or multifocal carcinoma not treatable with conservative surgery. ‘Multifocal’ was defined as the presence of 2 or more tumour foci in the same quadrant and less than 5cm from the primary focus; ‘multicentric’ was the presence of 2 or more tumour foci in different quadrants of the same breast or more than 5cm from the primary focus.
- –
Large in situ component of the infiltrating tumour found in the preoperative diagnostic biopsy.
- –
High risk due to family history (no known mutation), defined by 2 or more family members (at least one of them first degree affected by breast or ovarian cancer at an early age [before 50]).
- –
Known mutation in BRCA 1 and 2 genes or other mutations responsible for the increased risk.
Patients with advanced ages (over 70) and inflammatory carcinoma were excluded, as well as bilateral mastectomy and immediate reconstruction with no present or past oncological disease (purely prophylactic mastectomy).
Data were collected for demographic variables (age; comorbidities, such as obesity, hypertension, diabetes mellitus, vasculopathy and active smoking of more than 10 cigarettes a day; personal and family history), clinical-pathological variables (indication, tumour size and state of the axilla, etc.) and variables related with surgical and adjuvant treatment (type of surgical intervention, reconstructive techniques used and postoperative morbidity). Postoperative complications (those that appeared within 30 days after the intervention) and sequelae (after 30 days) were evaluated.
The surgical technique used was similar in all patients: resection of the breast tissue leaving thin skin flaps, varying skin incisions and complete preservation of the nipple-areola complex (NAC) or using a free NAC graft. The incisions varied according to the size and configuration of the affected and contralateral breasts, tumour size and location, previous scars and preference of the surgeon, and should be planned with immediate breast reconstruction (IBR) in mind.
- –
Subcutaneous mastectomy by external lateral incision. Modified Spira technique: double-layer prosthesis using a de-epithelialized flap attached to the pectoralis major muscle and free NAC graft after negative intraoperative biopsy of the base of the nipple.
- –
Skin-sparing mastectomy following the Wise pattern: this is a typical reduction mammoplasty pattern where, in addition to the periareolar incision, there is a vertical prolongation towards the inframammary fold with a lateral and medial extension along the sulcus. This is the treatment of choice in patients with hypertrophy and ptosis.
- –
Skin- and nipple-sparing mastectomy by external radial incision. Immediate reconstruction was performed by means of a direct silicone anatomical implant in the majority of patients 290/296 (98%), except for 6 cases in which a myocutaneous flap without prosthesis was used.
- –
Reconstruction with autologous tissue (with or without associated prosthesis). Two techniques were used, in most cases using the lattisimus dorsi flap, and in 2 cases a rectus abdominis muscle flap.
The criteria to consider the need for adjuvant treatment with radiotherapy followed the current guidelines and recommendations at the time of treatment of these patients, as well as the steps and their durations, taking into account that they varied over the years of the study.7–9 In patients treated by skin-sparing mastectomy with IBR, the indications were the same as after standard mastectomy: chest wall radiation in large T3-T4 tumours, affected or proximal margins, carcinoma in situ if there was an involved margin that could not be extended. Radiation therapy of the chest wall and axillary-supraclavicular lymph node chains if there is more than one affected node, T4 tumours, and individualizing cases in G3 tumours, lymphovascular invasion, Her2 (+) or triple negative.
Statistical AnalysisTo find significant differences in the categorical variables, the chi-squared or Fisher's statistical tests were used. The Mann–Whitney and Student's t tests were used for nonparametric and parametric variables, respectively. A P-value of .05 was considered statistically significant. Analyses were done with SPSS Statistics version 22.
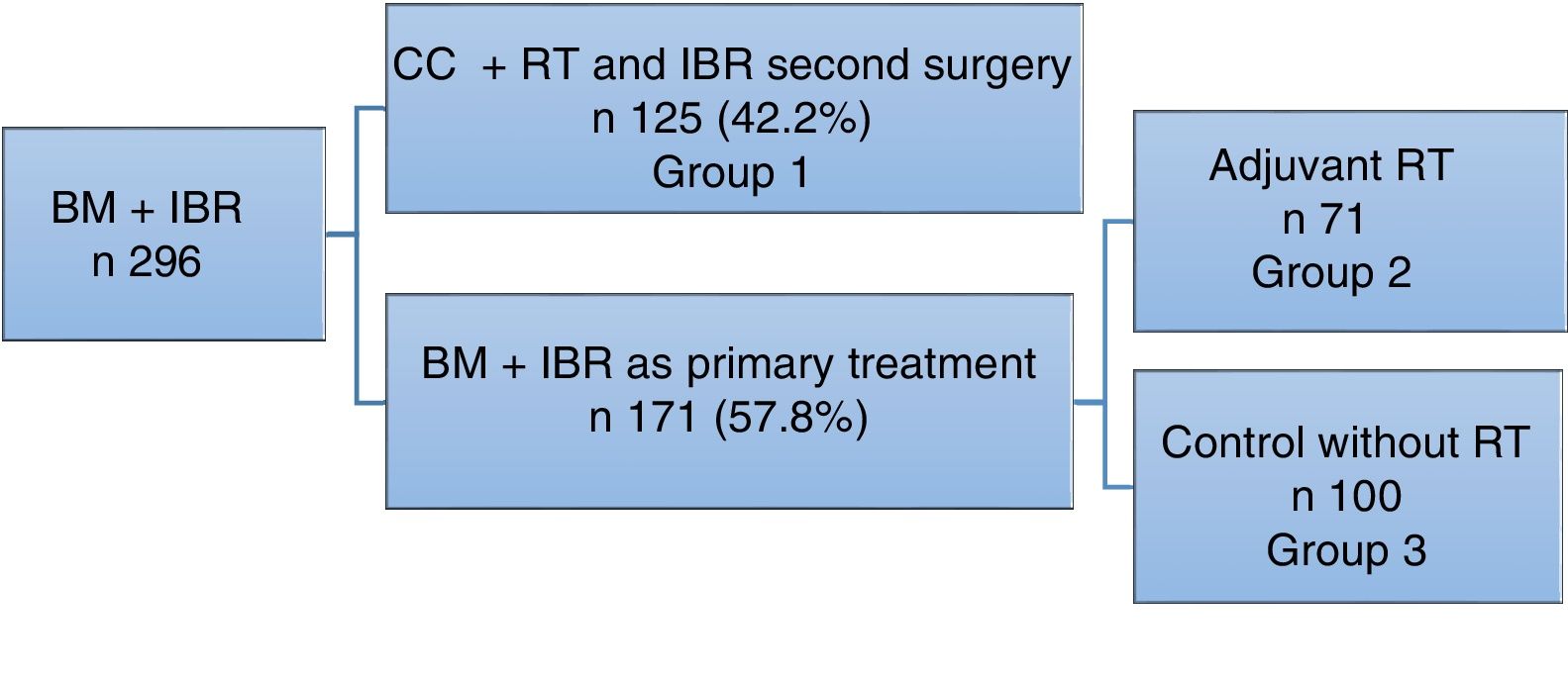
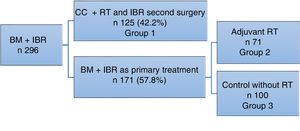
ResultsDuring the study period, 296 bilateral mastectomy procedures were performed with immediate breast reconstruction (BM+IBR) as a treatment for breast cancer at our hospital. Out of this total, 125 patients (42.2%) had been previously treated with breast-conservative surgery and radiotherapy, and in a second surgery bilateral mastectomy (completion mastectomy and contralateral mastectomy to reduce risk) was performed for different reasons: margin or proximal involvement, patient choice of mastectomy instead of widening of margins, local recurrence, or for cosmetic reasons to achieve symmetry.
Another 171 patients were treated in one operation (57.8%), meaning that BM+IBR was the primary treatment for breast cancer.
In this group, 71 patients required adjuvant radiotherapy. The remaining 100 patients, who did not require radiotherapy, were included as the control cohort (Fig. 1).
Overall results: the patients presented a mean age of 48.4±9.0 years (range 26–87) at the time of surgery.
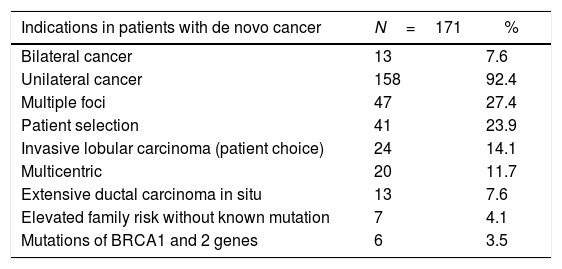
Bilateral mastectomy with immediate reconstruction is most frequently indicated by the choice of the patient, resection margin or proximal involvement, or to obtain symmetry in patients with previous cancer (group 1), and multifocal presentation followed by patient choice in the de novo cancer group (groups 2 and 3), as shown in Table 1.
Indications for BM+MRI in the Different Groups.
| Indications in patients with de novo cancer | N=171 | % |
|---|---|---|
| Bilateral cancer | 13 | 7.6 |
| Unilateral cancer | 158 | 92.4 |
| Multiple foci | 47 | 27.4 |
| Patient selection | 41 | 23.9 |
| Invasive lobular carcinoma (patient choice) | 24 | 14.1 |
| Multicentric | 20 | 11.7 |
| Extensive ductal carcinoma in situ | 13 | 7.6 |
| Elevated family risk without known mutation | 7 | 4.1 |
| Mutations of BRCA1 and 2 genes | 6 | 3.5 |
| Indications to complete the mastectomy for previously treated cancer | N=125 | % |
|---|---|---|
| Patient choice | 44 | 35.2 |
| Resection margin or proximal involvement | 32 | 25.6 |
| Achieve symmetry | 23 | 18.4 |
| Treatment of local recurrence | 16 | 12.8 |
| Contralateral cancer | 4 | 3.2 |
| Dense breast tissue and difficult follow-up | 3 | 2.4 |
| Mutations of BRCA 1 and 2 detected a posteriori | 3 | 2.4 |
The comorbidities that could affect the surgical technique and the development of complications are shown in Table 2. The distribution of these factors among the 3 groups of the study was homogeneous (P=.017).
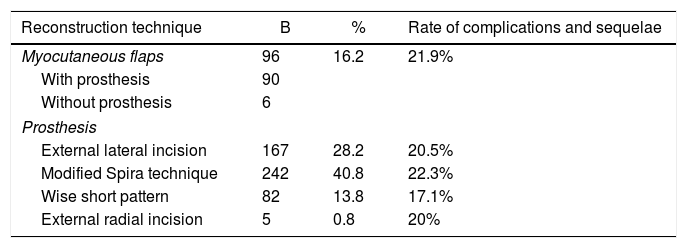
Surgical techniques: in some patients, different reconstruction techniques were used for each breast; for example, a flap in the previously operated breast (e.g., after unilateral simple mastectomy) and a subcutaneous mastectomy in the contralateral healthy breast (contralateral prophylactic mastectomy), while symmetrical reconstruction was performed in other patients, for example, with the Spira technique. The percentages were obtained from the total of 592 procedures.
The distribution of the different types of reconstruction in the 3 groups of the study was homogeneous (for purely technical reasons), individualizing each patient and choosing the most appropriate technique. No statistically significant differences were found.
Table 3 presents the results of the different techniques used.
Surgical Techniques and Eate of Complications/sequelae for Each Technique.
| Reconstruction technique | B | % | Rate of complications and sequelae |
|---|---|---|---|
| Myocutaneous flaps | 96 | 16.2 | 21.9% |
| With prosthesis | 90 | ||
| Without prosthesis | 6 | ||
| Prosthesis | |||
| External lateral incision | 167 | 28.2 | 20.5% |
| Modified Spira technique | 242 | 40.8 | 22.3% |
| Wise short pattern | 82 | 13.8 | 17.1% |
| External radial incision | 5 | 0.8 | 20% |
Results of group 1. Patients with conservative surgery and radiotherapy prior to mastectomy with reconstruction: between radiotherapy and BM+IBR, a mean of 21.69 months (range 3–180 months) transpired in this patient group.
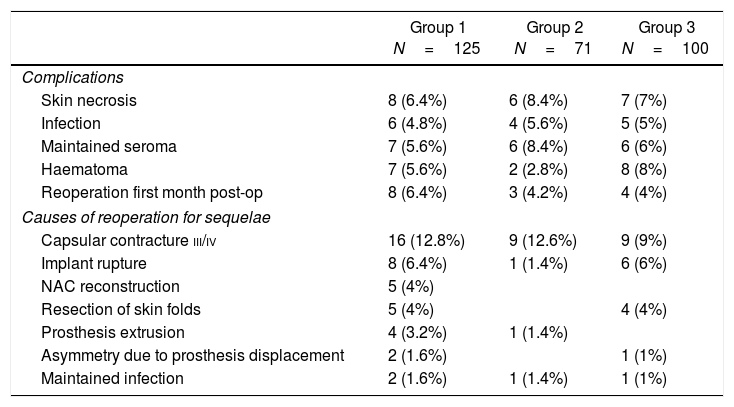
The overall rate of postoperative complications was 28.8% (36 out of 125 cases), while the rate of sequelae and poor long-term cosmetic results was 33.6% (42/125) and the reoperation rate after the first postoperative month was 33.6% (42/125). The results are shown in Table 4.
Complications and Sequelae in the 3 Study Groups.
| Group 1 N=125 | Group 2 N=71 | Group 3 N=100 | |
|---|---|---|---|
| Complications | |||
| Skin necrosis | 8 (6.4%) | 6 (8.4%) | 7 (7%) |
| Infection | 6 (4.8%) | 4 (5.6%) | 5 (5%) |
| Maintained seroma | 7 (5.6%) | 6 (8.4%) | 6 (6%) |
| Haematoma | 7 (5.6%) | 2 (2.8%) | 8 (8%) |
| Reoperation first month post-op | 8 (6.4%) | 3 (4.2%) | 4 (4%) |
| Causes of reoperation for sequelae | |||
| Capsular contracture iii/iv | 16 (12.8%) | 9 (12.6%) | 9 (9%) |
| Implant rupture | 8 (6.4%) | 1 (1.4%) | 6 (6%) |
| NAC reconstruction | 5 (4%) | ||
| Resection of skin folds | 5 (4%) | 4 (4%) | |
| Prosthesis extrusion | 4 (3.2%) | 1 (1.4%) | |
| Asymmetry due to prosthesis displacement | 2 (1.6%) | 1 (1%) | |
| Maintained infection | 2 (1.6%) | 1 (1.4%) | 1 (1%) |
NAC: nipple-areola complex.
Results of group 2. Patients with immediate reconstruction and adjuvant radiotherapy: adjuvant radiotherapy was performed after mastectomy with reconstruction after an average of 134.2±126.9 days after the intervention (range 19–344 days).
The overall rate of postoperative complications was 29.6% (21 out of 71 cases) and the rate of sequelae and poor long-term cosmetic results was 16.9% (12/71); the rate of reoperation after the first postoperative month was 16.9% (12/71). The results are shown in Table 4.
Results of group 3. Control cohort. Patients with mastectomy and immediate reconstruction without radiotherapy: patients treated with bilateral mastectomy and reconstruction with no indication for adjuvant radiotherapy had a general complication rate of 30% (30/100) and a sequelae rate of 21% (21/100); the rate of reoperation after the first postoperative month was 20% (20/100). Results are shown in Table 4.
DiscussionIn recent years, we have witnessed an increase in the number of patients undergoing mastectomy as rescue surgery due to local recurrence after conservative surgery and radiotherapy, as well as skin-sparing mastectomy in patients who will need postoperative radiotherapy for a locally advanced stage.10 It is therefore necessary to know whether immediate reconstruction has an acceptable complication rate in the described scenarios.
In general, published rates of early complications in patients treated with skin-sparing mastectomy after radiation either prior to or after reconstruction are higher than those of non-radiated patients and may exceed 30% of cases.11,12 In contrast, the 3 cohorts in our study experienced similar rates of immediate postoperative complications (28.8% in group 1, 29.6% in group 2, and 30% in group 3, with P=.21), although the size of our study is not enough to establish significant differences and conclude that previous radiotherapy does not influence this fact.
In the literature, we have found some studies similar to ours comparing the results of immediate reconstruction in cohorts of patients who received breast radiation therapy either previously or as adjuvant treatment.
Sbitany et al.5 focused their study on immediate postoperative complications, reporting a similar increase in relative risk in the 2 patient groups for the appearance of complications like haematoma, seroma, infection, skin or NAC necrosis, suture dehiscence or extrusion of the implant. Likewise, in our study we found that the rate of necrosis and infection was higher in the group of patients receiving adjuvant radiotherapy, and seroma or haematoma appeared with a similar frequency in both groups of patients.
The recently published study by Sosin et al.13 analyzed both early complications and sequelae as well as the needs for revision surgery in the 2 patient cohorts. In this study, both the early complication rate and the percentage of sequelae requiring reoperations after the first month were higher in the cohort of patients receiving adjuvant radiotherapy (33.3% vs 26.3% and 28.6% vs 10.5%, respectively). In contrast, our findings indicate a greater incidence of sequelae in patients with radiotherapy prior to reconstruction, especially in aesthetic complications (fundamentally, capsular contracture), with a reoperation rate of almost double (33.6% in group 1 vs 16.9% in group 2, P=.067), which was statistically significant. Like Sosin et al., Spear et al.14 found that capsular contracture was also more frequent in patients who received adjuvant radiotherapy (40% of cases vs 7.8% of patients with previous radiation).
In addition to radiotherapy, some specific predictors for complications after skin- and nipple-sparing mastectomy have been described, such as a high body mass index, active smoking and/or the type of incision made, and the periareolar incision is associated with greater risk of NAC necrosis.15 We have not analyzed the effect of these factors on the appearance of complications, although we have observed that the 3 cohorts are comparable in terms of the presence of these risk factors. If we analyze the surgical technique used, the complication rates in our series were similar to those published in the literature for the different techniques, as shown in Table 2.
All the studies consulted conclude that, although radiotherapy (either prior to10,16 or after mastectomy17) was the cause of a greater number of complications, these did not contraindicate immediate reconstruction, which is justified by adequate aesthetic results and patient satisfaction (factors that we have not analyzed in our study).
Our findings identify the cohort of patients treated by radiotherapy prior to mastectomy with immediate reconstruction as more likely to develop a long-term complication/sequela and require reoperation than patients who received radiotherapy after or those who were not treated by radiation.
However, this study does present certain limitations, as it is the experience of a single institution with a relatively small number of cases. Therefore, our results cannot be generalized.
In conclusion, the rate of long-term complications and the rate of reoperations are higher in the BM+IBR group with prior radiotherapy than in the BM+IBR groups with subsequent radiotherapy or BM+IBR without radiotherapy.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Allué Cabañuz M, Arribas del Amo MD, Navarro Barlés A, Guemes Sanchez AT. Influencia de la radioterapia sobre la reconstrucción mamaria inmediata posmastectomía ahorradora de piel. ¿Afecta igual antes que después? Cir Esp. 2019;97:156–161.